Published online Dec 15, 1996. doi: 10.3748/wjg.v2.i4.209
Revised: September 10, 1996
Accepted: October 25, 1996
Published online: December 15, 1996
AIM: To observe the effects of both dimethylamine hydrochloride and sodium nitrite on the glandular stomach of Wistar rats.
METHODS: Both dimethylamine hydrochloride and sodium nitrite were administered to male Wistar rats at a concentration of 0.06% in the drinking water ad libitum for 56 wks, and the rats were killed subsequently. The sequential histological changes induced in the glandular stomach of the rats by both dimethylamine hydrochloride and sodium nitrite were also observed.
RESULTS: It was found that the rate of intestinal metaplasia in the glandular stomach of rats was 88.9% (40/45), 55.0% (22/40) of which had the sulphomucin-positive intestinal metaplasia. The rates of mild, moderate and severe dysplasia were 44.5% (20/45), 33.3% (15/45), and 22.2% (10/45), respectively. Cystic dilatation of gastric glands was found in 28.9% (13/45). No gastric cancer occurred. The sequential histological changes were also reported.
CONCLUSION: Both dimethylamine hydrochloride and sodium nitrite can induce precancerous lesions of the glandular stomach in Wistar rats and may be one of the pathogenic factors of gastric cancer.
- Citation: Li CQ, Liu WW. Precancerous lesions in the glandular stomach of Wistar rats induced with dimethylamine hydrochloride and sodium nitrite. World J Gastroenterol 1996; 2(4): 209-211
- URL: https://www.wjgnet.com/1007-9327/full/v2/i4/209.htm
- DOI: https://dx.doi.org/10.3748/wjg.v2.i4.209
Since the demonstration by Magee et al[1] in 1956 of the carcinogenicity of N-nitrosodimethylamine (NDMA), the carcinogenic potential of over 200 N-NCs has been established in all 40 animal species so far tested with varying degrees of susceptibility in different organs including the liver, stomach, oesophagus, intestines, bladder, brain, lung and kidney[2,3]. There is now convincing experimental epidemiological evidence that N-NCs associated with human cancer affect the oral cavity, urinary bladder and oesophagus, and their possible role in the pathogenesis of gastric cancer has also been strongly postulated[4]. Recently NDMA and some other volatile N-nitrosamines and the precursors of N-NCs were detected in fish-sauce by Chinese researchers in the high-risk areas of gastric cancer[5,6]. Lu et al[7] further found that the concentration of N-NCs was relatively low in foods but that in the fasting gastric juice was very high in the high-risk population of oesophageal cancer, postulating that the endogenous synthesis in vivo was the main source of N-NCs. Both dimethylamine and sodium nitrite are the precursors of NDMA synthesis, and whether they can induce gastric cancer is unknown. In the present study, we observed the effects of both dimethylamine hydrochloride and sodium nitrite on the glandular stomach of rats.
One hundred and forty male Wistar rats, weighing 120-140 g, were randomized into three groups. Groups 1 and 2 consisted of 50 rats, respectively. They were given a standard diet and received a mixed drinking water containing 0.06% dimethylamine hydrochloride or 0.06% sodium nitrite ad libitum. The control group consisted of 40 rats, and was fed a regular diet and water. All the rats were housed in plastic cages (ten animals in each cage) in a room with controlled temperature and humidity. Animals were maintained under the guidance set forth in the “Guide for the Care and Use of Laboratory Animals” by the National Laboratory Animal Administration Council, China. From the beginning of experiments every 2-4 wk, 3-4 rats in group 1 were killed during 56 wk. The rats in group 2 and control group were killed at the 56th week.
The stomaches of the rats were quickly excised and opened along the great curvature. Each stomach was washed several times with normal saline for gross examination, and fixed in 10% neutral formalin and cut into 8 longitudinal strips about 2 mm in width. The strips were embedded in paraffin and serially sectioned at 4 μm in thickness. Sections were routinely stained with hematoxylin and eosin, and with alcian blue/periodic acid-Schiff (AB/PAS) and high iron diamine/alcian blue (HID/AB). The livers of the rats were also treated routinely for the histopathological observations. The pathological diagnosis and the grading of the lesions were performed according to the criteria of the National Anti-cancer Association of China in 1990.
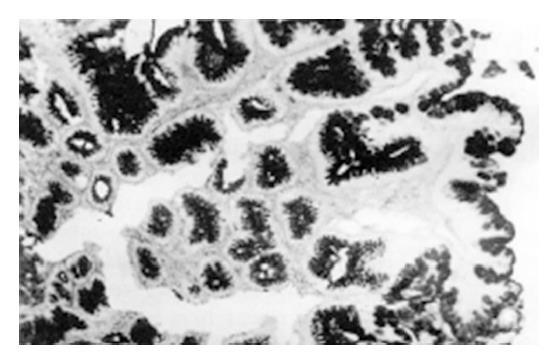
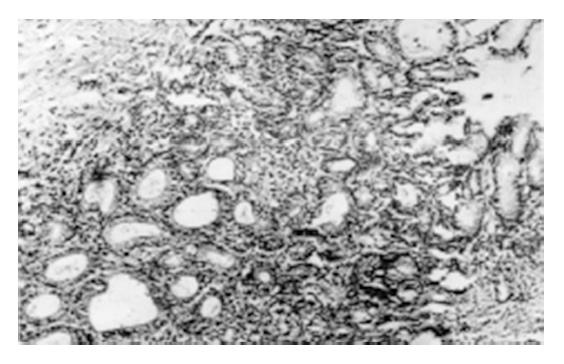
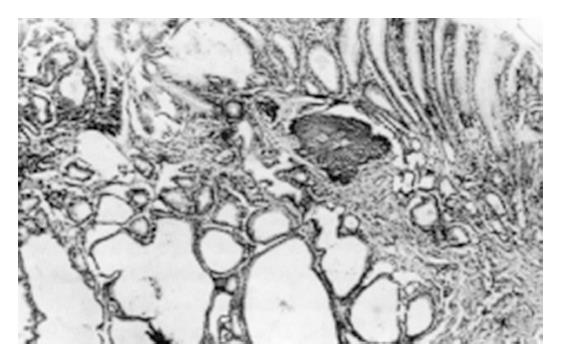
During the 2nd to 12th week, the gastric mucosal congestion, edema and erosion were grossly observed under a light microscope. There was a lot of inflammatory cell infiltration. Intestinal metaplasia (IM) of the glandular stomach was first found in the glandular neck with characteristic goblet cells at the 18th week and afterwards extended to the surface or bottom of the mucosa (Figure 1). Mild dysplasia of the gastric mucosa occurred at the 24th week, moderate dysplasia at the 32th week and severe dysplasia at the 46th week (Figure 2). Single and multiple cystic dilatation of the gastric glands was noted at the 22nd week (Figure 3). In the forestomaches of rats, hyperplasia or mild dysplasia of squamous epithelia appeared after the 36th week. At the 48th week, under a light microscope the obvious degeneration and necrosis of liver cells and inflammatory cell infiltration in the portal area were observed. At the 54th week, greyish white nodules in the liver could be seen at gross examination and under a light microscope, and the most typical feature was the hyperplasia of liver cells and fibro-tissues and adenoma-like dilatation of some intrahepatic biliary ducts.
Total intake of dimethylamine hydrochloride or sodium nitrite was 5.88 g and the mean daily intake was 15 mg for a rat. Five rats died in the course of experimentation. It was found that the rate of IM in the glandular stomaches of the rats was 88.9% (40/45), 55.0% (22/40) of which was the sulphomucin-positive IM. The rates of mild, moderate and severe dysplasia were 44.5 (20/45), 33.3% (15/45) and 22.2% (10/45), respectively. Cystic dilatation of gastric glands was found in 28.9% (13/45). No gastric cancer occurred. Hyperplasia of the squamous epithelia was 26.7% (12/45) and mild dysplasia 17.8% (8/45) in the forestomaches of the rats. Ten (22.2%) cases of liver cirrhosis without liver cancer were diagnosed.
IM was observed in 3 (7.5%) rats in the pyloric gland areas of stomaches.
N-NCs have been identified in various environmental situations, such as food products, water, drugs, cosmetics, tobacco products, agricultural and industrial products and so on. Exposure in human beings may be through ingestion, inhalation, skin contact and in vivo formation, with the latter being mainly in the gastrointestinal tract which probably represents the main source of human exposure. The site most commonly regarded as risk from endogenous N-NC synthesis in vivo is the stomach. N-NC synthesis in vivo can be carried out by chemical or biological synthesis[8]. Dimethylamine hydrochloride or sodium nitrite itself is not carcinogenic but they can be changed into NDMA by chemical synthesis in the acidic environment of the stomach.
It is widely accepted that epithelial dysplasia of the gastric mucosa is a precancerous lesion and IM, especially sulphomucin-positive IM, is closely related to gastric cancer[9-11]. We previously found that the sequential lesions of gastric glandular mucosa induced with both N-methyl-N-nitro-N-nitrosoguanidine (MNNG) and ranitidine were from IM, dysplasia to gastric cancer or from dysplasia to gastric cancer[12]. In this study both dimethylamine hydrochloride and sodium nitrite were administered to Wistar rats in the drinking water ad libitum for 56 wk, and the incidences of IM and moderate and severe dysplasia were 88.9% and 55.5%, respectively, suggesting that the two precursors of NDMA synthesis can induce the precancerous lesions of the stomach in rats. This finding enriches the contents of etiology of gastric cancer and supplies new experimental evidence for the primary prevention of gastric cancer.
We previously reported that after 0.01% NDMA liquid was administered to rats for 16 wk ad libitum, the incidence of liver cancer was 100% at the 18th week[13]. In the present study, both dimethylamine hydrochloride and sodium nitrite, the precursors of NDMA synthesis, were fed to the rats for 56 wk, and 22.2% of the rats developed liver cirrhosis. Epidemical investigations suggested that NDMA could be one of the pathogenic factors of oesophageal cancer. Oesophageal lesions were not observed in the study but only hyperplasia and mild dysplasia of the squamous epithelium were found in forestomaches of the rats. We supposed that this may be related to the low synthesizing quantity of NDMA in the stomach of the rats. The lesions of the liver and forestomach may be more severe if higher concentrations of both dimethylamine hydrochioride and sodium nitrite are administrated to the rats.
Original title:
S- Editor: Yang ZD L- Editor: Wang TQ E- Editor: Zhang FF
| 1. | Magee PN, Barnes JM. The production of malignant primary hepatic tumours in the rat by feeding dimethylnitrosamine. Br J Cancer. 1956;10:114-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 658] [Cited by in RCA: 560] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 2. | Bogovski P, Bogovski S. Animal Species in which N-nitroso compounds induce cancer. Int J Cancer. 1981;27:471-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 176] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Schmaal D, Scherf HR. Carcinogenic activity of N-nitroso-diethylamine in snakes. In Oneill IK (eds.) N-nitroso compounds: occurrence, biological effects and relevance to human cancer. IARC Scient Publ. 1984;57:677-682. |
| 4. | Correa P. A human model of gastric carcinogenesis. Cancer Res. 1988;48:3554-3560. [PubMed] |
| 5. | Cheng CS, Yu L, Cheng Y. Investigation on N-nitroso compounds in fish-sauce colleged from a high-risk area of stomach cancer. Zhongguo Aizheng Zazhi. 1988;7:81-83. |
| 6. | Zhang RF, Deng DJ, Cheng Y. Analysis of precursors of N-nitroso compounds in fish sauce from a high gastric cancer mortality area. Zhongguo Aizheng Zazhi. 1993;12:395-397. |
| 7. | Lu SX. [N-nitrosamines in gastric juice of subjects from high incidence area of esophageal cancer]. Zhonghua Zhongliu Zazhi. 1988;10:322-325. [PubMed] |
| 8. | Reed PI. Nitroso compounds, gastriccarcinogenesis and chemoprevention. In Reed PI, Carboni M, Johnstone BJ (eds). New trends in gastric cancer: background and vidosurgery. London: Kluwer Academic Publishers 1990; 21-30. [DOI] [Full Text] |
| 9. | Li C, Zhang X, Liu W. Recent progress in researches on precancerous lesions of gastric cancer in China. Zhonghua Yixue Zazhi (Engl). 1996;109:407-410. [PubMed] |
| 10. | Li C, Liu W, Fang D. Histological and electron-microscopic observations on the mucosa of pediculate gastric wall graft transplanted to the intestines in Wistar rats. Zhonghua Yixue Zazhi (Engl). 1996;109:77-82. [PubMed] |
| 11. | Li CQ, Liu WW, Wang BO, Li JC. Intestinal metaplasia, dysplasia and gastric cancer: a study of mucohistochemistry, immunohistochemistry and cell DNA quantitative analysis. Jiefangjun Yixueyuan Xuebao. 1992;7:103-106. |
| 12. | Li CQ, Liu WW, Fang DC. Experimental study on model establishment, mechanism of production and reverse therapy of gastric mucosal precancerous lesions in rats. Jiefangjun Yixueyuan Xuebao. 1994;9:103-107. |
| 13. | Liang HJ, Yuan AL, Liu WW. Changes of selenium level and glutathione peroxidase activity in blood and liver tissues during hepatocarcinogenesis in rats. Zhonghua Xiaohua Zazhi. 1990;10:215-217. |