Published online Apr 14, 2014. doi: 10.3748/wjg.v20.i14.4102
Revised: September 14, 2013
Accepted: October 17, 2013
Published online: April 14, 2014
Processing time: 350 Days and 5.7 Hours
Cystic hepatic neoplasms are rare tumors, and are classified into two separate entities: mucinous cystic neoplasms (MCNs) and intraductal papillary mucinous neoplasms of the bile duct (IPMN-B). We report the case of a 56-year-old woman who presented with abdominal pain and jaundice due to the presence of a large hepatic multilocular cystic tumor associated with an intraductal tumor. Partial hepatectomy with resection of extrahepatic bile ducts demonstrated an intrahepatic MCN and an intraductal IPMN-B. This is the first report of the simultaneous occurrence of these two histologically distinct entities in the liver.
Core tip: Cystic hepatic neoplasms are rare tumors, and are classified as mucinous cystic neoplasms (MCN) characterized by intracystic septae, ovarian-like stroma, and no communication with bile ducts; and intraductal papillary mucinous neoplasms of the bile duct (IPMN-B) characterized by intraductal growth, dilated bile ducts, and papillary projections. We report a 56-year-old woman diagnosed with a large multilocular cystic tumor in the liver and in dilated extrahepatic bile ducts that were two histologically distinct cystic tumors. This is the first report of the simultaneous occurrence of MCN and IPMN-B. We present diagnostic considerations, therapeutic approaches, and the prognosis of cystic tumors of the liver.
- Citation: Budzynska A, Hartleb M, Nowakowska-Dulawa E, Krol R, Remiszewski P, Mazurkiewicz M. Simultaneous liver mucinous cystic and intraductal papillary mucinous neoplasms of the bile duct: A case report. World J Gastroenterol 2014; 20(14): 4102-4105
- URL: https://www.wjgnet.com/1007-9327/full/v20/i14/4102.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i14.4102
Cystic hepatic neoplasms are categorized into two separate entities: hepatic mucinous cystic neoplasms (MCN) and intraductal papillary mucinous neoplasms of the bile duct (IPMN-B)[1]. MCNs are rare tumors that account for less than 5% of all cystic liver lesions. MCNs are mucin-containing septate cystic tumors, covered with cuboidal or columnar epithelium that present with ovarian-like stroma between the inner epithelial lining and the outer connective tissue capsule. This type of tumor has no communication with the biliary tree. IPMN-Bs are a newly described pathologic entity characterized by intraductal microscopic or macroscopic papillary growth, forming a cystic tumor that communicates with the bile ducts and shows no ovarian-like stroma. IPMN-Bs may produce a large amount of mucin, resulting in extensive dilatation of the bile ducts[2,3]. These two types of cystic neoplasms may present variable stages of dysplasia, at times progressing via carcinoma in situ to invasive cholangiocarcinoma. We present a unique case of the simultaneous occurrence of both types of hepatic cystic neoplasms.
A 56-year-old woman presented at the outpatient clinic complaining of upper abdominal discomfort. Upper digestive tract endoscopy revealed Helicobacter pylori (H. pylori)-positive duodenitis. The patient was given H. pylori eradication therapy, but showed no remarkable improvement. After two months she developed jaundice and mild itching. Ultrasonography showed a large multilocular cystic tumor located in the median part of the left hepatic lobe (segment IVB).
The liver function tests demonstrated significantly increased serum liver enzyme levels: γ-glutamyl transpeptidase, 776 IU/L; alkaline phosphatase, 281 IU/L; alanine transaminase, 198 IU/L; aspartate transaminase, 125 IU/L; and serum bilirubin, 6.4 mg/dL. Carcinoembryonic antigen 19-9 and alfa-fetoprotein were normal. Hydatid cystic disease was excluded by negative serologic tests.
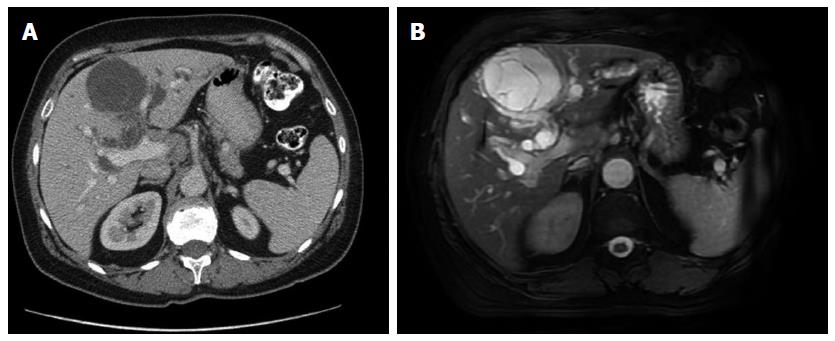
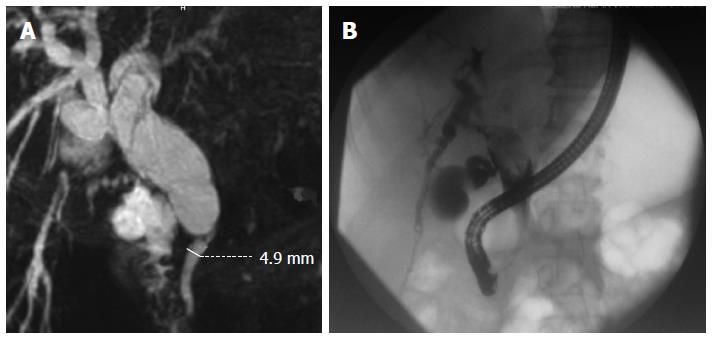
Computed tomography and magnetic resonance imaging revealed an 8-cm cystic tumor occupying hepatic segments IVB (Figure 1). The tumor had a thin capsule and septations that showed enhancement with intravenous contrast medium. The intrahepatic bile ducts were dilated, especially within the left hepatic lobe. The hepatic duct and proximal part of the common bile duct were dilated to 25 mm. Magnetic resonance cholangiopancreatography demonstrated significant dilation of the peripheral biliary ducts in the left lobe of the liver and cystic-like dilation of the proximal part of the extrahepatic biliary duct with a sharp change in the lumen diameter at the midportion of the common bile duct (Figure 2A).
Based on suspected malignancy, surgical resection was selected. Preoperative endoscopic retrograde cholangiopancreatography revealed a large oval tumor within the hepatic bile duct, presenting as a bulky filling defect (Figure 2B). On intraoperative inspection, we observed a large intrahepatic cystic tumor in the median part of the left hepatic lobe and a palpable floating soft mass in the main biliary duct that almost completely obstructed biliary flow. After incising the confluence of the hepatic ducts, the cystic tumor entering the left hepatic duct was visualized. The bile was thick, suggesting mucinous content. The two tumors were resected by left hemihepatectomy, excluding segment I, with concomitant resection of the extrahepatic bile ducts, followed by anastomosis of the right hepatic duct with Roux-Y intestinal loop. The postoperative course was uneventful. Neither tumor had recurred after 7 mo of postoperative follow-up.
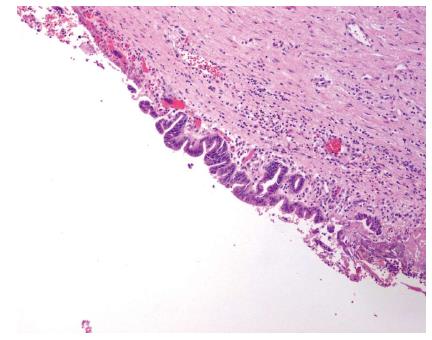
The tumors were grossly and microscopically separate. Microscopically, the intrahepatic tumor was a MCN composed of a single-layered mucin-producing epithelium that showed low-grade dysplasia without signs of malignancy (Figure 3A and B). The presence of ovarian-like stroma was confirmed by positive immunostaining for progesterone and estrogen receptors (Figure 3C). The tumor originating from the main hepatic duct was composed of epithelial papillary proliferations with focal ulcerations (Figure 4). None of the histologic features suggested a malignant change. The fibrous intratumoral interstitium lacked any mesenchymal or ovarian-like stroma.
The term IPMN-B refers to a recently classified subtype of biliary neoplasm, which is considered the biliary counterpart of pancreatic IPMN because they share several clinicopathologic features. It is a slow-growing tumor with relatively indolent clinical behavior and a lower malignant potential than ductal cell carcinoma and mucinous cystadenocarcinoma[4,5]. IPMN-B has been reported with several different names, such as mucin-producing intrahepatic cholangiocarcinoma, biliary papillary tumor, mucin-producing bile duct tumor, and biliary intraductal papillary mucinous neoplasia. An excellent prognosis with a 5-year survival rate higher than 80% can be expected after complete resection of the tumor with negative margins[6].
IPMN-B is associated with other malignancies (5.6% of cases), including hepatocellular carcinoma, gallbladder cancer, cervical cancer, and salivary gland cancer[4]. Several reports on the coincidence of IMPN-B with its pancreatic counterpart have been published[7,8]. To the best of our knowledge, this is the first case in the English-language literature of the simultaneous occurrence of IPMN-B and MCN in the liver. In contrast to MCNs, which are more frequently detected in women, IPMN-Bs occur in both sexes and are usually located in the left hepatic duct or perihilar region presenting as a multicystic tumor with a grape-like appearance[3,9]. Ovarian-like stroma is considered essential for the diagnosis of MCN, whereas papillary-like projections and intraductal growth are necessary for the diagnosis of IPMN-B. Missed diagnosis of IPMN-B may be common. In our patient, preoperative endoscopic retrograde cholangiopancreatography was more useful in the diagnosis of the intraductal tumor than routine computed tomography and magnetic resonance imaging, possibly due to its cystic nature. In IPMN-Bs, the bile ducts are frequently dilated due to mucin hypersecretion and accumulation. In our case, the hepatic and common bile ducts were dilated mainly due to the large size of the neoplasm obstructing biliary outflow. Dilated peripheral bile ducts in IPMN-B may be mistaken for other biliary duct diseases, including Caroli disease. The tumors were symptomatic in our patient, as she complained of abdominal pain, itching, and jaundice. Although the size of the tumors suggested malignant transformation, the histologic features did not suggest malignancy.
In conclusion, we present a unique case of the simultaneous occurrence of a MCN and an IPMN-B in the liver that could be radically resected.
P- Reviewers: Metaxas G, Serin KR S- Editor: Gou SX L- Editor: Webster JR E- Editor: Ma S
| 1. | Tsui WMS, Adsay NV, Crawford JM. Mucinous cystic neoplasms of the liver. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Digestive System. Lyon: IARC Press 2010; 236-238. |
| 2. | Chen TC, Nakanuma Y, Zen Y, Chen MF, Jan YY, Yeh TS, Chiu CT, Kuo TT, Kamiya J, Oda K. Intraductal papillary neoplasia of the liver associated with hepatolithiasis. Hepatology. 2001;34:651-658. [PubMed] |
| 3. | Zen Y, Pedica F, Patcha VR, Capelli P, Zamboni G, Casaril A, Quaglia A, Nakanuma Y, Heaton N, Portmann B. Mucinous cystic neoplasms of the liver: a clinicopathological study and comparison with intraductal papillary neoplasms of the bile duct. Mod Pathol. 2011;24:1079-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 4. | Yeh TS, Tseng JH, Chiu CT, Liu NJ, Chen TC, Jan YY, Chen MF. Cholangiographic spectrum of intraductal papillary mucinous neoplasm of the bile ducts. Ann Surg. 2006;244:248-253. [PubMed] |
| 5. | Matsubara T, Sato Y, Sasaki M, Harada K, Nomoto K, Tsuneyama K, Nakamura K, Gabata T, Matsui O, Nakanuma Y. Immunohistochemical characteristics and malignant progression of hepatic cystic neoplasms in comparison with pancreatic counterparts. Hum Pathol. 2012;43:2177-2186. [PubMed] |
| 6. | Lee SS, Kim MH, Lee SK, Jang SJ, Song MH, Kim KP, Kim HJ, Seo DW, Song DE, Yu E. Clinicopathologic review of 58 patients with biliary papillomatosis. Cancer. 2004;100:783-793. [PubMed] |
| 7. | Valente R, Capurso G, Pierantognetti P, Iannicelli E, Piciucchi M, Romiti A, Mercantini P, Larghi A, Federici GF, Barucca V. Simultaneous intraductal papillary neoplasms of the bile duct and pancreas treated with chemoradiotherapy. World J Gastrointest Oncol. 2012;4:22-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Xu XW, Li RH, Zhou W, Wang J, Zhang RC, Chen K, Mou YP. Laparoscopic resection of synchronous intraductal papillary mucinous neoplasms: a case report. World J Gastroenterol. 2012;18:6510-6514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Zen Y, Fujii T, Itatsu K, Nakamura K, Konishi F, Masuda S, Mitsui T, Asada Y, Miura S, Miyayama S. Biliary cystic tumors with bile duct communication: a cystic variant of intraductal papillary neoplasm of the bile duct. Mod Pathol. 2006;19:1243-1254. [PubMed] |