Published online Mar 28, 2014. doi: 10.3748/wjg.v20.i12.3164
Revised: December 20, 2013
Accepted: January 19, 2014
Published online: March 28, 2014
Processing time: 240 Days and 19 Hours
The risk of developing dysplasia leading to colorectal cancer (CRC) is increased in both ulcerative colitis and Crohn’s disease. The prognosis of CRC may be poorer in patients with inflammatory bowel disease (IBD) than in those without IBD. Most CRCs, in general, develop from a dysplastic precursor lesion. The interpretation by the pathologist of the biopsy will guide decision making in clinical practice: colonoscopic surveillance or surgical management. This review summarizes features of dysplasia (or intraepithelial neoplasia) with macroscopic and microscopic characteristics. From an endoscopic (gross) point of view, dysplasia may be classified as flat or elevated (raised); from a histological point of view, dysplasia is separated into 3 distinct categories: negative for dysplasia, indefinite for dysplasia, and positive for dysplasia with low- or high-grade dysplasia. The morphologic criteria for dysplasia are based on a combination of cytologic (nuclear and cytoplasmic) and architectural aberrations of the crypt epithelium. Immunohistochemical and molecular markers for dysplasia are reviewed and may help with dysplasia diagnosis, although diagnosis is essentially based on morphological criteria. The clinical, epidemiologic, and pathologic characteristics of IBD-related cancers are, in many aspects, different from those that occur sporadically in the general population. Herein, we summarize macroscopic and microscopic features of IBD-related colorectal carcinoma.
Core tip: The risk of developing dysplasia leading to colorectal cancer is increased in both ulcerative colitis and Crohn’s disease. The biopsy interpretations will guide decision making in clinical practice: colonoscopic surveillance or surgical management. This review summarizes histological features of dysplasia and colorectal cancer in inflammatory bowel disease.
- Citation: Bressenot A, Cahn V, Danese S, Peyrin-Biroulet L. Microscopic features of colorectal neoplasia in inflammatory bowel diseases. World J Gastroenterol 2014; 20(12): 3164-3172
- URL: https://www.wjgnet.com/1007-9327/full/v20/i12/3164.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i12.3164
The most common types of inflammatory bowel disease (IBD) are ulcerative colitis (UC) and Crohn’s disease (CD). The risk of colorectal cancer is increased in both UC[1] and CD[2,3]. The prognosis of colorectal cancer (CRC) may be poorer in patients with IBD than in those without IBD[4]. It is the pathologist’s biopsy interpretations that guide the management of patients during surveillance[5]. Pathologic interpretation of specimens for evaluation of dysplasia constitutes a critical step in endoscopic surveillance programs or surgery. Ultimately, it is the pathologist’s interpretation of mucosal biopsy specimens that distinguishes high-risk from low-risk populations and triggers recommendations for either continued surveillance or surgery. Thus, an accurate diagnosis of dysplasia (or intraepithelial neoplasia) is the most important step in the surveillance process.
We review here the pathological characteristics of IBD-related colorectal cancer and dysplasia.
Most CRCs, in general, develop from a dysplasic precursor lesion. Patients with IBD develop dysplastic lesions that can be polypoid, flat, localized, or multifocal[6]. Colorectal dysplasia may be defined as an unequivocal neoplastic alteration of the intestinal epithelium that remains restricted within the basement membrane within which it originated[7]. It is synonymous with the term intraepithelial neoplasia adopted by the World Health Organization and Vienna nomenclature systems (Table 1) for gastrointestinal neoplasia[5].
| Category | |
| 1 | Negative for neoplasia/dysplasia |
| 2 | Indefinite for neoplasia/dysplasia |
| 3 | Non-invasive low-grade neoplasia (low-grade adenoma/dysplasia) |
| 4 | Non-invasive high-grade neoplasia |
| High-grade adenoma/dysplasia | |
| Non-invasive carcinoma (carcinoma in situ)1 | |
| Suspicion of invasive carcinoma | |
| 5 | Invasive neoplasia |
| Intramucosal carcinoma2 | |
| Submucosal carcinoma or beyond |
From an endoscopic (gross) point of view, dysplasia may be classified as flat or elevated (raised)[8-11]. Flat dysplasia refers to endoscopically undetectable lesions, whereas raised dysplasia refers to any type of endoscopically detectable lesion[12].
Raised dysplasia: Endoscopically visible dysplastic raised lesions within an area affected by UC can be divided in adenoma-like and non-adenoma-like lesions on the basis of their macroscopic characteristics[12]. Raised lesions with dysplasia in UC have been broadly separated into those that appear similar to non-IBD related sporadic adenomas, referred to as “adenoma like” and those which do not resemble adenomas: “non-adenoma-like (the former term “DALM”)[13]. Adenoma-like RLDs represent well circumscribed, smooth or papillary, nonnecrotic, sessile, or pedunculated polyps that are usually amenable to removal by routine endoscopic methods[13,14]. Non-adenoma-like lesions include velvety patches, plaques, irregular bumps and nodules, wart-like thickenings, stricturing lesions, and broad-based masses[9,15-17] and are not usually amenable to removal by colonoscopic polypectomy. Non-adenoma, and adenoma like RLDs are differentiated on the basis of their gross (endoscopic) appearance. Histologic comparaisons of individual morphologic features in DALMs and adenoma-like dysplastic polyps have indicated that DALMs show increased architectural disarray[18], villous architecture, and inflammation[19], but these criteria have not been evaluated longitudinally and have so far lacked the statistical power to guide the management of patients with raised dysplasia[10,18,19].
Dysplastic polyps that are either encountered in nondiseased areas of the colorectum, for example, proximal to the transition zone in UC[20], or have a non dysplastic pedicle[19,21,22], are considered to be sporadic adenomas unrelated to the colitis and are managed accordingly. The endoscopic, histologic, and prognostic similarities between adenoma-like polyps in IBD and sporadic adenomatous polyps suggest that some, if not all, of the former are merely fortuitous adenomas, a conclusion that is also supported by limited molecular-based evidence[23]. Follow-up studies after their endoscopic removal have reported no significant excess risk for the development of CRC[24-28]. This favorable outlook is maintained even when the resected polyps contain HGD[28,29]. Adenoma-like lesions can be adequately treated by polypectomy provided the lesion can be completely excised, shows the absence of dysplasia at the margins of the specimen, and there is no evidence of flat dysplasia elsewhere in the colon, either adjacent to, or distant from, the raised lesion[12].
Kisiel et al[30] showed that, while polypectomy may be safe for the management of adenomas occurring in most UC patients, the 5-years cumulative incidence of a combined endpoint (cancer or flat dysplasia) was 13%. Such patients should be followed closely.
Flat dysplasia: Flat dysplasia refers to dysplasia that is detected unexpectedly in random biopsies of mucosa without a corresponding macroscopic lesion, although occult dysplasia is a more suitable term considering that small or subtle raised lesions might easily go unnoticed in the inflammatory background of IBD[31]. Retrospective endoscopic studies have suggested that most dysplastic lesions are in fact endoscopically visible. On the basis of a review of random and targeted surveillance biopsies in 525 subjects with UC during a period of 15 years, Rutter et al[9] reported that 85 of 110 (77.3%) biopsy specimens of dysplasia or cancer corresponded to macroscopically visible lesions, whereas 25 (22.7%) were invisible. Similarly, on the basis of a review of surveillance biopsies in 46 subjects with UC during a period of 10 years, Rubin et al[32] reported that 38 of 65 dysplastic lesions (58.5%) and 8 of 10 cancers (80.0%) were visible as 23 polyps and masses, 1 stricture, and 22 mucosal irregularities. Only some of the dysplastic lesions described as flat by endoscopists correspond to expanded mucosa resembling diminutive adenomatous polyps, whereas most correspond to histologically flat mucosa in which the crypts have been colonized by dysplastic epithelium, without alteration of the overall mucosal architecture.
Currently, dysplasia is separated into 3 distinct categories: negative for dysplasia, indefinite for dysplasia, and positive for dysplasia (low or high grade) (Table 2)[7]. While endeavouring to minimize disagreement in both terminology and interpretation, rates of agreement using this grading system are only fair among both expert and community pathologists[33]. Crude rates of agreement among experts have ranged from 42% to 72%; kappa values, where there is a correction for chance agreement, have remained fair for both experts and community pathologists[33-36]. Unfortunately, rates of agreement are lowest for the indefinite for dysplasia and low-grade dysplasia categories[24,33]. Based on these data, the CCFA consensus guidelines and the United States Multisociety Task Force strongly recommend that a second examination of the biopsies should be performed by an independent pathology expert prior to definitive treatment[21,37].
| Negative for dysplasia | Positive for dysplasia |
| Normal mucosa | Low-grade dysplasia |
| Inactive (quiescent) colitis | High-grade dysplasia |
| Active colitis |
The morphologic criteria for dysplasia are based on a combination of cytologic (nuclear and cytoplasmic) and architectural aberrations of the crypt epithelium[7,19,38]. Cytologic features that pathologists use to evaluate the presence or absence and degree of dysplasia include the nuclear/cytoplasmic (N/C) ratio of the cells; loss of cell polarity; an increase in the number and location of mitoses (typical and atypical); the degree of nuclear stratification within the epithelium; the degree of chromasia of the nuclei (an increase is referred to as “hyperchromasia”); the presence, size, and multiplicity of nucleoli; the size and regularity (or lack thereof) of the contour of nuclei; and the variation in the size and shape of nuclei between different cells (nuclear pleomorphism). Cytoplasmic characteristics include the degree of mucinous depletion; the number, location, and shape (normal or dystrophic) of goblet cells; and the presence or absence of surface maturation, which is defined as the progressive acquisition of cytoplasmic mucin, a decrease in the size of nuclei, and the degree of stratification of the cells, from the crypt base to the mucosal surface. Architectural features that are important for the determination of dysplasia include villiform change of the epithelium and the presence or absence and degree of crypt budding, branching, and crowding; the latter is referred to commonly as a “back-to-back” glandular growth pattern. In addition, the contour of the crypts, the degree of irregularity, and the presence or absence of intraluminal bridges (“cribriforming”) are important architectural features that are used to evaluate dysplasia in IBD[39].
Negative for dysplasia:“Negative for dysplasia” applies to epithelium that is regenerative in nature. In the presence of active inflammation, cryptitis, crypt abscesses, or ulceration, all of which are common in the active phase of IBD, the epithelium can undergo marked reactive changes that, in some circumstances, may mimic some of the “atypical” features of dysplasia. In general, nondysplastic (“reactive”) epithelium in IBD exhibits only mild or moderate cytologic atypia coupled with preservation of crypt architecture; however, a significant degree of atypia may be present in markedly reactive epithelium adjacent to ulcerated mucosa, an area in which the architecture of the crypts may be altered as well. One of the hallmarks of reactive crypts is a base-to-surface epithelial maturation gradient in which phenotypically immature, mitotically active, basal colonocytes differentiate into mature surface cells, featuring small, normochromatic nuclei, distinct absorptive and goblet cell phenotypes, and absent mitoses[5]. Pathologists need to exercise caution when evaluating dysplasia in ulcerated mucosa, and these areas should be avoided by the endoscopist when obtaining mucosal biopsies. Given the subtle gradation of changes, the progressive acquisition of molecular mutations that occurs in the progression of dysplasia in IBD[38,40,41], and the wide range of morphologic patterns of atypia that is related to epithelial regeneration and repair, regenerating epithelium, particularly in the setting of active inflammation or ulceration, may reveal a level of atypia that occasionally is difficult to distinguish from true dysplasia[41]. In these situations, pathologists use the “indefinite for dysplasia” diagnostic category. In reality, this diagnostic category is used most often as a result of one of the following circumstances: the presence of technical (tangential sectioning) or staining issues that makes interpretation of cytologic or architectural features difficult, atypia related to inflammation or ulceration, or for the rare instances in which dysplasia-like changes are present only in the crypt bases. Naturally, the frequency of the use of this diagnostic category is directly proportional to the “comfort” level and experience of the reviewing pathologist, and is one of the reasons why it is highly recommended to confirm any potential diagnosis of dysplasia with at least one other experienced IBD pathologist before definitive treatment[21,38].
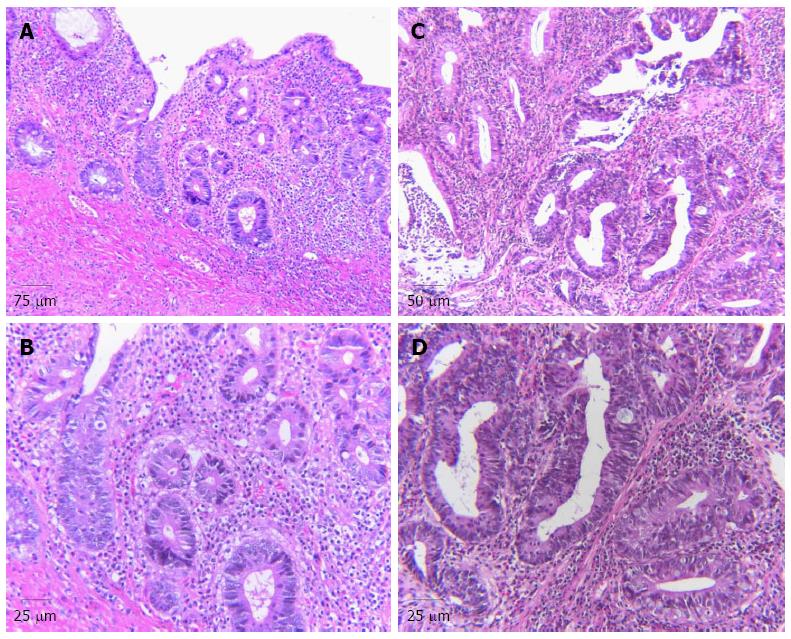
Low-grade dysplasia: Low-grade dysplasia is characterized by epithelium that contains cells with significant nuclear hyperchromaticity, enlargement, and elongation; the last is referred to as “pencil-shaped” or “adenomatous” nuclei. Nuclei in low-grade dysplasia often show a clumped chromatin pattern, multiple nucleoli, or a single large nucleolus. Typically, the cytoplasm is mucin depleted, and, as a result, is hypereosinophilic. A decrease in the number of goblet cells and unusually oriented goblet cells, referred to as “dystrophic” goblet cells, may also be observed (Figure 1B). Dysplastic cells are usually organized in a stratified manner, but in general, the nuclei are limited to the basal half of the cell cytoplasm, without full thickness stratification. Mitotic figures may be prominent, but there are only usually a few atypical mitotic figures. Most importantly, dysplastic epithelium usually does not show surface maturation, except in rare circumstances. A minor degree of architectural aberration may occur in low-grade dysplasia (Figure 1A), but significant architectural aberration is normally diagnostic of high-grade dysplasia (Figure 1C)[39].
High-grade dysplasia: With progression to high-grade dysplasia, the degree of cytologic or architectural aberration is more prominent (Figure 1C). Cytologically, full-thickness nuclear stratification, significant loss of cell polarity, nuclear pleomorphism, and an increase in the number of normal appearing and atypical mitoses, often at the level of the surface epithelium, are characteristic features of high-grade dysplasia (Figure 1D). In some instances, high-grade nuclei are more round or oval in contour, and also show a higher N/C ratio. Architectural aberrations, such as a complex crypt budding or branching, or a back-to-back growth pattern that is characterized by dysplastic crypts that show little or no intervening lamina propria, may also be present (Figure 1C). Cystic change, villiform surface change, and cribriforming are also features of high-grade dysplasia[42-44].
Immunohistochemical or molecular markers of dysplasia: Many studies have been published in an effort to help identify sensitive and specific immunohistochemical or molecular markers that may aid in the differentiation of dysplastic from reactive epithelium in IBD. p53 and Ki67 have been studied the most extensively. Most of the markers that were evaluated previously have been linked, in some capacity, to the development of cancer, and include those involved in control of cell proliferation (e.g., Ki67, cyclin D1), intercellular adhesion (β-catenin, e-cadherin), DNA content, mucin or glycoprotein histochemistry, and tumor suppression (p53)[40,45-59].
The p53 gene shows an increase in the frequency of mutations in the dysplasia-carcinoma progression in IBD[45-51]. p53 is a common early mutation in the dysplasia-carcinoma sequence in IBD, and, as a result, many investigators have evaluated the role of p53 in helping to differentiate reactive from dysplastic epithelium. For instance, in a study by Wong et al[45], a moderate degree of p53 staining was detected in almost 50% of reactive cases, but strong p53 staining was seen only in cases of true dysplasia. Unfortunately, although p53 expression increases progressively from low- to high-grade dysplasia and carcinoma, some studies showed that the epithelium that is considered indefinite, or even negative, for dysplasia, may be p53 positive; this diminishes its usefulness as a marker of true dysplasia[42,45,47]. Furthermore, p53 overexpression can be detected in a small proportion of cases that are considered morphologically negative for dysplasia[45,47,49,50]. In addition, several studies in other tissues have revealed a high rate of false-positive staining in the absence of p53 mutations, and a high frequency of false-negative staining as well[60,61]. Nonspecific binding of p53 to non-p53 mutation-related antigens may also lead to false-positive results. Furthermore, p53 results may vary substantially depending on the specific type of antibody used. For instance, some p53 mutations result in the production of a protein that does not bind to some antibodies that are directed against the wild-type protein. Finally, there is no known antibody, or combination of antibodies, in use that can detect all p53 mutations[60]. For these reasons, p53 immunostaining is not routinely used but can be helpful in rare cases to differentiate reactive from dysplastic epithelium in IBD.
Several studies showed that dysplasia expresses markers of cell proliferation at higher levels in the crypt, and in the surface epithelium, compared with biopsies that are considered negative for dysplasia[45,46,58]. Unfortunately, there is much overlap between reactive epithelium and dysplasia in this regard, so evaluation of cell proliferation is not useful in individual cases to distinguish these lesions.
Recently, immunostaining for alpha-methylocyl-CoA racemase (AMACR), an antibody that is often used in the assessment of diagnostically difficult atypical, and potentially neoplastic, lesions of the prostate, was shown to have a high degree of specificity for detection of dysplasia in the GI tract, such as in Barrett’s esophagus and IBD[62]. In this recent study by Dorer and Odze[62], AMACR was not expressed in any mucosal biopsy in UC that was considered negative for dysplasia; however, it was increased significantly in foci of low-grade dysplasia (96%), high-grade dysplasia (80%), and adenocarcinoma (71%) with a specificity for neoplasia of 100%. Thus, AMACR is a new, potentially useful immunohistochemical marker that pathologists may use in their arsenal when trying to differentiate reactive from dysplastic epithelium in IBD. More recently, Chen et al[63] showed that Chitinase 3-like-1 may contribute to the proliferation, migration and neoplastic progression of colonic epithelial cells under inflammatory conditions and could be a useful biomarker for neoplastic changes in patients with IBD. More recently, Ludwig et al[64] show that PDCD4 nuclear expression may be usefully applied as ancillary marker in the histological assessment of IBD-associated dysplastic lesions.
Overall, dysplasia diagnosis is essentially based on morphological criteria.
Less studied than in UC, dysplasia in CD occurs more often in areas close to, rather than distant from, the primary tumor mass. Dysplasia in CD is often multifocal[65]. In a study by Sigel et al[66], dysplasia was found adjacent to carcinoma in 87% of cases and distant from carcinoma in 41% of cases. Microscopic features that are used for a diagnosis of dysplasia (or intraepithelial neoplasia) in CD are the same that those used in UC dysplasia.
The clinical, epidemiologic, and pathologic characteristics of IBD-related cancers are, in many aspects, different from those that occur sporadically in the general population. For instance, cancers that occur in IBD, and particularly UC, tend to be distributed more evenly throughout the length of colon, are more likely to be multiple in number and tend to be of higher histologic grade than with sporadic carcinomas[67]. In some studies, up to 27% of IBD-related cancers are multiple in number[68,69]. In addition, there is a higher prevalence of mucinous carcinomas in IBD[67,70,71]. More recently, there has been a shift to a higher incidence of early-stage tumors (stage I-II) compared with IBD-related cancers from previous decades[41,67,68]. Contemporary studies show that 50%-60% of newly diagnosed IBD-related cancers are stages I or II. Of course, this may be due to a combination of many factors, such as an increased level of awareness and early detection by colonoscopic surveillance. In one study, by Delaunoit et al[67], of 290 patients who had IBD (241 with UC and 49 with CD) and an equal number of age- and sex-matched patients who had sporadic colorectal cancer, UC-related carcinomas were diagnosed at a younger age and tended to be distributed more evenly in the colon, compared with sporadic tumors.
Pathologically, IBD-related tumors often grow in a more diffuse fashion than sporadic cancers, and may be more difficult to detect grossly because they may be raised only minimally above the level of the surrounding mucosa[41,67]. The gross appearance of cancers in IBD is heterogenous. They may be strictured, ulcerated, irregular, polypoid (pedunculated or sessile), or nodular or they may appear as an irregular plaque or bump[41,71]. Some tumors may be entirely microscopic, without any grossly evident mucosal abnormality[17,72]. A disproportionately higher percentage of cancers in IBD, including UC, occurs in strictured segments of colon[73,74].
Microscopically, most IBD-related carcinomas are adenocarcinomas. Mucinous carcinomas make up a high proportion, up to 50% in some studies[40]. In addition, signet ring cell adenocarcinomas are 10 times more common in IBD than in the general population[41,75]. Rarely, IBD-related adenocarcinomas may be extremely well differentiated and consist of widely separated, regularly arranged, bland-appearing glandular profiles that contain only mildly atypical unilayered neoplastic epithelium with low-grade cytologic atypia, and without desmoplasia[41,76]. These tumors may arise from mucosa that shows little or no definite evidence of dysplasia. Other types of carcinomas, such as neuroendocrine carcinomas, mixed adenocarcinoma/squamous cell carcinoma, undifferentiated carcinoma, and even pure squamous cell carcinoma (particularly in the distal rectum and anal canal in CD), have been encountered in IBD; some occur with increased frequency[40,41,73-83]. However, these tumors are rare and are often reported as single case reports or as small series.
Regarding CD cancers specifically, recent evidence suggests that the risk of cancer in CD is similar to that in UC, particularly for patients who have long-standing and extensive colonic disease[38,40,84,85]. In contrast to UC, CD patients who develop cancer are often older in age, but are much younger than patients who develop sporadic colon cancer in the general population[41,68,86]. Some early reports cited a higher left-sided predominance for cancers in CD compared with UC; however, several contemporary studies showed a more equal distribution of tumors in the colon in CD, similar to UC[73,67,87]. Earlier studies may have been biased by the fact that cancers in CD often occur in and around the anal canal related to fissures or fistulas[86-88]. As a result, there is an increase in the incidence of pure squamous cell carcinomas in CD compared with UC. Although most cancers in CD are believed to occur within inflamed portions of intestine[40,89,90], in some studies, up to 42% of patients who had CD developed tumors in areas of mucosa devoid of endoscopic or pathologic evidence of inflammation[73,86-88,91]; however, this may be due to treatment effect[92]. Finally, surgically excluded segments of colon or small bowel also are considered particularly prone to the development of carcinoma in CD, but this is postulated to be related to the fact that excluded segments of inflamed bowel remain at risk for carcinogenesis for longer periods of time over the course of the patient’s life[40,41,67,87,93]. Because surgical procedures that result in preservation of inflamed but excluded segments of bowel are performed only infrequently in patients who have CD, cancers in surgically excluded segments of bowel are now uncommon.
Patients with IBD, including UC and CD, are at increased risk of developing colorectal cancer. The risk of developing carcinoma is related to the extent of the patient’s disease (pancolitis vs left-sided disease), duration of disease, and level of activity. In IBD there is abundant evidence to support the theory that cancer develops through an inflammation-dysplasia-carcinoma sequence. Several molecular events involved in the chronic active inflammatory process contribute to multistage progression of carcinoma development. Morphologic identification of dysplasia in IBD is the best and most reliable marker of an increased risk for malignancy. Future advances in, for example, stool DNA assays or the use of confocal endomicroscopy or endoscopic ultrasound may help in the identification of high risk patients and the assessment of dysplasic lesion[11].
P- Reviewers: Braet F, Bujanda L, Ciccone MM S- Editor: Zhai HH L- Editor: A E- Editor: Zhang DN
| 1. | Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2012;10:639-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 660] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 2. | Jess T, Gamborg M, Matzen P, Munkholm P, Sørensen TI. Increased risk of intestinal cancer in Crohn’s disease: a meta-analysis of population-based cohort studies. Am J Gastroenterol. 2005;100:2724-2729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 403] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 3. | Mudter J. What’s new about inflammatory bowel diseases in 2011. World J Gastroenterol. 2011;17:3177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (2)] |
| 4. | Peyrin-Biroulet L, Lepage C, Jooste V, Guéant JL, Faivre J, Bouvier AM. Colorectal cancer in inflammatory bowel diseases: a population-based study (1976-2008). Inflamm Bowel Dis. 2012;18:2247-2251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Harpaz N, Polydorides AD. Colorectal dysplasia in chronic inflammatory bowel disease: pathology, clinical implications, and pathogenesis. Arch Pathol Lab Med. 2010;134:876-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 6. | Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140:1807-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 856] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 7. | Riddell RH, Goldman H, Ransohoff DF, Appelman HD, Fenoglio CM, Haggitt RC, Ahren C, Correa P, Hamilton SR, Morson BC. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol. 1983;14:931-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1348] [Cited by in RCA: 1213] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 8. | Körsgen S, Keighley MR. Causes of failure and life expectancy of the ileoanal pouch. Int J Colorectal Dis. 1997;12:4-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 67] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Rutter MD, Saunders BP, Wilkinson KH, Kamm MA, Williams CB, Forbes A. Most dysplasia in ulcerative colitis is visible at colonoscopy. Gastrointest Endosc. 2004;60:334-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 215] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 10. | Odze RD. Adenomas and adenoma-like DALMs in chronic ulcerative colitis: a clinical, pathological, and molecular review. Am J Gastroenterol. 1999;94:1746-1750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 85] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Rutter M, Bernstein C, Matsumoto T, Kiesslich R, Neurath M. Endoscopic appearance of dysplasia in ulcerative colitis and the role of staining. Endoscopy. 2004;36:1109-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Van Assche G, Dignass A, Bokemeyer B, Danese S, Gionchetti P, Moser G, Beaugerie L, Gomollón F, Häuser W, Herrlinger K. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 3: special situations. J Crohns Colitis. 2013;7:1-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 340] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 13. | Cairns SR, Scholefield JH, Steele RJ, Dunlop MG, Thomas HJ, Evans GD, Eaden JA, Rutter MD, Atkin WP, Saunders BP. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut. 2010;59:666-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 808] [Article Influence: 53.9] [Reference Citation Analysis (2)] |
| 14. | Farraye FA, Odze RD, Eaden J, Itzkowitz SH. AGA technical review on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:746-74, 774.e1-4; quiz e12-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 341] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 15. | Blackstone MO, Riddell RH, Rogers BH, Levin B. Dysplasia-associated lesion or mass (DALM) detected by colonoscopy in long-standing ulcerative colitis: an indication for colectomy. Gastroenterology. 1981;80:366-374. [PubMed] |
| 16. | Lennard-Jones JE, Melville DM, Morson BC, Ritchie JK, Williams CB. Precancer and cancer in extensive ulcerative colitis: findings among 401 patients over 22 years. Gut. 1990;31:800-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 281] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Butt JH, Konishi F, Morson BC, Lennard-Jones JE, Ritchie JK. Macroscopic lesions in dysplasia and carcinoma complicating ulcerative colitis. Dig Dis Sci. 1983;28:18-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 86] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Schneider A, Stolte M. Differential diagnosis of adenomas and dysplastic lesions in patients with ulcerative colitis. Z Gastroenterol. 1993;31:653-656. [PubMed] |
| 19. | Torres C, Antonioli D, Odze RD. Polypoid dysplasia and adenomas in inflammatory bowel disease: a clinical, pathologic, and follow-up study of 89 polyps from 59 patients. Am J Surg Pathol. 1998;22:275-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 121] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Mathy C, Schneider K, Chen YY, Varma M, Terdiman JP, Mahadevan U. Gross versus microscopic pancolitis and the occurrence of neoplasia in ulcerative colitis. Inflamm Bowel Dis. 2003;9:351-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Itzkowitz SH, Present DH. Consensus conference: Colorectal cancer screening and surveillance in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:314-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 395] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 22. | Friedman S, Odze RD, Farraye FA. Management of neoplastic polyps in inflammatory bowel disease. Inflamm Bowel Dis. 2003;9:260-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Odze RD, Brown CA, Hartmann CJ, Noffsinger AE, Fogt F. Genetic alterations in chronic ulcerative colitis-associated adenoma-like DALMs are similar to non-colitic sporadic adenomas. Am J Surg Pathol. 2000;24:1209-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Connell WR, Lennard-Jones JE, Williams CB, Talbot IC, Price AB, Wilkinson KH. Factors affecting the outcome of endoscopic surveillance for cancer in ulcerative colitis. Gastroenterology. 1994;107:934-944. [PubMed] |
| 25. | Medlicott SA, Jewell LD, Price L, Fedorak RN, Sherbaniuk RW, Urbanski SJ. Conservative management of small adenomata in ulcerative colitis. Am J Gastroenterol. 1997;92:2094-2098. [PubMed] |
| 26. | Engelsgjerd M, Farraye FA, Odze RD. Polypectomy may be adequate treatment for adenoma-like dysplastic lesions in chronic ulcerative colitis. Gastroenterology. 1999;117:1288-194; discussion 1288-194;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 179] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 27. | Odze RD, Farraye FA, Hecht JL, Hornick JL. Long-term follow-up after polypectomy treatment for adenoma-like dysplastic lesions in ulcerative colitis. Clin Gastroenterol Hepatol. 2004;2:534-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 172] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 28. | Rubin PH, Friedman S, Harpaz N, Goldstein E, Weiser J, Schiller J, Waye JD, Present DH. Colonoscopic polypectomy in chronic colitis: conservative management after endoscopic resection of dysplastic polyps. Gastroenterology. 1999;117:1295-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 226] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 29. | Blonski W, Kundu R, Furth EF, Lewis J, Aberra F, Lichtenstein GR. High-grade dysplastic adenoma-like mass lesions are not an indication for colectomy in patients with ulcerative colitis. Scand J Gastroenterol. 2008;43:817-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Kisiel JB, Loftus EV, Harmsen WS, Zinsmeister AR, Sandborn WJ. Outcome of sporadic adenomas and adenoma-like dysplasia in patients with ulcerative colitis undergoing polypectomy. Inflamm Bowel Dis. 2012;18:226-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 31. | Ullman TA. Chromoendoscopy should be the standard method and more widely used for cancer surveillance colonoscopy in ulcerative colitis--con. Inflamm Bowel Dis. 2007;13:1273-1274; discussion 1273-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Rubin DT, Rothe JA, Hetzel JT, Cohen RD, Hanauer SB. Are dysplasia and colorectal cancer endoscopically visible in patients with ulcerative colitis? Gastrointest Endosc. 2007;65:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 33. | Eaden J, Abrams K, McKay H, Denley H, Mayberry J. Inter-observer variation between general and specialist gastrointestinal pathologists when grading dysplasia in ulcerative colitis. J Pathol. 2001;194:152-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 158] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 34. | Dixon MF, Brown LJ, Gilmour HM, Price AB, Smeeton NC, Talbot IC, Williams GT. Observer variation in the assessment of dysplasia in ulcerative colitis. Histopathology. 1988;13:385-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 128] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Melville DM, Jass JR, Morson BC, Pollock DJ, Richman PI, Shepherd NA, Ritchie JK, Love SB, Lennard-Jones JE. Observer study of the grading of dysplasia in ulcerative colitis: comparison with clinical outcome. Hum Pathol. 1989;20:1008-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 140] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 36. | Odze RD, Goldblum J, Noffsinger A, Alsaigh N, Rybicki LA, Fogt F. Interobserver variability in the diagnosis of ulcerative colitis-associated dysplasia by telepathology. Mod Pathol. 2002;15:379-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 109] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 37. | Winawer S, Fletcher R, Rex D, Bond J, Burt R, Ferrucci J, Ganiats T, Levin T, Woolf S, Johnson D. Colorectal cancer screening and surveillance: clinical guidelines and rationale-Update based on new evidence. Gastroenterology. 2003;124:544-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1501] [Cited by in RCA: 1438] [Article Influence: 65.4] [Reference Citation Analysis (0)] |
| 38. | Ullman TA. Dysplasia and colorectal cancer in Crohn’s disease. J Clin Gastroenterol. 2003;36:S75-S58; discussion S75-S58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Odze RD. Pathology of dysplasia and cancer in inflammatory bowel disease. Gastroenterol Clin North Am. 2006;35:533-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 40. | Judge TA, Lewis JD, Lichtenstein GR. Colonic dysplasia and cancer in inflammatory bowel disease. Gastrointest Endosc Clin N Am. 2002;12:495-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 41. | Harpaz N, Talbot IC. Colorectal cancer in idiopathic inflammatory bowel disease. Semin Diagn Pathol. 1996;13:339-357. [PubMed] |
| 42. | Rubio CA, Johansson C, Slezak P, Ohman U, Hammarberg C. Villous dysplasia. An ominous histologic sign in colitic patients. Dis Colon Rectum. 1984;27:283-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 32] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | Andersen SN, Lovig T, Clausen OP, Bakka A, Fausa O, Rognum TO. Villous, hypermucinous mucosa in long standing ulcerative colitis shows high frequency of K-ras mutations. Gut. 1999;45:686-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 44. | Rubio CA, Befrits R, Jaramillo E, Nesi G, Amorosi A. Villous and serrated adenomatous growth bordering carcinomas in inflammatory bowel disease. Anticancer Res. 2000;20:4761-4764. [PubMed] |
| 45. | Wong NA, Mayer NJ, MacKell S, Gilmour HM, Harrison DJ. Immunohistochemical assessment of Ki67 and p53 expression assists the diagnosis and grading of ulcerative colitis-related dysplasia. Histopathology. 2000;37:108-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 62] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 46. | Andersen SN, Rognum TO, Bakka A, Clausen OP. Ki-67: a useful marker for the evaluation of dysplasia in ulcerative colitis. Mol Pathol. 1998;51:327-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 47. | Wong NA, Harrison DJ. Colorectal neoplasia in ulcerative colitis-recent advances. Histopathology. 2001;39:221-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 48. | Burmer GC, Rabinovitch PS, Haggitt RC, Crispin DA, Brentnall TA, Kolli VR, Stevens AC, Rubin CE. Neoplastic progression in ulcerative colitis: histology, DNA content, and loss of a p53 allele. Gastroenterology. 1992;103:1602-1610. [PubMed] |
| 49. | Löfberg R, Broström O, Karlén P, Ost A, Tribukait B. DNA aneuploidy in ulcerative colitis: reproducibility, topographic distribution, and relation to dysplasia. Gastroenterology. 1992;102:1149-1154. [PubMed] |
| 50. | Itzkowitz SH, Young E, Dubois D, Harpaz N, Bodian C, Chen A, Sachar DB. Sialosyl-Tn antigen is prevalent and precedes dysplasia in ulcerative colitis: a retrospective case-control study. Gastroenterology. 1996;110:694-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 51. | Itzkowitz SH, Greenwald B, Meltzer SJ. Colon carcinogenesis in inflammatory bowel disease. Inflamm Bowel Dis. 1995;1:142-158. [PubMed] |
| 52. | Greenwald BD, Harpaz N, Yin J, Huang Y, Tong Y, Brown VL, McDaniel T, Newkirk C, Resau JH, Meltzer SJ. Loss of heterozygosity affecting the p53, Rb, and mcc/apc tumor suppressor gene loci in dysplastic and cancerous ulcerative colitis. Cancer Res. 1992;52:741-745. [PubMed] |
| 53. | Noffsinger AE, Belli JM, Miller MA, Fenoglio-Preiser CM. A unique basal pattern of p53 expression in ulcerative colitis is associated with mutation in the p53 gene. Histopathology. 2001;39:482-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 54. | Harpaz N, Peck AL, Yin J, Fiel I, Hontanosas M, Tong TR, Laurin JN, Abraham JM, Greenwald BD, Meltzer SJ. p53 protein expression in ulcerative colitis-associated colorectal dysplasia and carcinoma. Hum Pathol. 1994;25:1069-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 85] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 55. | Chaubert P, Benhattar J, Saraga E, Costa J. K-ras mutations and p53 alterations in neoplastic and nonneoplastic lesions associated with longstanding ulcerative colitis. Am J Pathol. 1994;144:767-775. [PubMed] |
| 56. | Walsh S, Murphy M, Silverman M, Odze R, Antonioli D, Goldman H, Loda M. p27 expression in inflammatory bowel disease-associated neoplasia. Further evidence of a unique molecular pathogenesis. Am J Pathol. 1999;155:1511-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 57. | Fogt F, Zhuang Z, Poremba C, Dockhorn-Dworniczak B, Vortmeyer A. Comparison of p53 immunoexpression with allelic loss of p53 in ulcerative colitis-associated dysplasia and carcinoma. Oncol Rep. 1998;5:477-480. [PubMed] |
| 58. | Noffsinger AE, Miller MA, Cusi MV, Fenoglio-Preiser CM. The pattern of cell proliferation in neoplastic and nonneoplastic lesions of ulcerative colitis. Cancer. 1996;78:2307-2312. [PubMed] |
| 59. | Rubin CE, Haggitt RC, Burmer GC, Brentnall TA, Stevens AC, Levine DS, Dean PJ, Kimmey M, Perera DR, Rabinovitch PS. DNA aneuploidy in colonic biopsies predicts future development of dysplasia in ulcerative colitis. Gastroenterology. 1992;103:1611-1620. [PubMed] |
| 60. | Younes M, Lebovitz RM, Lechago LV, Lechago J. p53 protein accumulation in Barrett’s metaplasia, dysplasia, and carcinoma: a follow-up study. Gastroenterology. 1993;105:1637-1642. [PubMed] |
| 61. | Greenblatt MS, Bennett WP, Hollstein M, Harris CC. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994;54:4855-4878. [PubMed] |
| 62. | Dorer R, Odze RD. AMACR immunostaining is useful in detecting dysplastic epithelium in Barrett’s esophagus, ulcerative colitis, and Crohn’s disease. Am J Surg Pathol. 2006;30:871-877. [PubMed] |
| 63. | Chen CC, Pekow J, Llado V, Kanneganti M, Lau CW, Mizoguchi A, Mino-Kenudson M, Bissonnette M, Mizoguchi E. Chitinase 3-like-1 expression in colonic epithelial cells as a potentially novel marker for colitis-associated neoplasia. Am J Pathol. 2011;179:1494-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 64. | Ludwig K, Fassan M, Mescoli C, Pizzi M, Balistreri M, Albertoni L, Pucciarelli S, Scarpa M, Sturniolo GC, Angriman I. PDCD4/miR-21 dysregulation in inflammatory bowel disease-associated carcinogenesis. Virchows Arch. 2013;462:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 65. | Kiran RP, Nisar PJ, Goldblum JR, Fazio VW, Remzi FH, Shen B, Lavery IC. Dysplasia associated with Crohn’s colitis: segmental colectomy or more extended resection? Ann Surg. 2012;256:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 66. | Sigel JE, Petras RE, Lashner BA, Fazio VW, Goldblum JR. Intestinal adenocarcinoma in Crohn’s disease: a report of 30 cases with a focus on coexisting dysplasia. Am J Surg Pathol. 1999;23:651-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 77] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 67. | Delaunoit T, Limburg PJ, Goldberg RM, Lymp JF, Loftus EV. Colorectal cancer prognosis among patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2006;4:335-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 111] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 68. | Sugita A, Greenstein AJ, Ribeiro MB, Sachar DB, Bodian C, Panday AK, Szporn A, Pozner J, Heimann T, Palmer M. Survival with colorectal cancer in ulcerative colitis. A study of 102 cases. Ann Surg. 1993;218:189-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 69. | Greenstein AJ, Slater G, Heimann TM, Sachar DB, Aufses AH. A comparison of multiple synchronous colorectal cancer in ulcerative colitis, familial polyposis coli, and de novo cancer. Ann Surg. 1986;203:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 70. | Fogt F, Vortmeyer AO, Goldman H, Giordano TJ, Merino MJ, Zhuang Z. Comparison of genetic alterations in colonic adenoma and ulcerative colitis-associated dysplasia and carcinoma. Hum Pathol. 1998;29:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 77] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 71. | Nugent FW, Haggit RC, Colcher H, Kutteruf GC. Malignant potential of chronic ulcerative colitis. Preliminary report. Gastroenterology. 1979;76:1-5. [PubMed] |
| 72. | Richards ME, Rickert RR, Nance FC. Crohn’s disease-associated carcinoma. A poorly recognized complication of inflammatory bowel disease. Ann Surg. 1989;209:764-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 90] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 73. | Sjödahl RI, Myrelid P, Söderholm JD. Anal and rectal cancer in Crohn’s disease. Colorectal Dis. 2003;5:490-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 74. | Yamazaki Y, Ribeiro MB, Sachar DB, Aufses AH, Greenstein AJ. Malignant colorectal strictures in Crohn’s disease. Am J Gastroenterol. 1991;86:882-885. [PubMed] |
| 75. | Nakahara H, Ishikawa T, Itabashi M, Hirota T. Diffusely infiltrating primary colorectal carcinoma of linitis plastica and lymphangiosis types. Cancer. 1992;69:901-906. [PubMed] |
| 76. | Levi GS, Harpaz N. Intestinal low-grade tubuloglandular adenocarcinoma in inflammatory bowel disease. Am J Surg Pathol. 2006;30:1022-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 77. | Sigel JE, Goldblum JR. Neuroendocrine neoplasms arising in inflammatory bowel disease: a report of 14 cases. Mod Pathol. 1998;11:537-542. [PubMed] |
| 78. | Grassia R, Bodini P, Dizioli P, Staiano T, Iiritano E, Bianchi G, Buffoli F. Neuroendocrine carcinomas arising in ulcerative colitis: coincidences or possible correlations? World J Gastroenterol. 2009;15:4193-4195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 79. | Cheng H, Sitrin MD, Satchidanand SK, Novak JM. Colonic squamous cell carcinoma in ulcerative colitis: Report of a case and review of the literature. Can J Gastroenterol. 2007;21:47-50. [PubMed] |
| 80. | Michelassi F, Montag AG, Block GE. Adenosquamous-cell carcinoma in ulcerative colitis. Report of a case. Dis Colon Rectum. 1988;31:323-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 81. | Hock YL, Scott KW, Grace RH. Mixed adenocarcinoma/carcinoid tumour of large bowel in a patient with Crohn’s disease. J Clin Pathol. 1993;46:183-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 82. | Palazzo JP, Mittal KR. Lymphoepithelioma-like carcinoma of the rectum in a patient with ulcerative colitis. Am J Gastroenterol. 1996;91:398-399. [PubMed] |
| 83. | Kulaylat MN, Doerr R, Butler B, Satchidanand SK, Singh A. Squamous cell carcinoma complicating idiopathic inflammatory bowel disease. J Surg Oncol. 1995;59:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 84. | Hamilton SR. Colorectal carcinoma in patients with Crohn’s disease. Gastroenterology. 1985;89:398-407. [PubMed] |
| 85. | Ekbom A, Helmick C, Zack M, Adami HO. Increased risk of large-bowel cancer in Crohn’s disease with colonic involvement. Lancet. 1990;336:357-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 448] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 86. | Connell WR, Sheffield JP, Kamm MA, Ritchie JK, Hawley PR, Lennard-Jones JE. Lower gastrointestinal malignancy in Crohn’s disease. Gut. 1994;35:347-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 98] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 87. | Stahl TJ, Schoetz DJ, Roberts PL, Coller JA, Murray JJ, Silverman ML, Veidenheimer MC. Crohn’s disease and carcinoma: increasing justification for surveillance? Dis Colon Rectum. 1992;35:850-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 45] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 88. | Nikias G, Eisner T, Katz S, Levin L, Eskries D, Urmacher C, McKinley M. Crohn’s disease and colorectal carcinoma: rectal cancer complicating longstanding active perianal disease. Am J Gastroenterol. 1995;90:216-219. [PubMed] |
| 89. | Ribeiro MB, Greenstein AJ, Sachar DB, Barth J, Balasubramanian S, Harpaz N, Heimann TM, Aufses AH. Colorectal adenocarcinoma in Crohn’s disease. Ann Surg. 1996;223:186-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 64] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 90. | Choi PM, Zelig MP. Similarity of colorectal cancer in Crohn’s disease and ulcerative colitis: implications for carcinogenesis and prevention. Gut. 1994;35:950-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 276] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 91. | Friedman S, Rubin PH, Bodian C, Goldstein E, Harpaz N, Present DH. Screening and surveillance colonoscopy in chronic Crohn’s colitis. Gastroenterology. 2001;120:820-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 168] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 92. | Odze R, Antonioli D, Peppercorn M, Goldman H. Effect of topical 5-aminosalicylic acid (5-ASA) therapy on rectal mucosal biopsy morphology in chronic ulcerative colitis. Am J Surg Pathol. 1993;17:869-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 102] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 93. | Greenstein AJ, Sachar DB, Smith H, Janowitz HD, Aufses AH. Patterns of neoplasia in Crohn’s disease and ulcerative colitis. Cancer. 1980;46:403-407. [PubMed] |
| 94. | Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, Dixon MF, Fenoglio-Preiser CM, Fléjou JF, Geboes K. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1463] [Cited by in RCA: 1548] [Article Influence: 61.9] [Reference Citation Analysis (0)] |