Published online Sep 15, 1996. doi: 10.3748/wjg.v2.i3.185
Revised: May 25, 1996
Accepted: July 16, 1996
Published online: September 15, 1996
- Citation: Zhao SL, Pan XF, Li SX, Liu DG. Combination assay for serum tumor markers in patients with hepatic carcinoma. World J Gastroenterol 1996; 2(3): 185-186
- URL: https://www.wjgnet.com/1007-9327/full/v2/i3/185.htm
- DOI: https://dx.doi.org/10.3748/wjg.v2.i3.185
In general, hepatic solid space occupying lesions (HSSOL) can be found by both ultrasonoscopy (US) and computed tomography (CT). If the customary alpha-fetoprotein (AFP) standard for diagnosis of primary hepatic carcinoma is used, those cancers with lower AFP concentration may go undiagnosed[1]. On the other hand, using the standard AFP-positive (≥ 20 μg/L) + HSSOL to diagnose primary hepatocellular carcinoma (PHCC) may yield false positive results[2,3]. In order to elevate both the preoperative and differential diagnosis levels for hepatic carcinoma, we assayed the preoperative levels of serum AFP, carbohydrate antibody (CA) 19-9, and carcinoembryonic antigen (CEA) in patients with HSSOL.
Four groups of patients (30 patients in each group) with benign HSSOL (BHSSOL group; including 17 cases of hepatic cyst, 10 of benign hepatic tumor, and three of hepatic tuberculosis), secondary hepatic carcinoma (SHC group; the cancer stemmed from the stomach in 12 cases; the pancreas in eight cases; the colon in seven cases; the bile duct in two cases; and the uterus in one case), primary non-hepatocellular carcinoma (PNHCC group; including 29 cases of intrahepatic duct cell carcinoma and one case of mixed type cancer), and primary hepatocellular carcinoma (PHCC group) were verified by percutaneoustranshepatic biopsy and/or hepatic postoperative pathology. All patients met the following criteria: (1) HSSOL was confirmed by US or CT. (2) The peripheral venous blood samples were collected before the operation. The concentrations of serum AFP, CA19-9, and CEA were assayed. Patients with serum AFP levels between 20 μg/L and 200 μg/L were reexamined by the same method 1 mo later. And s(3) The main organs, including stomach, pancreas, biliary tree, colon, lung, kidney, uterus, etc. were examined by endoscopy, X-ray, or CT combined with biopsy to check for the existence of tumor lesions. In this series, 74 patients were male and 46 were female, and they were between 17 and 76 years old, with a mean age of 53.5.
CA19-9 radioimmunoassay (RIA) kit was purchased from Symtron (USA), and other kits were provided by the Institute of Chinese Atomy (China). Serum AFP, CA19-9, and CEA levels were measured according to the manufacturer’s protocols.
Serum AFP ≥ 200 μg/L or between 20 μg/L and 200 μg/L, which was enhanced or unchanged after 1 month, was defined as AFP positive. Antigen levels higher than the cutoff value (serum CA19-9 ≥ 37 KU/L and CEA ≥ 15 μg/L) or a single item two times higher than the cutoff value were considered positive.
Analysis of variance was used for the multivariant measurement data, and a t test was used to make comparisons between two groups.
Using a cutoff value of 20 μg/L, the sensitivity of AFP to PHCC was 100% (30/30 cases, Table 1), and the specificity was 85.7% (78/90 cases). With a cutoff of 200 μg/L, the sensitivity was 76.7% (23/30 cases), and the specificity was 100% (90/90 cases).
| Grouping | n | AFP (μg/L) | CA19-9 (KU/L) | CEA (μg/L) |
| 1 BHSSOL | 30 | 11.07 ± 4.30 | 28.43 ± 15.76 | 10.49 ± 6.93 |
| 2 SHC | 30 | 15.23 ± 13.13 | 231.20 ± 196.64 | 27.08 ± 13.35 |
| 3 PNHCC | 30 | 14.03 ± 8.13 | 169.67 ± 140.08 | 19.27 ± 8.67 |
| 4 PHCC | 30 | 320.65 ± 180.12 | 23.73 ± 12.91 | 10.73 ± 3.94 |
With a cutoff value of 37 KU/L for CA19-9, the sensitivity in detecting PNHCC was 93.3% (28/30 cases, Table 1), and the specificity was 65.6% (59/90 cases). In SHC, the sensitivity and specificity of CA19-9 were 90.0% (27/30 cases) and 63.3% (57/90 cases), respectively. At a cutoff value of 74 KU/L, the sensitivity and specificity of CA 19-9 were 43.3% (13/30 cases) and 77.8% (70/90 cases), respectively, in detecting PNHCC, and 13.3% (18/30 cases) and 81.1% (73/90 cases), respectively, in detecting SHC.
At a cutoff of 15 μg/L, the sensitivity of CEA in detecting PNHCC was 73.3% (22/30 cases, Table 1), and the specificity was 63.3% (58/90 cases). In SHC, the sensitivity and specificity were 80.0% (24/30 cases) and 66.7% (53/90 cases), respectively. At a cutoff of 30 μg/L, the sensitivity and specificity of CEA were 16.7% (5/30 cases) and 84.4% (76/90 cases), respectively, in detecting PNHCC; and 13.3% (18/30 cases) and 83.3% (75/90 cases), respectively, in detecting SHC.
The results from the combined assay are shown in Table 2. With US, X-ray, CT, and endoscopy in combination with biopsy, 29 cases (96.7%) of extrahepatic primary cancer nests were clinically discovered in the SHC group; and four cases (13.3%) of extrahepatic transition cancer nests were discovered in the PNHCC group. The difference between the two groups was significant (P < 0.05, Table 2).
| Grouping | No. of cases | AFP | CA19-9 + CEA | ||
| Positive | Negative | Positive | Negative | ||
| BHSSOL | 30 | 0 | 30 | 0 | 30 |
| SHC | 30 | 3 | 27 | 30 | 0 |
| PNHCC | 30 | ||||
| Biliary cell cancer | 29 | 0 | 29 | 29 | 0 |
| Mixed cancer | 1 | 1 | 0 | 1 | 0 |
| PNCC | 30 | 30 | 0 | 0 | 30 |
There are four types of HSSOL, namely, PHCC, PNHCC, SHC, and BHSSOL. We found that serum AFP level was much higher in PHCC than in the other three types (P < 0.01, Table 1), while there was no statistically significant difference among the other three groups (P > 0.05). By raising the cutoff value of AFP, the specificity for detecting cancer was improved, but the sensitivity was reduced. Using the standard in our study (serum AFP ≥ 200 μg/L or between 20 μg/L and 200 μg/L, which was elevated 1 mo later), specificity and sensitivity for diagnosing PHCC were the highest. Therefore, we recommend that this standard be adopted as the diagnosis standard of PHCC. Assaying serum AFP level helps to differentiate PNHCC and SHC from BHSSOL and PHCC. Serum CA19-9 levels were much higher in the SHC and PNHCC groups than in PHCC and BHSSOL groups (P < 0.01). CA19-9 is a gastrointestinal tumor marker, and its content is the highest in tumor epithelial cells of the digestive tract[4,5]. Since PNHCC is an abdominal adenocarcinoma, the serum CA19-9 level in PNHCC is elevated, which is normal in PHCC and BHSSOL. Therefore, these diseases can be differentiated by assaying serum CA19-9 levels. Serum CEA levels were higher in the PNHCC and SHC groups than in the PHCC and BHSOL groups (P < 0.01). It has been reported that serum levels of CEA might be elevated significantly in patients with digestive tract tumors 2-18 mo before the lesions were found clinically by X-ray examination[6,7]. CEA is an important marker for the diagnosis of liver metastasis. Sometimes the level of CEA is elevated by 100% in patients with hepatic metastasis. CEA may also be a marker of intestinal tumors. Therefore, we can assay serum CEA levels to differentiate between SHC and PNHCC, and BHSSOL and PHCC.
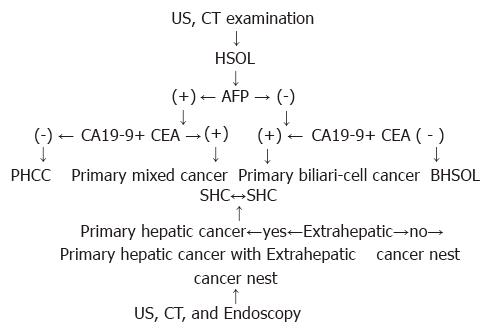
As shown in Table 2, negative-AFP and positive CA 19-9 + CEA occurred in 90% of SHC and primary biliary cell cancer (96.7%) among PNHCC. Both positive AFP and CA19-9 + CEA occurred in 10% of SHC and mixed cancer (3.3%) among PNHCC. SHC usually originated from the tumors of lower organs, i.e., stomach, colon, pancreas, biliary tree, etc. As some of the tumors of the digestive system belong to hepatoid adenocarcinomas in SHC, AFP levels were positive[8]. Nagai et al[9] reported that serum AFP was elevated in 5.4% of gastric carcinoma, and liver metastasis occurred in 72% of these patients. The appearances of SHC and PNHCC intersect, making differentiation between the two difficult. Our study indicates that extrahepatic cancer nests (OTCN) are more common in SHC than in PNHCC (P < 0.01). In general, patients with OTCN discovered by US, CT, X-ray, and endoscopy may be considered to have SHC, while those without OTCN may be considered to have PNHCC. Strictly speaking, however, OTCH transferred from primary hepatic cancer should be differentiated from primary OTCN by pathologic examination alone. Based on the above results, the procedure of early diagnosis and differential diagnosis of hepatic carcinoma is shown by the Figure 1. By detecting serum levels of AFP, CA19-9, and CEA in patients with BHSOL, preoperative and differential diagnosis of PHCC, PNHCC, SHC, and BHSSOL might be improved.
Original title:
S- Editor: Tao T L- Editor: Filipodia E- Editor: Li RF
| 1. | Ma ZC, Tang BH, Tang JY and Ye KL. Studies on diagnostic criteria of hepatocellular carcinoma with positive AFP. Zhonghua Xiaohau Zazhi. 1993;13:286. |
| 2. | Li WD. Deliberation to diagnostic criteria of the formula: “AFP+SOL=HCC”. Zhonghua Xiaohau Zazhi. 1994;14:262. |
| 3. | Filella X, Fuster J, Molina R, Grau JJ, García-Valdecasas JC, Grande L, Estapé J, Ballesta AM. TAG-72, CA 19.9 and CEA as tumor markers in gastric cancer. Acta Oncol. 1994;33:747-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Satake K, Takeuchi T. Comparison of CA19-9 with other tumor markers in the diagnosis of cancer of the pancreas. Pancreas. 1994;9:720-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Pasquali C, Sperti C, D’Andrea AA, Costantino V, Filipponi C, Pedrazzoli S. CA50 as a serum marker for pancreatic carcinoma: comparison with CA19-9. Eur J Cancer. 1994;30A:1042-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Guadagni F, Roselli M, Cosimelli M, Spila A, Cavaliere F, Arcuri R, Abbolito MR, Greiner JW, Schlom J. Biologic evaluation of tumor-associated glycoprotein-72 and carcinoembryonic antigen expression in colorectal cancer, Part I. Dis Colon Rectum. 1994;37:S16-S23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Koizumi F, Odagiri H, Fujimoto H, Kawamura T, Ishimori A. [Clinical evaluation of four tumor markers (CEA, TPA, CA50 and CA72-4) in colorectal cancer]. Rinsho Byori. 1992;40:523-528. [PubMed] |
| 8. | Chang YC, Nagasue N, Abe S, Taniura H, Kumar DD, Nakamura T. Comparison between the clinicopathologic features of AFP-positive and AFP-negative gastric cancers. Am J Gastroenterol. 1992;87:321-325. [PubMed] |
| 9. | Nagai E, Ueyama T, Yao T, Tsuneyoshi M. Hepatoid adenocarcinoma of the stomach. A clinicopathologic and immunohistochemical analysis. Cancer. 1993;72:1827-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |