Published online Mar 14, 2014. doi: 10.3748/wjg.v20.i10.2688
Revised: November 28, 2013
Accepted: January 2, 2014
Published online: March 14, 2014
Processing time: 195 Days and 0.6 Hours
AIM: To investigate the relationship between CD14-260 and -651 polymorphisms and the risk of developing gastric cancer.
METHODS: DNA was extracted from peripheral blood samples obtained from 225 Tibetans with gastric cancer and 237 healthy Tibetans, and analyzed using the polymerase chain reaction/ligase detection (PCR/LDR) method to determine the genotypes at -260 and -651 loci of the CD14 promoter. The allele frequencies, genotype frequencies, and haplotypes were analyzed for their association with gastric cancer risk using online SHEsis software. The luciferase reporter assay and point mutation analysis were used to construct in vitro plasmids expressing a C/T homozygote at the -260 locus of the CD14 promoter.
RESULTS: The frequencies of CC, CT and TT genotypes in the CD14-260 C/T locus in gastric cancer patients were 19.1%, 38.7% and 42.2%, respectively, whereas they were 33.3%, 32.5% and 34.2%, respectively, in healthy control subjects. CT genotype carriers were more frequently found among gastric cancer patients than healthy controls (OR = 2.076; 95%CI: 1.282-3.360). Also, TT genotype carriers were more frequently found among gastric cancer patients (OR = 2.155; 95%CI: 1.340-3.466). Compared to the C allele of CD14/-260, the T allele was associated with an increased risk for gastric cancer (OR = 1.574; 95%CI: 1.121-2.045). Furthermore, the frequencies of CC, CT and TT in the CD14-651 C/T locus in gastric cancer patients were 64.4%, 29.3% and 6.2%, respectively, while they were 56.5%, 35.0% and 8.4%, respectively, in the healthy control subjects (P > 0.05). Data obtained using the luciferase reporter assay showed that the p260T homozygote was associated with greater CD14 promoter activity (P < 0.01).
CONCLUSION: CD14/-260 polymorphism is associated with gastric cancer risk in Highland Tibetans and affects CD14 promoter activity, thereby regulating CD14 expression.
Core tip: This is the first study to report that CD14 gene polymorphisms in Highland Tibetans are associated with gastric cancer risk. We also identified a biomarker that can be used in gastric cancer research and clinical practice.
- Citation: Li K, Dan Z, Hu XJ, Gesang LB, Ze YG, Bianba ZX, Ciren CM, Nie YQ. Association of CD14/-260 polymorphism with gastric cancer risk in Highland Tibetans. World J Gastroenterol 2014; 20(10): 2688-2694
- URL: https://www.wjgnet.com/1007-9327/full/v20/i10/2688.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i10.2688
Gastric cancer is a significant health problem and was the fourth most common malignancy worldwide in 2008, with an estimated 989600 incident cases. Approximately 72% of incident cases are diagnosed in developing countries; however, the incidence of gastric cancer in developed countries, including North America and Europe, has actually declined during the past 50 years[1]. The known risk factors for gastric cancer include Heliobacter pylori (H. pylori) infection, exposure to tobacco smoke, and high consumption of smoked foods, salted meat or fish, and pickled vegetables. Additionally, these risk factors augment the risk of gastric cancer in individuals who are genetically predisposed to the disease. However, consumption of fresh fruits and vegetables appears to lower the risk of gastric cancer[2-5]. Epidemiological studies have reported the presence of family clusters of gastric cancer and coincident cases of gastric cancer in identical twin siblings[6], suggesting that genetic susceptibility may impact the risk of developing gastric cancer. The genomic region of differentiation antigen 14 (CD14), especially the CD14 C→T polymorphisms in the -159/-260 and -651 promoter regions, has attracted significant attention regarding a possible role in gastric cancer. Such genetic polymorphisms might affect CD14 expression in cells and subsequently alter expression of genes downstream of CD14, thereby altering CD14 biological function and the development/outcome of CD14-related diseases[7,8].
CD14 is a glycoprotein located on the cell surface and functions as a receptor for lipopolysaccharides (LPSs). CD14 is mainly produced by monocytes, macrophages and neutrophils, and CD14-TLR4 is an important receptor complex in the LPS-presenting pathway[9]. H. pylori is a micro-aerophilic Gram-negative bacterium recognized by the World Health Organization (WHO) as a group I carcinogen for gastric cancer and lymphoma of gastric mucosa-associated lymphoid tissue. CD14 has multiple roles in the mediation of primary immune and inflammatory responses[10-12]. During an immune response, CD14 mediates cellular recognition of LPS, phosphorylation of cellular tyrosine, and translocation of nuclear factor (NF)-κB, to trigger release of cytokines and production of oxygen radicals. During an inflammatory response, CD14 functions in conjunction with LPS binding protein (LPB) to form a LPS-LPB-CD14-TLR4-MD2 complex, which then activates monocytes and macrophages to produce inflammatory cytokines.
To date, CD14-159 and/or -260 polymorphisms have been associated with susceptibility to or development of various diseases, including inflammatory bowel disease, allergies[13-16], and gastric cancer[17,18]. Tibetans have one of the highest prevalences of gastric cancer in China, and the prevalence in Tibet is higher than the average prevalence in China[19]. In this study, we used the ligase detection reaction (LDR) for nucleotide typing to examine the distribution of alleles and genotypes for the CD14 loci in Highland Tibetan gastric cancer patients, and compared the results with those obtained from healthy Highland Tibetans. This study was conducted to explore the association between CD14 polymorphisms and the risk of gastric cancer, and provide information regarding the molecular basis of gastric cancer risk in Highland Tibetans.
A total of 225 gastric cancer patients and 237 healthy individuals were recruited from the Gastroenterology Unit and Oncology Unit of Tibet People’s Hospital. This study was approved by the hospital’s Institutional Review Board, and signed informed consent was obtained from all patients and healthy subjects before enrolling in the study. Gastric cancer was diagnosed based on criteria described by the WHO in 1979. Subjects with autoimmune disorders, including systemic lupus erythematous, rheumatoid arthritis, and inflammatory bowel disease, were excluded from this study. All study subjects were Highland Tibetans who had been living in Tibet for several generations and were not biologically related to one another.
Gastric cancer cell line MGC-803 was obtained from the Shanghai Cell Bank of the Chinese Academy of Science (Shanghai, China) and cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 mg/L streptomycin in a humidified incubator with 5% CO2 at 37 °C.
Samples of venous blood (2 mL) were drawn via the cubital vein from fasting study subjects and stored in EDTA-containing anti-coagulative tubes at -70 °C. DNA was extracted using a genomic DNA extraction kit (Beijing TIANGEN Biology Co., Ltd, Beijing, China) according to the manufacturer’s protocol. CD14 gene polymorphisms were identified by searching GenBank for relevant CD14 sequence and polymorphism information. This information was then used to design PCR primers, which were synthesized and purified by Shanghai Sangon Biotech (Shanghai, China) (Table 1). PCR amplification was carried out in a 20 μL reaction volume containing 2.0 μL of 1 × PCR buffer, 0.4 μL of each primer (10 pmol), 2.0 μL of each dNTP (2.0 mmol/L), 9.3 μL of sterilized water, 0.6 μL of MgCl2, 0.3 μL of Taq enzyme (2.5 U/μL), and 5 μL of template DNA. The PCR reaction conditions consisted of an initial denaturation at 95 °C for 15 min, followed by 35 cycles of 94 °C for 30 s, 53 °C for 1 min and 65 °C for 1 min, and a final extension cycle at 72 °C for 7 min. The PCR products were separated by 3% agarose gel electrophoresis and used for the LDR.
| Polymorphism | Primers and probes | Length, bp |
| -260 C/T | 5'-CACCCACCAGAGAAGGCTTA-3' | 212 |
| 5'-ATCACCTCCCCACCTCTCTT-3' | ||
| Common probe | 5'-CCCCCTCCCTGAAACATCCTTTTTTTTTTTTTTTTTTT-FAM-3' | |
| Discriminating | 260C/T-R_G | 77 |
| probes | 5'-TTTTTTTTTTTTTTTTTGCAGAATCCTTCCTGTTACGGC | |
| 260C/T-R_A-3' | 79 | |
| 5'-TTTTTTTTTTTTTTTTTTTGCAGAATCCTTCCTGTTACGGT-3' | ||
| -651C/T | 5'-GGGTAGAATTAGGTTCAAG-3' | 103 |
| 5'-CTTAATCAAAGGAGCAAGG-3' | 1 | |
| Common probe | 5'-GTCTAAAGAAAAATTCCCCCTTCCTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTT-FAM-3' | |
| Discriminating probes | 651C/T_C | 150 |
| 5'-TTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTAGGTTCAAGAAAAGGAAGTTG-3' | ||
| 651C/T_T | 152 | |
| 5'-TTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTAGGTTCAAGAAAAGGAAGTTA-3' |
The LDR was used to measure the distribution of CD14 alleles and genotypes in the aforementioned PCR products. The upstream and downstream probes for multiple LDRs were designed as shown in Table 1. The upstream probe was modified by phosphorylation at the 5’-terminal region. The LDR reaction mixture contained 2 μL of PCR product, 1 μL of LDR probe mixture (1 mmol/L), 1 μL of 1 × buffer, and 0.05 μL of ligase (2 U), and the total volume was adjusted to 10 μL using PCR-grade water. LDR conditions were 95 °C for 2 min, followed by 30 cycles of 95 °C for 15 s and 50 °C for 25 s. The PCR/LDR gel electrophoresis and DNA sequencing experiments were conducted by Shanghai Biowing Applied Biotechnology Co., Ltd. (Shanghai, China) using the following equipment: Gene Amp PCR System 9600 (Perkin-Elmer, Waltham, MA, United States); PTC-200 Gradient Cycler (MJ Research, Waltham, MA, United States); PRISM 3730 and 3100 DNA Sequencer (Applied Biosystems Inc., Carlsbad, CA, United States); JY600+ Electrophoresis System (Beijing Junyi-Dongfang Electrophoresis Equipment Co., Ltd, Beijing, China); FR-200A Automatic Ultra-violet and Visible Light Analyzer and the Biological Electrophoretic Image Analyzer (Shanghai Furi Technology Co., Ltd, Shanghai, China); Agarose LE (Shanghai Genebase Gene-Tech Co., Ltd, Shanghai, China); Flouroskan Ascent FL (Thermo Scientific, Waltham, MA, United States).
The luciferase reporter assay was used to examine the effects of different genotypes (C and T) of CD14 on regulation of CD14 expression in gastric cancer cells. Reporter vectors were constructed to verify the effect of -260C/T polymorphism on CD14 expression, and primers were used to amplify the -360 to +29 region of the CD14 promoter. The relevant primer sequences were: 5’-GACCGCTAGCCGAGTCAACAGGGCATTCAC-3’ and 5’-CGTCAAGCTTGTT CGACCCCAAGACCCTAC-3’, while primers for the mutated-260 site (changed to C) were 5’-CCTTCCTGTTACGGCCCCCCTCCCTG-3’ and 5’-GTTTCAGGGAGGGGGGCCGTAACAGGA-3’. Cleavage sites of NheI and HindIII were also introduced on both ends of the primers, respectively. The PCR products were inserted into a pGL3-Basic reporter vector (Promega, Madison, WI, United States). After cloning, amplification, and DNA sequence confirmation, these vectors were designated as p260C and p260T, respectively, and subsequently used to transfect gastric cancer MGC-803 cells. Co-transfection with the renilla luciferase reporter vector pRL-TK was also performed at this time. After 12 h, LPS was added to the cell culture at a final concentration of 1 μg/mL, and the cells were incubated for an additional 12 h and then analyzed using a dual-luciferase assay kit (Promega) according to the manufacturer’s instructions. Measurements of fluorescence intensity were expressed as the mean ± SE of firefly/renilla obtained from three readings at each setting.
Frequencies of CD14 genotypes and alleles in gastric cancer patients and control subjects were calculated and tested using the Hardy-Weinberg equilibrium equation. Allele frequencies, genotype frequencies, and haplotypes were analyzed using SHEsis online software (http://analysis.bio.-x.cn/myAnalysis.php). Comparisons of demographic and clinical data between groups were conducted using the χ2 test and Student’s t test. The Student’s t test was also used to analyze genotype and luciferase levels. P values were generated using the SPSS statistical software suite for Windows (SPSS Inc., Chicago, IL, United States); a P-value < 0.05 was considered statistically significant.
Genotyping for the CD14 polymorphisms was conducted in 225 patients pathologically diagnosed with gastric adenocarcinoma and 237 healthy control subjects. The gastric cancer patients had a mean age of 54.8 ± 11.2 years, and included 172 males and 53 females. The 237 healthy control subjects were recruited among individuals who received a routine health examination at the hospital during the concurrent period. The control subjects had a mean age of 54.8 ± 11.2 years, and included 175 males and 62 females (Table 2). The results of genotyping studies showed that genotype frequencies of CD14 -260C/T and -651 C/T were not significantly different (P > 0.05) between gastric cancer and control subjects. The population was in Hardy-Weinberg equilibrium.
| Variable | Controls | GC patients | aP value |
| n = 237 | n = 225 | ||
| Age, yr | 54.8 ± 11.2 | 54.0 ± 12.3 | NS |
| Male | 175 (73.8) | 172 (76.4) | NS |
| Clinical stage | |||
| I | 7 (3.1) | ||
| II | 21 (9.3) | ||
| III | 126 (56.0) | ||
| IV | 71 (31.6) |
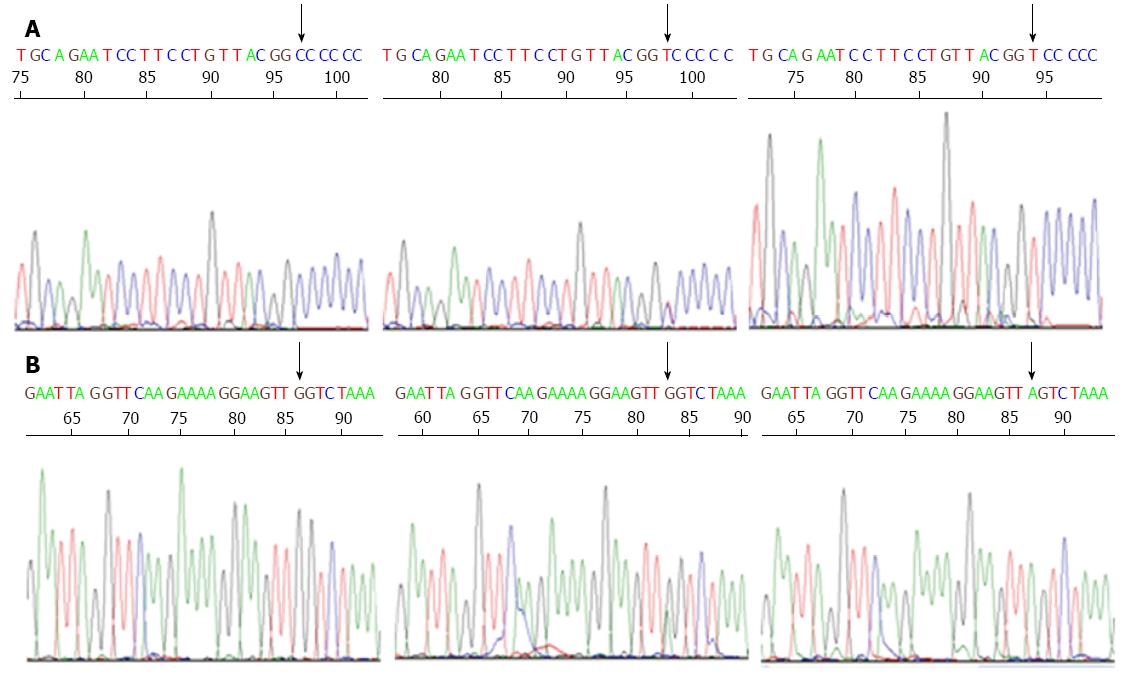
Gastric cancer patients showed CD14-260 C/T CC, CT and TT genotype frequencies of 19.1%, 38.7% and 42.2%, respectively, whereas these frequencies in healthy control subjects were 33.3%, 32.5% and 34.2%, respectively. These results showed that frequencies of the CT and TT genotypes of CD14 were significantly higher in gastric cancer patients than in healthy control subjects (for CT: OR = 2.076; 95%CI: 1.282-3.360, P = 0.003; for TT: OR = 2.155; 95%CI: 1.340-3.466, P = 0.001). Additionally, the T allele carried by gastric cancer patients was associated with a diagnosis of gastric cancer (OR = 1.574; 95%CI: 1.121-2.045, P = 0.001) (Table 3). The DNA sequences of CD14-260C/T polymorphism are shown in Figure 1A.
| Polymorphism | Case | Control | OR | 95%CI | P value |
| n = 225 | n = 237 | ||||
| -260 C/T | |||||
| Genotypes | |||||
| CC | 43 (19.1) | 79 (33.3) | 1.000 | (reference) | - |
| CT | 87 (38.7) | 77 (32.5) | 2.076 | 1.282-3.360 | 0.003 |
| TT | 95 (42.2) | 81 (34.2) | 2.155 | 1.340-3.466 | 0.001 |
| Alleles | |||||
| C | 173 (38.4) | 235 (49.6) | 1.000 | (reference) | - |
| T | 277 (61.6) | 239 (50.4) | 1.574 | 1.121-2.045 | 0.001 |
| -651 C/T | |||||
| Genotypes | |||||
| CC | 145 (64.4) | 134 (56.5) | 1.000 | (reference) | - |
| CT | 66 (29.3) | 83 (35.0) | 0.735 | 0.493-1.096 | 0.130 |
| TT | 14 (6.2) | 20 (8.4) | 0.647 | 0.314-1.332 | 0.234 |
| Alleles | |||||
| C | 356 (79.1) | 351 (74.1) | 1.000 | (reference) | - |
| T | 94 (20.9) | 123 (25.9) | 0.753 | 0.55-1.024 | 0.07 |
The frequencies of CD14-651C/T CC, CT and TT genotypes in gastric cancer patients were 64.4%, 29.3% and 6.2%, respectively, and 56.5%, 35.0% and 8.4%, respectively, in the healthy control subjects. The differences in genotype frequencies between the two groups of subjects were not statistically significant (P > 0.05). Moreover, the frequencies of C and T alleles were 79.1% and 20.9%, respectively, in gastric cancer patients, and 74.1% and 25.9%, respectively, in healthy control subjects, and these differences were also not statistically significant (P > 0.05; Table 3). The DNA sequences of CD14-651C/T polymorphism are shown in Figure 1B.
Linkage disequilibrium (LD) analysis showed linkage disequilibrium between the CD14/-260C/T and CD14/-651 C/T loci (|D’| > 0.50). Haplotype analysis showed that the -260C/T--651 C/TT-C haplotype was associated with an increased risk of developing gastric cancer (OR = 1.58; 95%CI: 1.21-2.07, P = 0.001; Table 3), and the -260C/T--651 C/TC-C haplotype was associated with a decreased risk of developing gastric cancer (OR = 0.70; 95%CI: 0.51-0.96, P = 0.028; Table 4).
| Haplotypes -260C/T - -651 C/T | Case | Control | OR (95%CI) | P value |
| T-C | 244 (57.9) | 206 (46.5) | 1.58 (1.21-2.07) | 0.001 |
| C-C | 87 (20.8) | 120 (27.2) | 0.70 (0.51-0.96) | 0.028 |
| C-T | 76 (18.1) | 97 (21.9) | 0.79 (0.56-1.10) | 0.157 |
| T-T | 13 (3.3) | 19 (4.4) | 0.73 (0.36-1.47) | 0.371 |
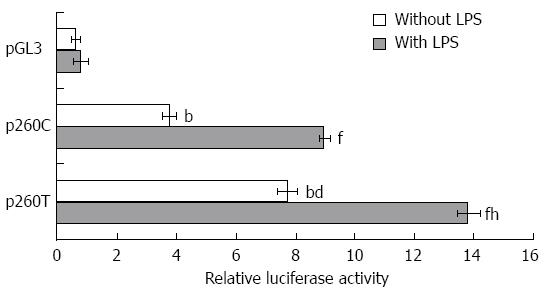
The luciferase reporter assay was used to determine the effect of the CD14-260C/T gene promoter on CD14 expression. The results showed that luciferase activity in p260T-transfected cells was 2.05-fold higher than that in p260C-transfected cells (P < 0.01). LPS treatment induced 5.19- and 6.09-fold increases in relative luciferase activity in p260C- and p260T-transfected cells, respectively (P < 0.01). The 1.54-fold increase in relative luciferase activity produced by LPS treatment in p260T-transfected cells compared to p260C-transfected cells (P < 0.01) indicated that the CD14-260 polymorphism did affect CD14 expression in gastric cancer cells (Figure 2).
This study analyzed polymorphisms of CD14/-260 and CD14/-651 loci in Tibetan gastric cancer patients and healthy control subjects. The results showed that compared to the C allele, the T allele of CD14/-260 was associated with an increased risk for gastric cancer. However, the CD14/-651 polymorphism was not associated with a risk of gastric cancer. Moreover, the CD14/-260 polymorphism was found to affect CD14 promoter activity and therefore regulate CD14 expression. Our current study confirmed that the CD14/-260 polymorphism is associated with a higher risk for developing gastric cancer in Highland Tibetans. A further study with a larger number of subjects will be conducted to investigate the molecular mechanism underlying the role of CD14 in development of gastric cancer.
Gastric cancer is associated with H. pylori infection and subsequent inflammation, and thus the role of cytokines in gastric cancer has received increased attention. Because environment, lifestyle, and other extrinsic factors also impact gastric cancer development, research on gene-environment interactions may provide a better understanding of the genetic background of various ethnic groups and why certain ethnic groups have increased susceptibility to developing gastric cancer.
Various epidemiological studies have shown inconsistent correlations between different diseases and CD14 polymorphisms, and some of these contradictory results might be due to the inclusion of heterogeneic genetic groups in such studies. Highland Tibetans live in a hypoxic environment and have a unique genetic makeup that permits adaptation to such environmental conditions. Highland Tibetans also frequently suffer from certain pre-cancerous conditions, such as chronic atrophic gastritis and gastric ulcer. These conditions may be due to high levels of circulating H. pylori, which are associated with the high prevalence of gastric cancer in Tibetans[20]. The current study demonstrated a higher rate of CD14/-260 CT genotype polymorphism in gastric cancer patients (38.7%) than in healthy control subjects (32.5%). Additionally, the CD14/-260 TT genotype was found more frequently in gastric cancer patients (42.2%) compared to healthy subjects (32.2%). The T allele of CD14/-260 was also found significantly more often in gastric cancer patients than in healthy subjects, and individuals carrying the T allele had a 1.6-fold increased risk of developing gastric cancer compared to individuals carrying the C allele. Highland Tibetans carrying the T allele of CD14/-260 might have an increased risk of gastric cancer, because the T allele promotes high levels of CD14 expression. CD14/-651 C/T polymorphism was not associated with a risk for gastric cancer in Highland Tibetans. Our current data support results from a previous study by Zhao et al[18], which showed an association between genetic polymorphism in this locus and H. pylori-related gastric cancer.
As a receptor for LPS, CD14 plays a crucial role in innate immunity and is mainly expressed in mature monocytes, macrophages, and activated neutrophils[21]. Inflammatory signals induced by LPS initiate signal transduction through TLR4 and CD14. These events trigger activation of transcription factors, such as NF-κB, which regulate secretion of interleukins-1, 6, 8 and 12, and TNF-α. The latter cytokine subsequently triggers a series of immune and inflammatory responses that can damage the gastric mucosa[22]. However, the mechanism by which H. pylori infection causes gastric cancer remains to be determined. One hypothesis is that inflammation triggered by H. pylori infection causes the gastric mucosa to undergo atrophy, intestinal metaplasia, and dysplasia, leading to eventual development of gastric cancer[23]. In this context, polymorphism of CD14/-260 may alter the host’s immune system, weaken defenses against H. pylori infection, and allow the gastric mucosa to become susceptible to infection, inflammation, and formation of cancerous lesions. At the molecular level, the C260T polymorphism harbors the S1 binding site of the CD14 promoter, and a C/T polymorphism may alter CD14 promoter activity, leading to increased CD14 gene transcription. Thus, the T allele homozygote can enhance CD14 expression on circulating monocytes and therefore promote inflammation. Our current data support this notion, because CD14/C260T was associated with very high luciferase activity, and such high transcriptional activity will induce high levels of CD14 expression, especially during H. pylori infection[18].
Gastric cancer is a multi-factorial disease, and our current study suggests that gastric cancer may be induced by several events, including oncogene activation and the down-regulation of tumor suppressor genes. Nevertheless, this study does not provide evidence associating CD14 polymorphisms with these events or explain how CD14 polymorphisms subvert the immune response to trigger gastric cancer development. However, our study does provide novel information which may link CD14 polymorphisms in Highland Tibetans with an increased risk of developing gastric cancer. Future studies will further investigate the role of CD14 in development of gastric cancer.
Helicobacter pylori infection is the major risk factor for gastric cancer. During an immune response, CD14 mediates cellular recognition of lipopolysaccharides (LPS), phosphorylation of cellular tyrosine, and translocation of nuclear factor (NF)-κB to trigger cytokine release and production of oxygen radicals. This study investigated CD14-260 and -651 polymorphisms in Highland Tibetans for their association with gastric cancer risk.
Tibetans have one of the highest rates of gastric cancer in China, and the prevalence in Tibet is higher than the average prevalence throughout China. This study was conducted to explore the association between CD14 polymorphisms in highland Tibetans and the risk of gastric cancer, and also to clarify the connection between genotype and phenotype.
This study demonstrated an association between CD14/-260 polymorphism and gastric cancer risk in Highland Tibetans. Studies conducted in vitro revealed that CD14/-260 polymorphism affects CD14 promoter activity and may therefore regulate CD14 expression. This study provides information regarding the molecular basis for an increased gastric cancer risk among Highland Tibetans.
This study provides molecular data linking genetic polymorphisms to an increased risk of gastric cancer in the high-plateau Tibetan population in China. The results lay a foundation for use of genetic screening to identify individuals at high risk of gastric cancer and for developing gene therapy techniques for prevention and treatment.
LPS is lipopolysaccharide. CD14 is a cell surface glycoprotein mainly produced by monocytes, macrophages, and neutrophils. CD14 has multiple roles in the mediation of primary immune and inflammatory responses. CD14-TLR4 is an important receptor complex in the LPS presenting pathway.
This is a well designed study with interesting results. The authors demonstrated an association of CD/-260 polymorphism with gastric cancer risk in Highland Tibetans. In vitro data revealed that CD14/-260 polymorphism affects CD14 promoter activity and therefore regulates CD14 expression. This manuscript has some novelty because the study population was mainly located in Tibet, and the results may help explain the high prevalence of gastric cancer in this highland area.
P- Reviewers: Aoyagi K, Domagk D, Chung YJ, Ji JF, Shrikhande SV S- Editor: Cui XM L- Editor: Wang TQ E- Editor: Ma S
| 1. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11834] [Article Influence: 845.3] [Reference Citation Analysis (4)] |
| 2. | Brenner H, Rothenbacher D, Arndt V. Epidemiology of stomach cancer. Methods Mol Biol. 2009;472:467-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 411] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 3. | Forman D, Burley VJ. Gastric cancer: global pattern of the disease and an overview of environmental risk factors. Best Pract Res Clin Gastroenterol. 2006;20:633-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 307] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 4. | Suzuki H, Iwasaki E, Hibi T. Helicobacter pylori and gastric cancer. Gastric Cancer. 2009;12:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Ferguson LR. Meat and cancer. Meat Sci. 2010;84:308-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 165] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 6. | Hamajima N, Matsuo K, Saito T, Tajima K, Okuma K, Yamao K, Tominaga S. Interleukin 1 polymorphisms, lifestyle factors, and Helicobacter pylori infection. Jpn J Cancer Res. 2001;92:383-389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 103] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Griga T, Klein W, Epplen JT, Hebler U, Stachon A, May B. CD14 expression on monocytes and soluble CD14 plasma levels in correlation to the promotor polymorphism of the endotoxin receptor CD14 gene in patients with inactive Crohn’s disease. Hepatogastroenterology. 2005;52:808-811. [PubMed] |
| 8. | Baldini M, Lohman IC, Halonen M, Erickson RP, Holt PG, Martinez FD. A Polymorphism* in the 5’ flanking region of the CD14 gene is associated with circulating soluble CD14 levels and with total serum immunoglobulin E. Am J Respir Cell Mol Biol. 1999;20:976-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 579] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 9. | Parajuli B, Sonobe Y, Kawanokuchi J, Doi Y, Noda M, Takeuchi H, Mizuno T, Suzumura A. GM-CSF increases LPS-induced production of proinflammatory mediators via upregulation of TLR4 and CD14 in murine microglia. J Neuroinflammation. 2012;9:268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 10. | Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2084] [Cited by in RCA: 2417] [Article Influence: 134.3] [Reference Citation Analysis (0)] |
| 11. | Guo S, Al-Sadi R, Said HM, Ma TY. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am J Pathol. 2013;182:375-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 508] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 12. | Berthet J, Damien P, Hamzeh-Cognasse H, Arthaud CA, Eyraud MA, Zéni F, Pozzetto B, McNicol A, Garraud O, Cognasse F. Human platelets can discriminate between various bacterial LPS isoforms via TLR4 signaling and differential cytokine secretion. Clin Immunol. 2012;145:189-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Sengler C, Haider A, Sommerfeld C, Lau S, Baldini M, Martinez F, Wahn U, Nickel R. Evaluation of the CD14 C-159 T polymorphism in the German Multicenter Allergy Study cohort. Clin Exp Allergy. 2003;33:166-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Wang Z, Hu J, Fan R, Zhou J, Zhong J. Association between CD14 gene C-260T polymorphism and inflammatory bowel disease: a meta-analysis. PLoS One. 2012;7:e45144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Bakolis I, Doekes G, Heinrich J, Zock JP, Heederik D, Kogevinas M, Guerra S, Norbäck D, Ramasamy A, Nevalainen A. Respiratory health and endotoxin: associations and modification by CD14/-260 genotype. Eur Respir J. 2012;39:573-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Wang G, Yu D, Tan W, Zhao D, Wu C, Lin D. Genetic polymorphism in chemokine CCL22 and susceptibility to Helicobacter pylori infection-related gastric carcinoma. Cancer. 2009;115:2430-2437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Tahara T, Shibata T, Hirata I, Nakano H, Arisawa T. CD14 promoter-159 polymorphism is associated with reduced risk of intestinal-type gastric cancer in a Japanese population. Dig Dis Sci. 2009;54:1508-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Zhao D, Sun T, Zhang X, Guo Y, Yu D, Yang M, Tan W, Wang G, Lin D. Role of CD14 promoter polymorphisms in Helicobacter pylori infection--related gastric carcinoma. Clin Cancer Res. 2007;13:2362-2368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Dan Z, Li K, Wang ZH, Xiangba ZX. Epidemiological features of gastric cancer in a community population in Lhasa. World Chin J Digestol. 2013;21:2104-2108. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Li K, Zhang YL, Dan Z, Zhaxi CM, Nie J. Risk factors for the gastric cancer: a case-control study in Tibet. Dig Liver Dis. 2009;41:78-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Wu MS, Cheng TY, Shun CT, Lin MT, Chen LC, Lin JT. Functional polymorphisms of CD14 and toll-like receptor 4 in Taiwanese Chinese with Helicobacter pylori-related gastric malignancies. Hepatogastroenterology. 2006;53:807-810. [PubMed] |
| 22. | Isomoto H, Mizuta Y, Miyazaki M, Takeshima F, Omagari K, Murase K, Nishiyama T, Inoue K, Murata I, Kohno S. Implication of NF-kappaB in Helicobacter pylori-associated gastritis. Am J Gastroenterol. 2000;95:2768-2776. [PubMed] |
| 23. | Tapping RI, Tobias PS. Soluble CD14-mediated cellular responses to lipopolysaccharide. Chem Immunol. 2000;74:108-121. [PubMed] |