Published online Apr 7, 2014. doi: 10.3748/wjg.v20.i13.3542
Revised: November 22, 2013
Accepted: March 5, 2014
Published online: April 7, 2014
Processing time: 174 Days and 7.8 Hours
Anemia, a common complication associated with inflammatory bowel disease (IBD), is frequently overlooked in the management of IBD patients. Unfortunately, it represents one of the major causes of both decreased quality of life and increased hospital admissions among this population. Anemia in IBD is pathogenically complex, with several factors contributing to its development. While iron deficiency is the most common cause, vitamin B12 and folic acid deficiencies, along with the effects of pro-inflammatory cytokines, hemolysis, drug therapies, and myelosuppression, have also been identified as the underlying etiology in a number of patients. Each of these etiological factors thus needs to be identified and corrected in order to effectively manage anemia in IBD. Because the diagnosis of anemia in IBD often presents a challenge, combinations of several hematimetric and biochemical parameters should be used. Recent studies underscore the importance of determining the ferritin index and hepcidin levels in order to distinguish between iron deficiency anemia, anemia due to chronic disease, or mixed anemia in IBD patients. With regard to treatment, the newly introduced intravenous iron formulations have several advantages over orally-administered iron compounds in treating iron deficiency in IBD. In special situations, erythropoietin supplementation and biological therapies should be considered. In conclusion, the management of anemia is a complex aspect of treating IBD patients, one that significantly influences the prognosis of the disease. As a consequence, its correction should be considered a specific, first-line therapeutic goal in the management of these patients.
Core tip: Anemia represents one of the major causes of both decreased quality of life and increased hospital admissions among inflammatory bowel disease (IBD) patients. This paper analyses the complex etiological and pathophysiological mechanisms underlying anemia in IBD, including iron and micronutrients deficiency, effects of proinflammatory mediators and bone marrow insufficiency secondary to the disease by itself and IBD therapy. By a comprehensive review of the current diagnostic and therapeutic evidences on anemia in IBD, an state-of-the-art approach will be provided to effectively manage this challenging and common condition.
- Citation: Guagnozzi D, Lucendo AJ. Anemia in inflammatory bowel disease: A neglected issue with relevant effects. World J Gastroenterol 2014; 20(13): 3542-3551
- URL: https://www.wjgnet.com/1007-9327/full/v20/i13/3542.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i13.3542
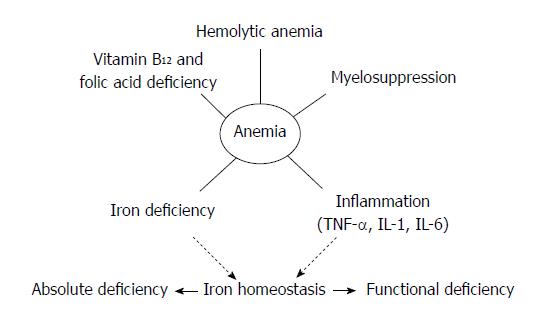
Anemia, a frequent systemic complication in patients with inflammatory bowel disease (IBD), has a complex and multifactorial pathogenesis (Figure 1). It is considered a prototype of a combination of iron deficiency (IDA) and anemia of chronic disease (ACD), which is caused by the negative effects of an activated immune system at different levels of erythropoiesis[1,2]. Besides IDA and ACD, metabolic disturbances, vitamin deficiencies, and various drug therapies commonly used in IBD can aggravate anemia in IBD patients[3]. The study of anemia in these patients thus requires a specific diagnostic and therapeutic approach.
It is important to highlight that anemia has a significant impact on the disease and is one of the most frequent comorbid conditions associated with mortality in IBD patients[4]. In addition, it also has a relevant effect on health related quality of life (QoL) and ability to work[5,6]. The fact that it is also a common cause of hospitalization and delay of discharge[7] only serves to underscore the need for prompt diagnosis and management of this condition.
Although the correction of anemia in IBD patients can improve the QoL and the quality of patient management, the specific diagnosis and treatment of anemia is often a low priority for gastroenterologists and has thus received little attention. A recent study showed that further diagnostic tests were undertaken in only one-third of patients with proven anemia and that 54.3% of patients diagnosed with IDA receive no iron supplements[8,9].
This article reviews current data on the diagnosis and treatment of anemia in IBD patients. A search was conducted in the PubMed, Cochrane, MEDLINE, and Scopus libraries with the following individual and combined key words: Crohn’s disease, ulcerative colitis, anemia, iron deficiency anemia, anemia of chronic disease, vitamin B12 deficiency, folic acid deficiency, myelodysplastic syndrome, refractory anemia, iron supplementation, intravenous iron therapy, erythropoietin, and inflammatory bowel disease. References cited in the articles retrieved were also searched in order to identify other potential sources of information. The results were limited to human studies available in English.
The prevalence of anemia in IBD is markedly variable, ranging from 6% to 74% in two systematic reviews[10,11]. The more recent review calculated a mean prevalence of 17% (16% in outpatients and 68% in hospitalized patients), with anemia occurring more frequently in patients with Crohn’s disease (CD) than in those with ulcerative colitis (UC)[3,12]. This variability in the prevalence of anemia depends on different factors. First, the definition of anemia is not homogenous in the various studies reviewed. In fact, because the widely accepted World Health Organization criterion for the diagnosis of anemia [hemoglobin (Hb) below 13 g/dL in men or 12 g/dL in non-pregnant women][13] has been questioned due to racial differences, environmental conditions, and eating habits[14,15], its use cannot completely reflect the real prevalence of anemia in different IBD populations. Furthermore, estimations of the prevalence of anemia often depend on the specific groups of patients studied (for example, hospitalized patients vs outpatients). In this sense, a study from a Swedish cohort showed that the prevalence of anemia in hospitalized UC and CD patients was higher than among outpatient populations (5% vs 35% and 9% vs 50%, respectively)[16]. Furthermore, it is important to note that anemia has been poorly studied in pediatric IBD patients. One recent epidemiological study showed that while the prevalence of anemia was 72% at the time of diagnosis, the proportion of severely anemic pediatric patients decreased from 34% to 9% while the number of patients with mild anemia doubled after 1 year of follow-up[17]. Finally, because Hb levels form part of a widely used disease activity index, the presence of anemia correlates directly with disease activity, which means that the prevalence of anemia may change throughout the natural history of the disease. In fact, the prevalence of mild and moderate anemia in IBD has decreased over time, reflecting improved treatment and management of the disease. However, the prevalence of severe anemia in IBD patients over the last 10 years has not decreased in the same manner[18].
Iron deficiency is the most common cause of anemia in IBD patients, with a reported prevalence of up to 90%[11]. Iron deficiency may be related to “absolute iron deficiency” due to low dietary intake and blood loss from ulcerated intestinal mucosa (especially in UC patients) along with reduced iron absorption (especially in CD localized in the upper GI tract), or it may be related to “functional iron deficiency.”
Iron is an essential mineral for the function of all body cells and is absorbed at the apical surface of enterocytes to be transported by ferroportin across the basolateral surface of the enterocyte into the circulation[19]. In the maintenance of iron homeostasis, the peptide hormone hepcidin is a master regulator that is produced in response to iron overload or upon induction by pro-inflammatory stimuli such as lipopolysaccharide or interleukin (IL)-6. In fact, inflammatory conditions can interfere with iron absorption by causing an increase in hepcidin that inhibits ferroportin activity[20], leading to its internalization and degradation[21]. The inhibition of ferroportin activity blocks the transfer of absorbed iron from the enterocyte into the circulation and causes iron retention in the macrophages and monocyte cells[22]. In addition, during inflammation other events contribute to the retention of iron in these cells, including the inhibition of ferroportin transcription by pro and anti-inflammatory cytokine action and a reduction in the half-life of erythrocytes due to oxidative stress and lipidperoxidation, with iron recycling through erythrophagocytosis[23]. These mechanisms all lead to “functional iron deficiency,” which means that despite an abundance of iron in the body, it is not available for erythropoiesis.
The exact prevalence of ACD in IBD patients is unknown[24], with its etiology being ascribed to altered erythropoiesis at different levels[25]. Firstly, chronic inflammation can decrease erythropoiesis by direct action of interferon (IFN)-γ, IFN-α, tumor necrosis factor (TNF)-α, and IL-1 in the bone marrow to exert pro-apoptotic effects on erythroid burst-forming units (BFU-E) and colony-forming units (CFU-E)[26]. Moreover, IL-1, IL-6, TNF-α, and hepcidin may decrease erythropoietin (EPO) synthesis and impair its biological activity[1,27]. In fact, EPO levels in ACD have been found to be inadequate in some chronic disease and IBD patients[11,28,29]. Low EPO production is due to direct inhibition of the activity of the promoter of the EPO gene by IL-1 and TNF-α, which in turn inhibits the synthesis of EPO in the kidney and acts indirectly on EPO-producer cells through cytokine-induced toxic radicals[30]. Impairment of the biological activity of EPO means that much higher amounts of EPO are needed to restore the formation of CFU-E in the bone marrow. Cytokines can also interfere with the signaling process mediated by the interaction of EPO and its receptor and can downregulate EPO receptors on erythroid progenitor cells[26], thus producing cell resistance to EPO activity. Finally, the limited availability of iron for heme biosynthesis induced by “functional” or “absolute’’ iron deficiency and the inhibition of iron uptake into erythroid progenitors due to the blocking of the transferrin receptor by alfa1-antitrypsin (an acute phase protein) negatively affect the biological functions of EPO along with cell growth and differentiation[1].
Vitamin B12 deficiency has been observed in 48% of CD patients and in 5% of UC patients[31] while folic acid deficiency has been noted in 67% of CD patients and in 30%-40% of UC patients[31-33]. These types of deficiencies depend on low dietary intake as well as increased turnover of epithelial cells due to chronic inflammation in the intestinal mucosa and a reduced absorption in the intestinal tract[34-36]. In CD, several factors influence these deficiencies, including the inflammatory involvement of ileal mucosa, the presence of fistulas, secondary bacterial overgrowth with direct consumption of vitamin B12, and extensive surgical resections in small bowel segments with impaired absorption[37]. Deficiencies in patients with UC derive from proctocolectomy and ileo-pouch anastomosis, with the prevalence of vitamin B12 deficiency being affected more by surgical changes leading to impaired function of ileal receptors, reduced intestinal transit time, and secondary bacterial overgrowth than on the length of the ileal segment resected[38].
Autoimmune hemolytic anemia (AIHA) is a rare type of anemia observed in UC patients. It can be due either to the development of antibodies with cross-reactivity with erythrocytes[39] or to the hemolytic effect of sulfasalazine in patients with glucose-6-phosphate dehydrogenase deficiency[40]. This association was first described in 1955 by Lorber et al[41] with the most recent studies calculating that the prevalence of AIHA in UC patients is between 0.2%-1.7%, as indicated by a positive Coombs test result in 1.8% of patients studied[40]. AIHA can occur before, after, or at the moment of diagnosing UC. Even when the potential relationship between disease activity and the occurrence of AIHA is not clear, a correlation with the extension of the disease has been demonstrated in several reports, which show a prevalence of AIHA of up to 28% in patients with extensive colitis[40].
Anemia can also represent a late manifestation of myelosuppression in IBD patients due to several factors. Firstly, myelosuppression can be associated with myelodysplastic syndrome (MDS), with ineffective erythropoiesis and a risk of progression to acute myeloid leukemia. Some studies have shown a frequent predominance of MDS in CD with colorectal involvement; however, it should be noted that the prognosis of IBD with concomitant MSD is determined by the MSD itself[42]. The prevalence of MDS in IBD patients has been estimated to be 0.17%, with a higher incidence in IBD patients than in the general population (170/100000 IBD patients/year vs 20-30/100000 of the general population over the age of 70/year)[43]. This is probably due to a undetermined common pathogenetic mechanism, the long-term use of immunosuppressive drugs, or chromosomal abnormalities in bone marrow cells that have been observed in 67% of patients with concomitant IBD and MSD[44,45]. These can induce the development of colitogenic monocytes, producing a large number of pro-inflammatory cytokines resistant to apoptosis upon stimulation with microbial antigens. Indeed, one of the first hypotheses about this association regarded IBD to be an extra-hematologic manifestation of MSD with a vasculitic process at the level of the mesenteric arteries[46]. Alternatively, myelosuppression may represent a complication of severe UC with the development of a systemic inflammatory response syndrome, or even been a side effect of immunosuppressive drugs. There is an increasing concern about therapy-induced leukemias and myelodysplastic syndromes in patients treated with thiopurines, which are extensively used as immunosuppresants in IBD, particularly for maintenance therapy[47]. Data from a large French cohort of patients (19486) with inflammatory bowel disease identified a relative risk of developing lymphoproliferative disorders as 5.2 for patients who were treated with thiopurines compared to those who were not[48].
Finally, additional gastrointestinal diseases that do not usually cause bleeding should be also considered in case of iron deficiency anemia in those IBD patients who maintain disease into remission, including colon or gastric cancer o polyps, peptic ulcer, hiatal hernia with linear erosions, atrophic or Helicobacter pylori-associated gastritis, and celiac disease[49,50].
Basic laboratory screening for anemia in IBD should consist of hemoglobin and full blood counts (including reticulocytes to differentiate between regenerative or hypo-regenerative anemia), with a determination of erythrocyte mean corpuscular volume (MCV) to distinguish between microcytic, normocytic, and macrocytic anemia as well as a determination of mean corpuscular Hb (MCH) and reticulocyte Hb content (CHr), if available. Moreover, assessments of both the level of inflammation by means of C-reactive protein (CRP) and of iron status are required. There is no single biomarker to diagnose iron deficiency in IBD; a combination of different biomarkers is needed. In most cases, total store of body iron with serum-ferritin (or ferritin) and the iron available in the bone marrow with transferrin saturation (TfS) is sufficient to differentiate between IDA and ACD[51]. However, many of the laboratory measures of iron status may be unreliable in IBD patients because the inflammation influences all parameters of iron metabolism to produce “functional iron deficiency[52,53]”. For this reason, in some cases it is essential to use other, more specific biomarkers of iron status to allow for the differentiation between predominantly IDA, predominantly ACD, and ACD combined with iron deficiency in order to provide appropriate, more effective treatment[54] (Table 1). Further testing for causes of anemia in IBD may include tests for vitamin B12, folic acid (especially erythrocyte levels, which, when available, represent the best stable marker of folic acid deficiency), haptoglobin, lactate dehydrogenase, indirect bilirubin (with Coombs test if hemolytic anemia is confirmed), and serum creatinine in order to rule out potential hemolysis or renal failure, which in itself can cause macrocytic or normocytic anemia[51]. It should be noted that if the origin of anemia is not obvious, IBD patients should be tested for MDS, especially if normocytic and hypo-regenerative anemia are both present, carrying out a bone marrow study in selected patients.
| Biomarkers | IDA | ACD | Mixed anemia |
| MCV (fL) | < 80 | Normal or reduced | Normal or reduced |
| MCH (pg) | < 27 | Normal | Normal |
| CHr (pg) | < 28 | Normal | Normal |
| C-RP (mg/dL) | Vormal | > 5 | > 5 |
| ferritin (ng/mL) | < 30 | > 100 | 30-100 |
| TfS (%) | < 20 | < 20 | < 20 |
| Ferritin index | > 3.2 | < 11 | > 2 |
| sTfR | Increased | Normal | Normal or increased |
| Hepcidin (nmol/L) | Reduced | Increased (> 4) | Increased (> 4) |
Once a diagnosis of IBD has been established, patients in clinical remission should be screened for anemia at least every 6 to 12 mo, whereas patients with active disease should be tested every 3 mo or at even shorter intervals, depending upon their iron status[55].
Patients are considered to suffer from IDA when they present with low Hb (men < 13 g/dL, non-pregnant women < 12 g/dL), TfS < 20%, and ferritin concentrations < 30 ng/mL without any biochemical or clinical signs of inflammation. A low MCH (< 27 pg) or even better a low CHr (< 28 pg) rather than MCV (< 80 fL) have became the most important red cell markers for detecting iron deficiency in circulating red blood cells. Although MCV is a reliable and widely available measurement, it tends to be a relatively late indicator in patients who are not actively bleeding[56]. A normal Hb level does not rule out iron deficiency and with an MCH in the lower limit of normal (normal range: 28-35 pg) or an increased red cell distribution width (RDW, normal range: 11-15), one can suspect the presence of mild iron deficiency without anemia[57]. Although the main laboratory marker for iron deficiency with or without anemia is a low ferritin level (< 30 ng/mL) in the absence of inflammation, in the presence of inflammation a normal ferritin level (as an acute phase reactant) does not rule out iron deficiency; therefore, TfS should also be measured. “Functional iron deficiency” in inflammatory conditions should be defined by low TfS (< 20%) and normal ferritin concentration (> 100 ng/mL), whereas low TfS (< 20%) and intermediate ferritin values (30-100 ng/mL) suggest “absolute iron deficiency[57]”. Some authors suggest a cut-off value of TfS < 16% combined with low iron value for the diagnosis of iron deficiency[51]. Iron deficiency can also be defined by a ferritin index > 3.2 (> 2 if CRP > 5 mg/L). The ferritin index, which reflects the iron supply for erythropoiesis, is calculated as the ratio between the soluble transferrin receptor (sTfR) and the log of ferritin[58]. The sTfR is a truncated fragment of the membrane receptor and its levels increase when the availability of iron for erythropoiesis is low, as occurs in IDA. CHr, which measures the Hb content of reticulocytes, reflects the direct measurement of available iron for erythropoiesis and is useful for differentiating IDA from ACD. In particular, CHr has a high sensitivity and specificity for diagnosing iron deficiency and is less affected by inflammation than TfS and ferritin, but no data are available for its use in IBD[58].
Patients are considered to suffer predominantly from ACD when they present evidence of inflammation (with increased levels of serum CRP and clinical signs), an Hb concentration < 13 g/dL for men and < 12 g/dL for non-pregnant women, and a low TfS < 20%, but normal or increased ferritin concentrations > 100 ng/mL. In the presence of intermediate ferritin concentrations (30-100 ng/mL), a diagnosis of ACD combined with “absolute iron deficiency” is confirmed if the ferritin index has a value < 2 with normal CHr[54,58-60]. Still, some cases may require supplementary testing for the differential diagnosis between IDA and ACD. It has recently been shown that hepcidin levels may replace the ferritin index for the confirmation of combined IDA and ACD if the hepcidin levels are > 4 nmol/L with a CHr < 28 pg[61]. In fact, hepcidin levels have been found to be significantly higher in IBD patients compared with healthy controls, with a significant correlation with ferritin levels, CRP, and disease activity, whereas those of prohepcidin were observed to be significantly lower[62]. In addition, although other hematological indices may help in the diagnosis of iron deficiency in ACD, many of them are only available in specific hematology analyzers and their precise clinical usefulness has yet to be determined. In a recent study carried out with a Beckman-Coulter LH 780, high values of RDW and low values of blood cell Size Factor were the best markers for the diagnosis of IDA, whereas both Reticulocyte Distribution Width-Coefficient of Variation (RDWR-CV) and Reticulocyte Distribution Width-Standard Deviation (RDWR-SD) were significantly correlated to disease activity and CRP levels[63].
Iron supplementation should be considered in every patient presenting iron deficiency with or without anemia. In patients with mild to moderate anemia (Hb ≥ 10 g/dL), the administration of oral iron at optimal low doses of 60-120 mg/d is the conventional approach recommended by the Centers for Disease Control and Prevention[9,64]. Oral iron compounds are mostly available as inorganic ferrous salts, such as ferrous fumarate, ferrous sulphate, and ferrous gluconate containing 33%, 20%, and 12% of elemental iron, respectively. A single tablet of most of these ferrous salt preparations provides a sufficient dose for the treatment of iron deficiency[65,66]. In fact, there is no evidence to support the administration of high doses of iron in comparative trials[65-67]; on the contrary, excessive doses may actually decrease tolerance and compliance while increasing gastrointestinal side effects, with a discontinuation of iron treatment in 20% of patients with or without IBD[68]. Nevertheless, there are several drawbacks associated with oral iron therapy that must be taken into account. In addition to the generally low bioavailability of oral iron, intestinal absorption is further compromised in IBD patients due to an inflammation-driven blockade caused by increased hepcidin levels. For this reason, in patients with active inflammation and combined ACD/iron deficiency, intravenous administration of iron may be preferable to oral iron therapy. Moreover, when achieved, the therapeutic effect of oral iron supplementation is slow, requiring two to three weeks to obtain increased Hb concentrations and up to two months to achieve normal values. At least six months are needed to replenish iron stores completely[69]. Moreover, non-absorbed iron salts can be toxic to the intestinal mucosa and oral iron has been shown to increase intestinal inflammation and possibly colon carcinogenesis in animal models through the production of reactive oxygen species that mediate intestinal damage and the alteration of the intestinal bacterial milieu in rodents[70-75].
Intravenous iron therapy is more effective, has a higher response rate, and is better tolerated by patients, with a lower discontinuation rate due to adverse events than oral iron supplementations in IBD patients, as demonstrated in a recent systematic review with meta-analysis[76]. However, it is important to highlight that of 757 articles identified, only three industry-funded articles met the inclusion criteria for this systematic review[69,77-83]. Nevertheless, intravenous iron therapy should be considered in patients with severe anemia (< 10 g/dL), with intolerance or inadequate response to oral iron, or with concomitant erythropoietin treatment and/or presence of active IBD. It should be noted that the new intravenous iron formulations (iron carboxymaltose, iron ferumoxytol, and iron isomaltoside) reduce both the risk of free iron reactions as well as that of immunogenicity without the need for administering a test dose before starting treatment. Treatment duration is also reduced because the new formulations are safer at higher doses than traditional intravenous iron formulations (iron sucrose, ferric gluconate, and low molecular weight iron dextran). Iron carboxymaltose is the only new intravenous iron formulation approved for use in Europe that has been studied in IBD patients. Its superiority at higher standardized doses over individually calculated doses of iron sucrose has been demonstrated along with its efficacy in reducing anemia recurrence as compared to a placebo[84-86]. After 12 wk of follow-up, ferric carboxymaltose led to higher response rates (66.1% vs 54.1%), a higher proportion of non-anemic patients (72.8% vs 61.8%), and better treatment adherence (92.5% vs 79.1%) than iron sucrose, with no difference in treatment-related adverse events (13.9% vs 11.3%). With regard to side effects, in the ferric carboxymaltose group there were more skin and subcutaneous tissue disorders (rash, dermatitis, pruritus) and more cases of hypophosphatemia, but fewer infusion site reactions than in the iron sucrose group. The superiority of high-dose intravenous iron supplementation in IBD probably depends on the iron overload produced in the macrophages of the reticulo-endothelial system. This induces an over-expression of ferroportin, which may, in turn, by-pass the hepcidin block in ACD[57]. Recently, an alternative dosage scheme to the traditional Ganzoni formula has been presented for ferric carboxymaltose treatment. In the new protocol, for a baseline Hb > 10 g/dL, the dose is 1.0 g for patients with a body weight < 70 kg and 1.5 g for patients > 70 kg; the corresponding total doses for serum Hb ≤ 10 g/dL are 1.5 g and 2.0 g[84]. Phase 3 clinical trials are currently underway to evaluate the use of ferumoxytol in patients with iron deficiency anemia, including a subgroup with IBD (ClinicalTrial.org identifier: NCT01114139, NCT01114217 and NCT01114204). However, ferumoxytol may be problematic in IBD patients because it can interfere with MRI signals due to the paramagnetic nature of its iron core[19]. In addition, a Phase 3 clinical trial of iron isomaltoside (NCT01017614) and a Phase 4 study of iron sucrose (NCT01067547) are currently being carried out on IBD patients with IDA. All treatments being tested strive to achieve ferritin concentrations > 100 μg/L, measured as early as 8 wk after intravenous iron treatment to obtain a reliable result[55]. Considering that the recurrence of iron deficiency with or without anemia is frequent in IBD patients[87], regular controls at 12 wk intervals are advisable so that treatment can be restarted promptly if needed.
Several studies have shown that recombinant human erythropoietin may be effective for treating ACD in IBD patients[88-95]. In the anemia treatment algorithm, intravenous iron therapy should be considered as a first-line therapy in patients with severe anemia whereas erythropoietin treatment should be considered only in patients unresponsive to intravenous treatment, with low EPO levels, or who are unresponsive to aggressive management of IBD[29,53,96] since EPO can be used as an adjunct therapy to control the inflammation[97]. Recently, a prospective study on CD patients showed that EPO combined with enteral nutrition can improve the Hb levels in CD patients with a treatment success rate of 63.16% in the EPO group compared to none of the patients in the non-EPO group[98]. When a decision has been made to administer EPO therapy, it should always be combined with intravenous iron supplementation to meet the increased demand caused by the “functional iron deficiency” typical in IBD patients[99].
Intramuscular vitamin B12 continues to be the gold standard therapy for vitamin B12 deficiency, especially in symptomatic patients[100]. A dose of 1000 μg/wk for 8 wk, then 1000 μg once monthly for maintenance lifelong is recommended[101]. No therapeutic advantages have been demonstrated for either cyanocobalamine or hydroxycobalamine in terms or serum levels during maintenance therapy[102]. Effectiveness data for sublingual[103,104] and intranasal[105] routes for vitamin B12 administration have revealed as promising non-invasive alternatives.
Specific treatment of IBD has been shown to gradually increase Hb levels over time, which indicates that the presence of anemia is positively associated with disease activity and disease-associated gut lesions. Some data suggest that anti-TNF-α treatment improves the anemia in a sub-group of patients with CD. In fact, patients who responded to treatment showed improvements in their anemia within 2 wk of the first infusion of Infliximab, with a parallel improvement in their CD activity index and an increase in endogenous EPO levels over time[12]. Infliximab seems to neutralize the inhibitory effects of TNF-α on EPO production, increasing the availability of iron for erythropoiesis and reducing anemia[30]. The drug produces these effects through various mechanisms, including reduced cytokine-induced formation of ferritin and hepcidin, with improvement of intestinal iron absorption and iron release from macrophages via ferroportin-mediated iron export[21,106,107]. Moreover, Infliximab improved the proliferation of cultured BFU-E, blocking the inhibitory effects of cytokines on erythroid progenitor cells[26]. Finally, it induced mucosal healing, thereby reducing the production of pro-inflammatory cytokines and the amount of blood loss through mucosal ulcers. Other therapies with potential for treating IBD associated with anemia include treatment with anti-IL-6, which is the major inflammation-driven inducer of hepcidin, and other new therapies that neutralize hepcidin, modify EPO and/or erythropoietin receptor sensitivity, or affect cytokines to effectively stimulate erythropoiesis.
The multi-factorial origin of anemia in IBD implies that several leading mechanisms can be simultaneously identified in a single patient, including chronic intestinal blood loss, decreased absorption capabilities of the small bowel secondary to inflammation or resection, bacterial overgrowth, and an inability of many IBD patients to tolerate the side effects of oral ferrous sulfate, among others[108]. Each of these causative factors usually requires a specific therapeutic approach. Disease inflammatory activity and iron deficiency should be the first aspects to be restored in every patient[109] since they are the main causes of anemia and easily identified. Although more uncommon, vitamin B12 or folate deficiency, hemolytic and drug-induced anemia should also be born in mind. Effective treatment is only possible if the contributing factors in a particular patient are clearly defined and corrected[110].
Anemia is a common multifactorial complication in IBD that increases disease morbidity. Awareness on the part of gastroenterologists needs to be increased to improve the specific diagnosis and management of anemia in these patients. New generation IV iron compounds are currently available to treat iron deficiency effectively in IBD patients. Further studies are needed to establish standardized treatments to reduce the development and recurrence of anemia as well as to improve the clinical course of IBD.
P- Reviewers: Cheifetz A, Dickey W S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
| 1. | Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2133] [Cited by in RCA: 2158] [Article Influence: 107.9] [Reference Citation Analysis (0)] |
| 2. | Weiss G, Gasche C. Pathogenesis and treatment of anemia in inflammatory bowel disease. Haematologica. 2010;95:175-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 3. | Gomollón F, Gisbert JP. Anemia and inflammatory bowel diseases. World J Gastroenterol. 2009;15:4659-4665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 126] [Cited by in RCA: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 4. | Cucino C, Sonnenberg A. Cause of death in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2001;7:250-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Pizzi LT, Weston CM, Goldfarb NI, Moretti D, Cobb N, Howell JB, Infantolino A, Dimarino AJ, Cohen S. Impact of chronic conditions on quality of life in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 145] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 6. | Wells CW, Lewis S, Barton JR, Corbett S. Effects of changes in hemoglobin level on quality of life and cognitive function in inflammatory bowel disease patients. Inflamm Bowel Dis. 2006;12:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 213] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 7. | Liu K, Kaffes AJ. Iron deficiency anaemia: a review of diagnosis, investigation and management. Eur J Gastroenterol Hepatol. 2012;24:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Ott C, Liebold A, Takses A, Strauch UG, Obermeier F. High prevalence but insufficient treatment of iron-deficiency anemia in patients with inflammatory bowel disease: results of a population-based cohort. Gastroenterol Res Pract. 2012;2012:595970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Gasche C, Lomer MC, Cavill I, Weiss G. Iron, anaemia, and inflammatory bowel diseases. Gut. 2004;53:1190-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 348] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 10. | Wilson A, Reyes E, Ofman J. Prevalence and outcomes of anemia in inflammatory bowel disease: a systematic review of the literature. Am J Med. 2004;116 Suppl 7A:44S-49S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 155] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Kulnigg S, Gasche C. Systematic review: managing anaemia in Crohn’s disease. Aliment Pharmacol Ther. 2006;24:1507-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 220] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 12. | Bergamaschi G, Di Sabatino A, Albertini R, Ardizzone S, Biancheri P, Bonetti E, Cassinotti A, Cazzola P, Markopoulos K, Massari A. Prevalence and pathogenesis of anemia in inflammatory bowel disease. Influence of anti-tumor necrosis factor-alpha treatment. Haematologica. 2010;95:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 13. | Nutritional anaemias. Report of a WHO scientific group. World Health Organ Tech Rep Ser. 1968;405:5-37. [PubMed] |
| 14. | Beutler E, Waalen J. The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration? Blood. 2006;107:1747-1750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 646] [Cited by in RCA: 639] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 15. | Patel KV, Harris TB, Faulhaber M, Angleman SB, Connelly S, Bauer DC, Kuller LH, Newman AB, Guralnik JM. Racial variation in the relationship of anemia with mortality and mobility disability among older adults. Blood. 2007;109:4663-4670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 16. | Rejler M, Tholstrup J, Andersson-Gäre B, Spångéus A. Low prevalence of anemia in inflammatory bowel disease: a population-based study in Sweden. Scand J Gastroenterol. 2012;47:937-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Gerasimidis K, Barclay A, Papangelou A, Missiou D, Buchanan E, Tracey C, Tayler R, Russell RK, Edwards CA, McGrogan P. The epidemiology of anemia in pediatric inflammatory bowel disease: prevalence and associated factors at diagnosis and follow-up and the impact of exclusive enteral nutrition. Inflamm Bowel Dis. 2013;19:2411-2422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Vijverman A, Piront P, Belaiche J, Louis E. Evolution of the prevalence and characteristics of anemia in inflammatory bowel diseases between 1993 and 2003. Acta Gastroenterol Belg. 2006;69:1-4. [PubMed] |
| 19. | Stein J, Hartmann F, Dignass AU. Diagnosis and management of iron deficiency anemia in patients with IBD. Nat Rev Gastroenterol Hepatol. 2010;7:599-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 184] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 20. | Bergamaschi G, Di Sabatino A, Albertini R, Costanzo F, Guerci M, Masotti M, Pasini A, Massari A, Campostrini N, Corbella M. Serum hepcidin in inflammatory bowel diseases: biological and clinical significance. Inflamm Bowel Dis. 2013;19:2166-2172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090-2093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3282] [Cited by in RCA: 3529] [Article Influence: 168.0] [Reference Citation Analysis (0)] |
| 22. | Theurl I, Mattle V, Seifert M, Mariani M, Marth C, Weiss G. Dysregulated monocyte iron homeostasis and erythropoietin formation in patients with anemia of chronic disease. Blood. 2006;107:4142-4148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 132] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 23. | Knutson MD, Oukka M, Koss LM, Aydemir F, Wessling-Resnick M. Iron release from macrophages after erythrophagocytosis is up-regulated by ferroportin 1 overexpression and down-regulated by hepcidin. Proc Natl Acad Sci USA. 2005;102:1324-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 356] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 24. | de la Morena F, Gisbert JP. [Anemia and inflammatory bowel disease]. Rev Esp Enferm Dig. 2008;100:285-293. [PubMed] |
| 25. | Reinisch W, Staun M, Bhandari S, Muñoz M. State of the iron: how to diagnose and efficiently treat iron deficiency anemia in inflammatory bowel disease. J Crohns Colitis. 2013;7:429-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 26. | Means RT. Recent developments in the anemia of chronic disease. Curr Hematol Rep. 2003;2:116-121. [PubMed] |
| 27. | Dallalio G, Law E, Means RT. Hepcidin inhibits in vitro erythroid colony formation at reduced erythropoietin concentrations. Blood. 2006;107:2702-2704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 28. | Cazzola M, Ponchio L, de Benedetti F, Ravelli A, Rosti V, Beguin Y, Invernizzi R, Barosi G, Martini A. Defective iron supply for erythropoiesis and adequate endogenous erythropoietin production in the anemia associated with systemic-onset juvenile chronic arthritis. Blood. 1996;87:4824-4830. [PubMed] |
| 29. | Sandborn W. Erythropoietin for inflammatory bowel disease anemia. Gastroenterology. 1997;112:660-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Jelkmann W. Proinflammatory cytokines lowering erythropoietin production. J Interferon Cytokine Res. 1998;18:555-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 286] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 31. | Dieleman LA, Heizer WD. Nutritional issues in inflammatory bowel disease. Gastroenterol Clin North Am. 1998;27:435-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Lucendo AJ, De Rezende LC. Importance of nutrition in inflammatory bowel disease. World J Gastroenterol. 2009;15:2081-2088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 78] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 33. | Guagnozzi D, González-Castillo S, Olveira A, Lucendo AJ. Nutritional treatment in inflammatory bowel disease. An update. Rev Esp Enferm Dig. 2012;104:479-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Lambert D, Benhayoun S, Adjalla C, Gelot MA, Renkes P, Felden F, Gerard P, Belleville F, Gaucher P, Guéant JL. Crohn’s disease and vitamin B12 metabolism. Dig Dis Sci. 1996;41:1417-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Shaw S, Jayatilleke E, Meyers S, Colman N, Herzlich B, Herbert V. The ileum is the major site of absorption of vitamin B12 analogues. Am J Gastroenterol. 1989;84:22-26. [PubMed] |
| 36. | Hoffbrand V, Provan D. ABC of clinical haematology. Macrocytic anaemias. BMJ. 1997;314:430-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Bermejo F, Algaba A, Guerra I, Chaparro M, De-La-Poza G, Valer P, Piqueras B, Bermejo A, García-Alonso J, Pérez MJ. Should we monitor vitamin B12 and folate levels in Crohn’s disease patients? Scand J Gastroenterol. 2013;48:1272-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 38. | M'Koma AE. Follow-up results of hematology data before and after restorative proctocolectomy. Clinical outcome. Dis Colon Rectum. 1994;37:932-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Yates P, Macht LM, Williams NA, Elson CJ. Red cell autoantibody production by colonic mononuclear cells from a patient with ulcerative colitis and autoimmune haemolytic anaemia. Br J Haematol. 1992;82:753-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Giannadaki E, Potamianos S, Roussomoustakaki M, Kyriakou D, Fragkiadakis N, Manousos ON. Autoimmune hemolytic anemia and positive Coombs test associated with ulcerative colitis. Am J Gastroenterol. 1997;92:1872-1874. [PubMed] |
| 41. | Lorber M, Schwartz LI, Wasserman LR. Association of antibody-coated red blood cells with ulcerative colitis; report of four cases. Am J Med. 1955;19:887-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 42. | Wang Z, Zhou Y, Liu Y. Concurrent inflammatory bowel disease and myelodysplastic syndrome: report of nine new cases and a review of the literature. Dig Dis Sci. 2008;53:1929-1932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | Harewood GC, Loftus EV, Tefferi A, Tremaine WJ, Sandborn WJ. Concurrent inflammatory bowel disease and myelodysplastic syndromes. Inflamm Bowel Dis. 1999;5:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 44. | Eng C, Farraye FA, Shulman LN, Peppercorn MA, Krauss CM, Connors JM, Stone RM. The association between the myelodysplastic syndromes and Crohn disease. Ann Intern Med. 1992;117:661-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 45. | Nakamura F, Watanabe T, Hori K, Ohara Y, Yamashita K, Tsuji Y, Ueda Y, Mikami S, Nakase H, Chiba T. Simultaneous occurrence of inflammatory bowel disease and myelodysplastic syndrome due to chromosomal abnormalities in bone marrow cells. Digestion. 2009;79:215-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 46. | Wakefield AJ, Sankey EA, Dhillon AP, Sawyerr AM, More L, Sim R, Pittilo RM, Rowles PM, Hudson M, Lewis AA. Granulomatous vasculitis in Crohn’s disease. Gastroenterology. 1991;100:1279-1287. [PubMed] |
| 47. | Karran P. Thiopurines, DNA damage, DNA repair and therapy-related cancer. Br Med Bull. 2006;153-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 137] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 48. | Beaugerie L, Brousse N, Bouvier AM, Colombel JF, Lémann M, Cosnes J, Hébuterne X, Cortot A, Bouhnik Y, Gendre JP. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet. 2009;374:1617-1625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 774] [Cited by in RCA: 805] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 49. | Annibale B, Capurso G, Chistolini A, D’Ambra G, DiGiulio E, Monarca B, DelleFave G. Gastrointestinal causes of refractory iron deficiency anemia in patients without gastrointestinal symptoms. Am J Med. 2001;111:439-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 135] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 50. | Guagnozzi D, Lucendo AJ. Colorectal cancer surveillance in patients with inflammatory bowel disease: What is new? World J Gastrointest Endosc. 2012;4:108-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 51. | Gasche C, Berstad A, Befrits R, Beglinger C, Dignass A, Erichsen K, Gomollon F, Hjortswang H, Koutroubakis I, Kulnigg S. Guidelines on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases. Inflamm Bowel Dis. 2007;13:1545-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 313] [Article Influence: 17.4] [Reference Citation Analysis (3)] |
| 52. | Oldenburg B, Koningsberger JC, Van Berge Henegouwen GP, Van Asbeck BS, Marx JJ. Iron and inflammatory bowel disease. Aliment Pharmacol Ther. 2001;15:429-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 53. | Tsiolakidou G, Koutroubakis IE. Stimulating erythropoiesis in inflammatory bowel disease associated anemia. World J Gastroenterol. 2007;13:4798-4806. [PubMed] |
| 54. | Theurl I, Aigner E, Theurl M, Nairz M, Seifert M, Schroll A, Sonnweber T, Eberwein L, Witcher DR, Murphy AT. Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: diagnostic and therapeutic implications. Blood. 2009;113:5277-5286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 280] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 55. | Gasche C, Evstatiev R, Haas T, Kaser A, Knoflach P, Petritsch W, Weiss G, Reinisch W. [Diagnosis and treatment of iron deficiency and anaemia in inflammatory bowel diseases. Consensus of the Austrian IBD Working Party]. Z Gastroenterol. 2011;49:627-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 56. | Muñoz M, García-Erce JA, Remacha ÁF. Disorders of iron metabolism. Part II: iron deficiency and iron overload. J Clin Pathol. 2011;64:287-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 190] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 57. | Muñoz M, García-Erce JA, Remacha AF. Disorders of iron metabolism. Part 1: molecular basis of iron homoeostasis. J Clin Pathol. 2011;64:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (2)] |
| 58. | Thomas C, Thomas L. Biochemical markers and hematologic indices in the diagnosis of functional iron deficiency. Clin Chem. 2002;48:1066-1076. [PubMed] |
| 59. | Punnonen K, Irjala K, Rajamäki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood. 1997;89:1052-1057. [PubMed] |
| 60. | Beguin Y, Clemons GK, Pootrakul P, Fillet G. Quantitative assessment of erythropoiesis and functional classification of anemia based on measurements of serum transferrin receptor and erythropoietin. Blood. 1993;81:1067-1076. [PubMed] |
| 61. | Oustamanolakis P, Koutroubakis IE, Messaritakis I, Malliaraki N, Sfiridaki A, Kouroumalis EA. Serum hepcidin and prohepcidin concentrations in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2011;23:262-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 62. | Thomas C, Kobold U, Balan S, Roeddiger R, Thomas L. Serum hepcidin-25 may replace the ferritin index in the Thomas plot in assessing iron status in anemic patients. Int J Lab Hematol. 2011;33:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 63. | Oustamanolakis P, Koutroubakis IE, Messaritakis I, Kefalogiannis G, Niniraki M, Kouroumalis EA. Measurement of reticulocyte and red blood cell indices in the evaluation of anemia in inflammatory bowel disease. J Crohns Colitis. 2011;5:295-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 64. | Recommendations to prevent and control iron deficiency in the United States. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1998;47:1-29. [PubMed] |
| 65. | Rimon E, Kagansky N, Kagansky M, Mechnick L, Mashiah T, Namir M, Levy S. Are we giving too much iron? Low-dose iron therapy is effective in octogenarians. Am J Med. 2005;118:1142-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 139] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 66. | Zlotkin S, Arthur P, Antwi KY, Yeung G. Randomized, controlled trial of single versus 3-times-daily ferrous sulfate drops for treatment of anemia. Pediatrics. 2001;108:613-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 67. | Makrides M, Crowther CA, Gibson RA, Gibson RS, Skeaff CM. Efficacy and tolerability of low-dose iron supplements during pregnancy: a randomized controlled trial. Am J Clin Nutr. 2003;78:145-153. [PubMed] |
| 68. | de Silva AD, Tsironi E, Feakins RM, Rampton DS. Efficacy and tolerability of oral iron therapy in inflammatory bowel disease: a prospective, comparative trial. Aliment Pharmacol Ther. 2005;22:1097-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 69. | Gisbert JP, Bermejo F, Pajares R, Pérez-Calle JL, Rodríguez M, Algaba A, Mancenido N, de la Morena F, Carneros JA, McNicholl AG. Oral and intravenous iron treatment in inflammatory bowel disease: hematological response and quality of life improvement. Inflamm Bowel Dis. 2009;15:1485-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 70. | Werner T, Wagner SJ, Martínez I, Walter J, Chang JS, Clavel T, Kisling S, Schuemann K, Haller D. Depletion of luminal iron alters the gut microbiota and prevents Crohn’s disease-like ileitis. Gut. 2011;60:325-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 232] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 71. | Seril DN, Liao J, Ho KL, Warsi A, Yang CS, Yang GY. Dietary iron supplementation enhances DSS-induced colitis and associated colorectal carcinoma development in mice. Dig Dis Sci. 2002;47:1266-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 139] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 72. | Carrier J, Aghdassi E, Platt I, Cullen J, Allard JP. Effect of oral iron supplementation on oxidative stress and colonic inflammation in rats with induced colitis. Aliment Pharmacol Ther. 2001;15:1989-1999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 119] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 73. | Erichsen K, Ulvik RJ, Grimstad T, Berstad A, Berge RK, Hausken T. Effects of ferrous sulphate and non-ionic iron-polymaltose complex on markers of oxidative tissue damage in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2005;22:831-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 74. | Reifen R, Matas Z, Zeidel L, Berkovitch Z, Bujanover Y. Iron supplementation may aggravate inflammatory status of colitis in a rat model. Dig Dis Sci. 2000;45:394-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 75. | Dostal A, Chassard C, Hilty FM, Zimmermann MB, Jaeggi T, Rossi S, Lacroix C. Iron depletion and repletion with ferrous sulfate or electrolytic iron modifies the composition and metabolic activity of the gut microbiota in rats. J Nutr. 2012;142:271-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 175] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 76. | Lee TW, Kolber MR, Fedorak RN, van Zanten SV. Iron replacement therapy in inflammatory bowel disease patients with iron deficiency anemia: a systematic review and meta-analysis. J Crohns Colitis. 2012;6:267-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 77. | Schröder O, Mickisch O, Seidler U, de Weerth A, Dignass AU, Herfarth H, Reinshagen M, Schreiber S, Junge U, Schrott M. Intravenous iron sucrose versus oral iron supplementation for the treatment of iron deficiency anemia in patients with inflammatory bowel disease--a randomized, controlled, open-label, multicenter study. Am J Gastroenterol. 2005;100:2503-2509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 162] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 78. | Gasche C, Waldhoer T, Feichtenschlager T, Male C, Mayer A, Mittermaier C, Petritsch W. Prediction of response to iron sucrose in inflammatory bowel disease-associated anemia. Am J Gastroenterol. 2001;96:2382-2387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 79. | Bodemar G, Kechagias S, Almer S, Danielson BG. Treatment of anaemia in inflammatory bowel disease with iron sucrose. Scand J Gastroenterol. 2004;39:454-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 80. | García-López S, Gomollón F, García-Erce JA, Araméndiz R, Sicilia B, Vicente R. Intravenous iron sucrose: a simple, safe and quick method to treat anemia secondary to digestive diseases. Gastroenterology. 2006;130:A84. |
| 81. | Lindgren S, Wikman O, Befrits R, Blom H, Eriksson A, Grännö C, Ung KA, Hjortswang H, Lindgren A, Unge P. Intravenous iron sucrose is superior to oral iron sulphate for correcting anaemia and restoring iron stores in IBD patients: A randomized, controlled, evaluator-blind, multicentre study. Scand J Gastroenterol. 2009;44:838-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 82. | Koutroubakis IE, Oustamanolakis P, Karakoidas C, Mantzaris GJ, Kouroumalis EA. Safety and efficacy of total-dose infusion of low molecular weight iron dextran for iron deficiency anemia in patients with inflammatory bowel disease. Dig Dis Sci. 2010;55:2327-2331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 83. | Muñoz M, Gómez-Ramírez S, García-Erce JA. Intravenous iron in inflammatory bowel disease. World J Gastroenterol. 2009;15:4666-4674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 84. | Evstatiev R, Marteau P, Iqbal T, Khalif IL, Stein J, Bokemeyer B, Chopey IV, Gutzwiller FS, Riopel L, Gasche C. FERGIcor, a randomized controlled trial on ferric carboxymaltose for iron deficiency anemia in inflammatory bowel disease. Gastroenterology. 2011;141:846-853.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 244] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 85. | Kulnigg S, Stoinov S, Simanenkov V, Dudar LV, Karnafel W, Garcia LC, Sambuelli AM, D’Haens G, Gasche C. A novel intravenous iron formulation for treatment of anemia in inflammatory bowel disease: the ferric carboxymaltose (FERINJECT) randomized controlled trial. Am J Gastroenterol. 2008;103:1182-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 252] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 86. | Evstatiev R, Alexeeva O, Bokemeyer B, Chopey I, Felder M, Gudehus M, Iqbal T, Khalif I, Marteau P, Stein J. Ferric carboxymaltose prevents recurrence of anemia in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11:269-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 87. | Kulnigg S, Teischinger L, Dejaco C, Waldhör T, Gasche C. Rapid recurrence of IBD-associated anemia and iron deficiency after intravenous iron sucrose and erythropoietin treatment. Am J Gastroenterol. 2009;104:1460-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 88. | Gasché C, Reinisch W, Lochs H, Parsaei B, Bakos S, Wyatt J, Fueger GF, Gangl A. Anemia in Crohn’s disease. Importance of inadequate erythropoietin production and iron deficiency. Dig Dis Sci. 1994;39:1930-1934. [PubMed] |
| 89. | Horina JH, Petritsch W, Schmid CR, Reicht G, Wenzl H, Silly H, Krejs GJ. Treatment of anemia in inflammatory bowel disease with recombinant human erythropoietin: results in three patients. Gastroenterology. 1993;104:1828-1831. [PubMed] |
| 90. | Schreiber S, Howaldt S, Schnoor M, Nikolaus S, Bauditz J, Gasché C, Lochs H, Raedler A. Recombinant erythropoietin for the treatment of anemia in inflammatory bowel disease. N Engl J Med. 1996;334:619-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 140] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 91. | Gasché C, Dejaco C, Waldhoer T, Tillinger W, Reinisch W, Fueger GF, Gangl A, Lochs H. Intravenous iron and erythropoietin for anemia associated with Crohn disease. A randomized, controlled trial. Ann Intern Med. 1997;126:782-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 112] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 92. | Gasche C, Dejaco C, Reinisch W, Tillinger W, Waldhoer T, Fueger GF, Lochs H, Gangl A. Sequential treatment of anemia in ulcerative colitis with intravenous iron and erythropoietin. Digestion. 1999;60:262-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 93. | Demirtürk L, Hülagü S, Yaylaci M, Altin M, Ozel M. Serum erythropoietin levels in patients with severe anemia secondary to inflammatory bowel disease and the use of recombinant human erythropoietin in patients with anemia refractory to treatment. Dis Colon Rectum. 1995;38:896-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 94. | Koutroubakis IE, Karmiris K, Makreas S, Xidakis C, Niniraki M, Kouroumalis EA. Effectiveness of darbepoetin-alfa in combination with intravenous iron sucrose in patients with inflammatory bowel disease and refractory anaemia: a pilot study. Eur J Gastroenterol Hepatol. 2006;18:421-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 95. | Dohil R, Hassall E, Wadsworth LD, Israel DM. Recombinant human erythropoietin for treatment of anemia of chronic disease in children with Crohn’s disease. J Pediatr. 1998;132:155-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 96. | Katsanos KH, Tatsioni A, Natsi D, Sigounas D, Christodoulou DK, Tsianos EV. Recombinant human erythropoietin in patients with inflammatory bowel disease and refractory anemia: a 15-year single center experience. J Crohns Colitis. 2012;6:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 97. | Cronin CC, Shanahan F. Anemia in patients with chronic inflammatory bowel disease. Am J Gastroenterol. 2001;96:2296-2298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 98. | Liu S, Ren J, Hong Z, Yan D, Gu G, Han G, Wang G, Ren H, Chen J, Li J. Efficacy of erythropoietin combined with enteral nutrition for the treatment of anemia in Crohn’s disease: a prospective cohort study. Nutr Clin Pract. 2013;28:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 99. | Christodoulou DK, Tsianos EV. Anemia in inflammatory bowel disease - the role of recombinant human erythropoietin. Eur J Intern Med. 2000;11:222-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 100. | Majumder S, Soriano J, Louie Cruz A, Dasanu CA. Vitamin B12 deficiency in patients undergoing bariatric surgery: preventive strategies and key recommendations. Surg Obes Relat Dis. 2013;9:1013-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 101. | Mullin GE. Micronutrients and inflammatory bowel disease. Nutr Clin Pract. 2012;27:136-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 102. | Hvas AM, Nexo E. Diagnosis and treatment of vitamin B12 deficiency--an update. Haematologica. 2006;91:1506-1512. [PubMed] |
| 103. | Delpre G, Stark P, Niv Y. Sublingual therapy for cobalamin deficiency as an alternative to oral and parenteral cobalamin supplementation. Lancet. 1999;354:740-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 104. | Sharabi A, Cohen E, Sulkes J, Garty M. Replacement therapy for vitamin B12 deficiency: comparison between the sublingual and oral route. Br J Clin Pharmacol. 2003;56:635-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 105. | Slot WB, Merkus FW, Van Deventer SJ, Tytgat GN. Normalization of plasma vitamin B12 concentration by intranasal hydroxocobalamin in vitamin B12-deficient patients. Gastroenterology. 1997;113:430-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 106. | Johnson D, Bayele H, Johnston K, Tennant J, Srai SK, Sharp P. Tumour necrosis factor alpha regulates iron transport and transporter expression in human intestinal epithelial cells. FEBS Lett. 2004;573:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 107. | Ludwiczek S, Aigner E, Theurl I, Weiss G. Cytokine-mediated regulation of iron transport in human monocytic cells. Blood. 2003;101:4148-4154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 309] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 108. | Warsch S, Byrnes J. Emerging causes of iron deficiency anemia refractory to oral iron supplementation. World J Gastrointest Pharmacol Ther. 2013;4:49-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 109. | Gomollón F, Gisbert JP. Current management of iron deficiency anemia in inflammatory bowel diseases: a practical guide. Drugs. 2013;73:1761-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 110. | Gisbert JP, Gomollón F. Common misconceptions in the diagnosis and management of anemia in inflammatory bowel disease. Am J Gastroenterol. 2008;103:1299-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 154] [Article Influence: 9.1] [Reference Citation Analysis (0)] |