Published online Feb 14, 2013. doi: 10.3748/wjg.v19.i6.855
Revised: December 17, 2012
Accepted: December 22, 2012
Published online: February 14, 2013
Processing time: 117 Days and 12.6 Hours
AIM: To assess the usefulness of contrast-enhanced ultrasound (CEUS) during follow-up after percutaneous ablation therapy for hepatocellular carcinoma (HCC).
METHODS: A total of 141 patients with HCCs who received percutaneous ablation therapy were assessed by paired follow-up CEUS and contrast-enhanced computed tomography (CECT). The follow-up scheme was designed prospectively and the intervals between CEUS and CECT examinations were less than 14 d. Both images of follow-up CEUS and CECT were reviewed by radiologists. The ablated lesions were evaluated and classified as local tumor progression (LTP) and LTP-free. LTP was defined as regrowth of tumor inside or adjacent to the successfully treated nodule. The detected new intrahepatic recurrences were also evaluated and defined as presence of intrahepatic new foci. On CEUS and CECT, LTP and new intrahepatic recurrence both were displayed as typical enhancement pattern of HCC (i.e., hyper-enhancing during the arterial phase and washout in the late phase). With CECT as the reference standard, the ability of CEUS in detecting LTP or new intrahepatic recurrence during follow-up was evaluated.
RESULTS: During a follow-up period of 1-31 mo (median, 4 mo), 169 paired CEUS and CECT examinations were carried out for the 141 patients. For a total of 221 ablated lesions, 266 comparisons between CEUS and CECT findings were performed. Thirty-three LTPs were detected on CEUS whereas 40 LTPs were detected on CECT, there was significant difference (P < 0.001). In comparison with CECT, the numbers of false positive and false negative LTPs detected on CEUS were 6 and 13, respectively; the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and overall accuracy of CEUS in detecting LTPs were 67.5%, 97.4%, 81.8%, 94.4% and 92.3%, respectively. Meanwhile, 131 new intrahepatic recurrent foci were detected on CEUS whereas 183 were detected on CECT, there was also significant difference (P < 0.05). In comparison with CECT, the numbers of false positive and false negative intrahepatic recurrences detected on CEUS were 13 and 65, respectively; the sensitivity, specificity, PPV, NPV and overall accuracy of CEUS in detecting new intrahepatic recurrent foci were 77.7%, 92.0%, 92.4%, 76.7% and 84.0%, respectively.
CONCLUSION: The sensitivity of CEUS in detecting LTP and new intrahepatic recurrence after percutaneous ablation therapy is relatively low in comparison with CECT.
- Citation: Zheng SG, Xu HX, Lu MD, Xie XY, Xu ZF, Liu GJ, Liu LN. Role of contrast-enhanced ultrasound in follow-up assessment after ablation for hepatocellular carcinoma. World J Gastroenterol 2013; 19(6): 855-865
- URL: https://www.wjgnet.com/1007-9327/full/v19/i6/855.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i6.855
Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide, the incidence of which is continuously increasing in both western and eastern countries[1]. Percutaneous ablation therapy, such as radiofrequency ablation (RFA) and microwave ablation (MWA), as a minimally invasive and effective treatment modality, has been accepted in the management of hepatic malignance and regarded as one of the best treatment options for patients with early stage HCC who are not suitable for resection or transplantation[2-5]. Several randomized clinical trials have also confirmed that for small HCC, treatment efficacy of thermal ablation is comparable to that of surgical resection[2,6,7].
The evaluation of treatment efficacy after percutaneous ablation therapy for HCC is essential for the determination of subsequent treatment and follow-up strategy[8], which is usually performed by means of imaging modalities such as contrast-enhanced computed tomography (CT) and magnetic resonance imaging (MRI)[2,9,10]. Both of them have been regarded as reliable and accurate imaging tools for post-treatment efficacy evaluation and follow-up[2]. However, contrast-enhanced CT (CECT) and contrast-enhanced MRI (CEMRI) are relatively expensive. In addition, CECT is unsuitable for patients with renal function impairment and allergic reaction to contrast agent. Finally, the radiation exposure in CT examination is always a concern for both clinicians and patients.
The technique of contrast-enhanced ultrasound (CEUS) has the potential to be a substitute for CECT or CEMRI. By using ultrasound contrast agents (UCAs) and contrast specific imaging techniques, CEUS is able to depict the micro- and macro-circulation in the liver and the treated lesion, thus allowing assessment of the treatment efficacy for HCC after percutaneous ablation therapy in a similar fashion with CECT or CEMRI[2,8,11-14]. Besides, UCAs are very safe and the incidence of severe hypersensitivity or allergic reaction is lower than that of current X-ray contrast agents and comparable to MR contrast agents[13,15]. CEUS has been confirmed to be comparable to CECT for characterization of HCC[16-19]. A prospective multi-center study also proved that CEUS is equal to CECT or CEMRI for the assessment of the local treatment response after ablation therapy[11,12]. After local treatment response assessment, the patient is always enrolled into a long-term follow-up scheme for surveillance of local tumor progression (LTP) or new intrahepatic foci[11,12]. In theory, CEUS may be inferior to CECT or CEMRI since the development of new foci may be multiple and may be located in different lobes of the liver, and the arterial enhancement on CEUS only lasts several seconds, thus it is hard to detect all the hypervascular lesions. In addition, the new foci in the blind areas such as liver dome may be invisible on CEUS. To our knowledge, there has been no study evaluating the role of CEUS in the follow-up assessment after percutaneous ablation therapy for HCC[2,12,19,20]. To confirm the hypothesis that CEUS might not be competent to CECT in follow-up assessment after local ablation for HCC, the study was carried out to assess the usefulness of CEUS in detecting LTP and new intrahepatic recurrence in the follow-up after ablation therapy for HCC, with CECT as the reference standard.
Between May 2007 and March 2011, 466 consecutive patients with HCCs were referred to the institution for ultrasound (US)-guided percutaneous ablation therapy. These patients met the following enrollment criteria: (1) single HCC not greater than 6 cm in diameter; (2) multiple HCCs up to 5 in number with each tumor measuring 3 cm or smaller; (3) absence of portal venous thrombosis or extrahepatic metastases; (4) liver cirrhosis classified as Child-Pugh class A or B; and (5) prothrombin time ratio greater than 50% and platelet count greater than 60 000/mm3 (60 × 109 /L). Among them, 141 patients (132 men, 9 women; mean age, 53.4 ± 12.1 years; age range, 27-81 years) were enrolled to this follow-up study after ablation. The inclusion criteria were as follows: (1) the patients had no allergic reaction to iodinated contrast agent; (2) CECT confirmed complete ablation of the tumors within 1 mo after ablation therapy; and (3) paired CEUS and CECT were performed during the follow-up and the time interval between CEUS and CECT was less than 14 d. All the data of the 141 patients, including baseline data, clinical data, and imaging data, were collected prospectively and stored in a dedicated database for further analysis. The study was approved by the Institutional Review Board, and written informed consent was obtained from all patients.
Among the 141 patients, 60 patients had recurrent HCCs after partial hepatectomy for primary HCCs and the remaining 81 patients with primary HCCs were treated by US-guided percutaneous ablation therapy as the first therapy. The diagnoses of HCC were confirmed by histopathological examination with specimens obtained from US-guided percutaneous biopsy (n = 35) or clinical data (n = 106). The clinical diagnostic criteria for HCC mainly followed the AASLD and EFSUMBS practice guideline: the presence of typical CECT and CEUS features (i.e., hyper-enhancement in arterial phase and washout in portal-venous or late phase)[2,15]. Among the clinically confirmed 106 patients, 46 were diagnosed by characteristic imaging findings on CECT and serum α-fetoprotein ≥ 200 ng/mL; 60 patients were diagnosed by history of partial hepatectomy for HCC and typical appearance of HCC recurrence on CECT. The US-guided percutaneous ablation therapies for them included radiofrequency ablation (RFA) (n = 83), percutaneous ethanol ablation (EA) (n = 29), RFA in combination with EA (n = 26), and microwave ablation (MWA) (n = 3).
Percutaneous ablation therapy for HCC was performed with local anesthesia and conscious sedation. RFA was carried out with a cooled-tip RFA ablation system (Cool-tip, Radionics, MA, United States), which is a 480 kHz alternative current generator that can produce a maximum power of 200W through a 17 Gmonopolar, cooled-tip needle electrode. The radiofrequency electrode temperature was maintained at less than 18 °C by the application of a circulating chilled saline solution to the cannula sheath. A single 3 cm exposed tip RFA electrode was applied[21,22]. MWA was carried out with a microwave delivery system (FORSEA; Qinghai Microwave Electronic Institute, Nanjing, China), which consisted of an MTC-3 microwave generator (FORSEA) with a frequency of 2450 MHz, a power output of 10-150 W, a flexible low-loss cable, and a 14-gauge cooled-shaft antenna. The RFA electrode or MWA antenna was firstly placed at the bottom of the tumor and withdrawn 1.5-2 cm each time to ablate the more superficial portion for large tumors. Multiple insertions were applied to treat tumors larger than 1.5 cm for RFA and 3.0 cm for MWA[21,22]. EA was performed using a Quadra-FuseTM multi-pronged needle (Rex Medical, Radnor, PA, United States). In general, no greater than 30 mL of 95% ethanol was injected until the hyperechoic cloud covered the whole tumor. For patients with tumor adjacent to critical structures such as hilum or great vessels, RFA was performed in combination with EA. In general, EA was carried out in advance of RFA and RFA was performed 5 min after EA, the aim of which was to increase the coagulation volume whereas limit the damage to adjacent critical structures[23-25]. To prevent possible bleeding or tumor seeding, the needle track was cauterized when the RFA electrode or the MWA antenna was withdrawn. The aim of the procedure was to completely ablate the tumor along with an ablative margin of 0.5-1.0 cm[26,27].
All the US examinations were performed by one of three skillful radiologists who had more than 7 years experience in CEUS and were unaware of clinical and other imaging information of the patients. Two US machines were used in this study. One was an Acuson Sequoia 512 machine (Siemens Medical Solutions, Mountain View, CA, United States) and the other was an Aplio XV machine (Toshiba Medical Systems, Tokyo, Japan). A 4V1 vector transducer with a frequency range of 1.0-4.0 MHz was applied for Sequoia 512 and a 375 BT convex transducer with a frequency range of 1.9-6.0 MHz was applied for Aplio XV. The installed contrast-specific imaging modes were contrast pulse sequencing (CPS) for Sequoia 512 and contrast harmonic imaging (CHI) for Aplio XV. Both modes work under low acoustic power, and the corresponding mechanical index (MI) ranges were 0.15-0.21 for CPS in Sequoia 512 and 0.05-0.08 for CHI in Aplio XV.
Baseline US (BUS) investigation in B-mode was firstly applied to scan the whole liver, including Doppler technique. Once the treated lesion was found, the lesion size, echogenicity, and location were recorded, and the images that showed best the above-mentioned features were stored digitally in the US machine. Then the transducer was moved to scan other liver to detect if there were suspected new recurrence foci and the above-mentioned features were also recorded if new foci were present. Afterward, the imaging mode was shifted to CEUS mode and the imaging settings were optimized to ensure sufficient tissue cancellation with the maintenance of adequate depth penetration, with the diaphragm remaining barely visible.
The US contrast agent used was SonoVue (Bracco, Milan, Italy), a sulfur hexafluoride-filled microbubble contrast agent. A total of 2.4 mL contrast agent was given intravenously as a 2.4 mL bolus within 2-3 s through the antecubital vein, followed by 5 mL saline flush. Upon start of the SonoVue injection, the stop clock was started and digital cine was recorded simultaneously. During early period of CEUS procedure, the transducer was firstly kept in a stable position to observe the enhancement pattern of the treated lesion and then switched to scan other liver parenchyma. The first 2 min was continuously observed and subsequent intermittent scanning was performed until the disappearance of contrast agent in liver parenchyma. According to the previous studies, the CEUS process was divided into arterial (i.e., 8-30 s from the beginning of contrast agent administration), portal (31-120 s), and late (121-360 s) phases[11,15]. A second or third injection of SonoVue was performed when suspicious new foci were documented on BUS or hypoenhancing new foci were detected in the late phase on CEUS. No patient received more than 3 injections.
For the CT examination, the Aquilion 64-slice helical CT machine (Tokyo, Japan) was used. The intervals between CEUS and CECT examinations were less than 14 d and no additional treatment was carried out during this period. The imaging protocol for CT examinations was as follows: 0.5 mm × 64 mm collimation, 120 kV, 150-200 mAs for 64-slice helical CT examination. The standard triphasic scan procedure was used. An unenhanced helical sequence scan through the liver was performed first; thereafter nonionic iodinated contrast material (Ultravist, Schering, Berlin, Germany) (1.5 mL/kg) was administered via antecubital vein with power injection at a rate of 4 mL/s for 64-slice helical CT. The arterial phase sequence was obtained 25-32 s after contrast material administration, followed by a portal venous phase sequence 70 s after contrast agent administration.
Two of the three skillful radiologists, who had more than 7-year experience in liver CEUS, evaluated the CEUS images and two experienced radiologists, who had more than 15-year experience in liver CECT, evaluated the treatment response using the CT images. The reviewers were not involved in the US or CT scanning, and were unaware of clinical and other imaging information of the patients. The findings of the treated lesions and new intrahepatic recurrence were observed and the treatment response was evaluated. Complete ablation was defined as nonenhancement in the ablated area; otherwise, ablation was considered incomplete. During the follow-up period, local tumor progression (LTP) was defined as regrowth of tumor inside or adjacent to the successfully treated nodule, which appeared as a hyper-enhancing area during the arterial phase and wash-out during portal-late phases inside the treated lesion on CEUS or CECT[3]. Non-enhancement in the treated area was defined as LTP-free. New intrahepatic recurrence was defined as presence of intrahepatic new foci with typical enhancement pattern of HCC on CEUS or CECT (i.e., hyper-enhancing during the arterial phase and wash-out in the late phase). Development of portal venous tumor thrombosis was also defined as new intrahepatic recurrence. Non-recurrence was defined as no additional HCC lesions found.
In the study design, the local effectiveness of ablation was assessed by CEUS or CECT within one month after ablation. Only the patients with complete ablation were enrolled into the prospectively designed follow-up scheme, and those with incomplete ablation were referred to further treatment, e.g., additional ablation, transcatheter arterial chemoembolization (TACE), Sorafenib, etc., according to the liver function status and tumor staging.
In the follow-up scheme, all patients were evaluated simultaneously with CECT and CEUS every 3 mo for the first 6 mo. If no positive findings were present and the ablation area shrank or disappeared, follow-up at an interval of 6-12 mo was made. At the same time, all patients were also monitored monthly with abdominal color Doppler US, serum AFP, chest radiography and liver function tests for the first 6 mo, and thereafter every 3 to 6 mo. When there were suspicious findings on US (i.e., enlargement of the treated area, changes in US pattern, presence of intralesional Doppler signal, and appearance of new lesion) or elevated AFP, then paired CEUS and CECT were performed to confirm the diagnosis. Once the LTP or new intrahepatic recurrences were detected, the follow-up was over and the patients were referred to further treatment.
Continuous data were expressed as mean ± SD. The χ2 test was used to compare the differences in detecting LTP and new intrahepatic recurrence between CECT and CEUS. Two-tailed P < 0.05 was considered statistically significant. With CECT as the reference standard, the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and overall accuracy of CEUS in detecting LTP were computed on the basis of the assessment results of the ablation lesions on each follow-up examination, and those of CEUS in detecting new intrahepatic recurrence were computed on the basis of the patients’ screening results on each follow-up examination. Statistical analysis were performed using the SPSS 13.0 software package (SPSS Inc., Chicago, IL, United States).
After percutaneous ablation therapy, the 141 patients with 221 HCCs (the maximum diameter ranged from 0.6 cm to 5.7 cm; mean ± SD, 2.4 ± 1.0 cm) were followed up for 1-31 mo (median, 4 mo; mean ± SD, 6.7 ± 6.4 mo) after complete ablation was confirmed by CECT 1 mo after ablation. The interval between every paired CEUS and CECT examination ranged from 0 to 14 d (median, 1 d; mean ± SD, 3.1 ± 4.3 d).
During the follow-up period, the 141 patients received 169 (once, n = 118; twice, n = 18; three times, n = 5) paired CEUS and CECT examinations. For the 221 ablated lesions (the diameter ranged from 1.2 cm to 7.4 cm; mean ± SD, 4.1 ± 1.1 cm), 266 comparisons between CEUS and CECT findings (185 ablated lesions were examined once; 27 ablated lesions were examined twice; 9 ablated lesions were examined three times) were performed.
During the follow-up, 40 LTPs (the diameter ranged from 0.4 cm to 5.5 cm; mean ± SD, 2.0 ± 1.1 cm) and 183 new intrahepatic recurrences (the diameter ranged from 0.3 cm to 3.8 cm; mean ± SD, 1.5 ± 0.8 cm) were detected on CECT, whereas only 33 and 131 were detected on CEUS. The locations of the LTPs and the new intrahepatic recurrences are summarized in Table 1.
| Location | Local tumor progression | New intrahepatic recurrence | ||
| CEUS | CECT | CEUS | CECT | |
| S1 | 0 | 0 | 1 | 2 |
| S2 | 1 | 2 | 14 | 22 |
| S3 | 2 | 2 | 7 | 13 |
| S4 | 8 | 10 | 27 | 38 |
| S5 | 5 | 7 | 17 | 18 |
| S6 | 7 | 5 | 14 | 23 |
| S7 | 3 | 4 | 17 | 18 |
| S8 | 7 | 10 | 25 | 36 |
| PV | 0 | 0 | 9 | 11 |
| HV | 0 | 0 | 0 | 2 |
| Total | 33 | 40 | 131 | 183 |
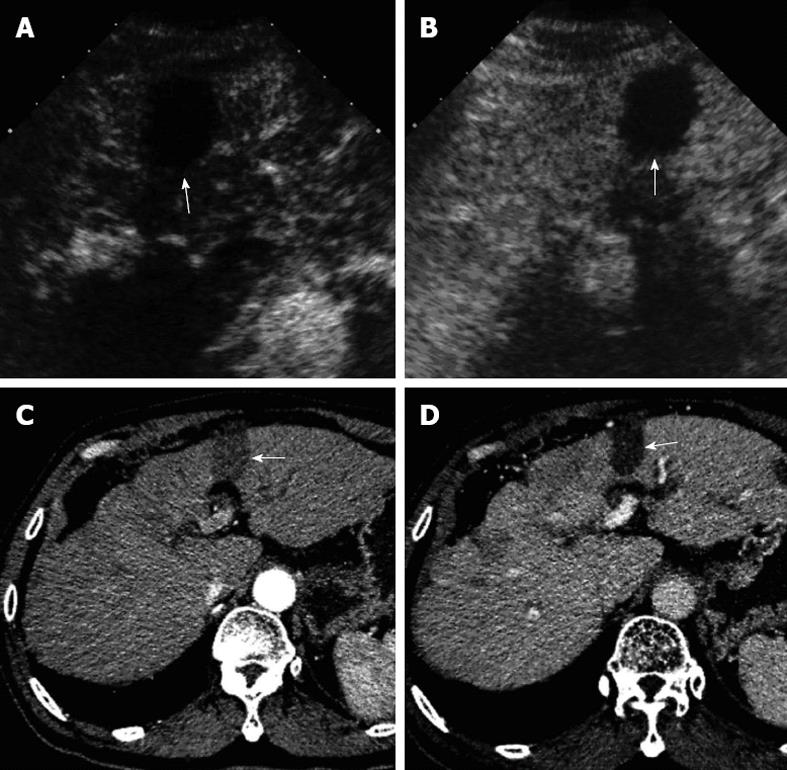
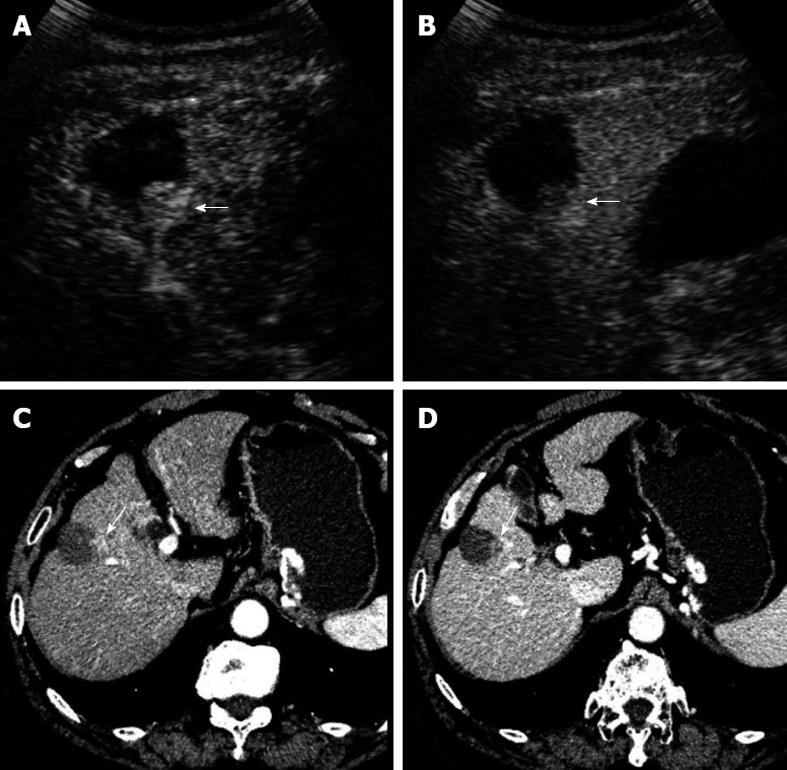
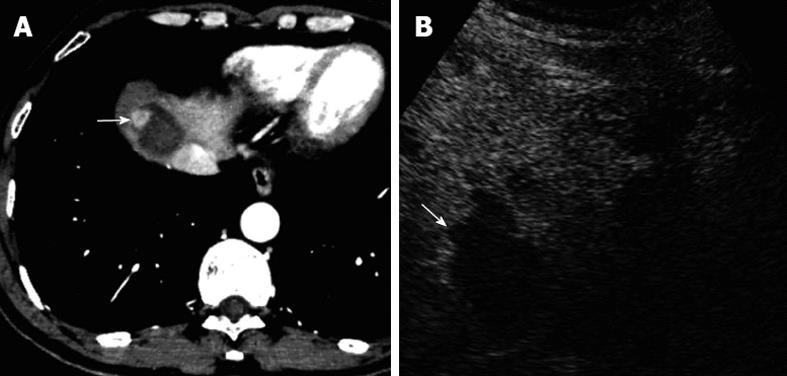
In the 266 comparisons between paired CECT and CEUS for all ablation lesions, CEUS determined that 233 lesions were LTP-free and all showed non-enhancement in the arterial, portal, and late phases (Figure 1A and B). The remaining 33 lesions were determined to be LTP on CEUS and all showed hyper-enhancement in the arterial phase and wash-out (n = 28) (Figure 2A and B) or iso-enhancement (n = 5) in the portal-late phases. However, on CECT, 226 lesions were determined to be LTP-free and all showed non-enhancement in the arterial, portal, and late phases (Figure 1C and D); and the remaining 40 lesions were determined to be LTP and all showed hyper-enhancement in the arterial phase and wash-out in the portal-late phase (Figure 2C and D).
By comparing the number of the ablated lesions, there was significant difference between CECT and CEUS in detecting LTP (P < 0.001, Table 2). Differences between CECT and CEUS were also found in the subgroups (< 3 cm vs≥ 3 cm in diameter; single vs multiple ablated lesions), (both P < 0.001) (Table 2). With CECT as the reference standard, the sensitivity, specificity, PPV, NPV and overall accuracy of CEUS in detecting LTP after percutaneous ablation were 67.5% (27/40), 97.3% (220/226), 81.8% (27/33), 94.4% (220/233), 92.9% (247/266), respectively.
| CEUS | CECT | ||||||||||||||
| All | < 3 cm | ≥3 cm | Single | Multiple | |||||||||||
| LTP | LTP-free | Total | LTP | LTP-free | Total | LTP | LTP-free | Total | LTP | LTP-free | Total | LTP | LTP-free | Total | |
| LTP | 27 | 6 | 33 | 18 | 4 | 22 | 9 | 2 | 11 | 15 | 5 | 20 | 12 | 1 | 13 |
| LTP-free | 13 | 220 | 233 | 10 | 165 | 175 | 3 | 55 | 58 | 3 | 74 | 76 | 10 | 146 | 156 |
| Total | 40 | 226 | 266 | 28 | 169 | 197 | 12 | 57 | 69 | 18 | 79 | 97 | 22 | 147 | 169 |
| χ2 | 125.6 | 86.7 | 32.66 | 48.51 | 70.79 | ||||||||||
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||||||||||
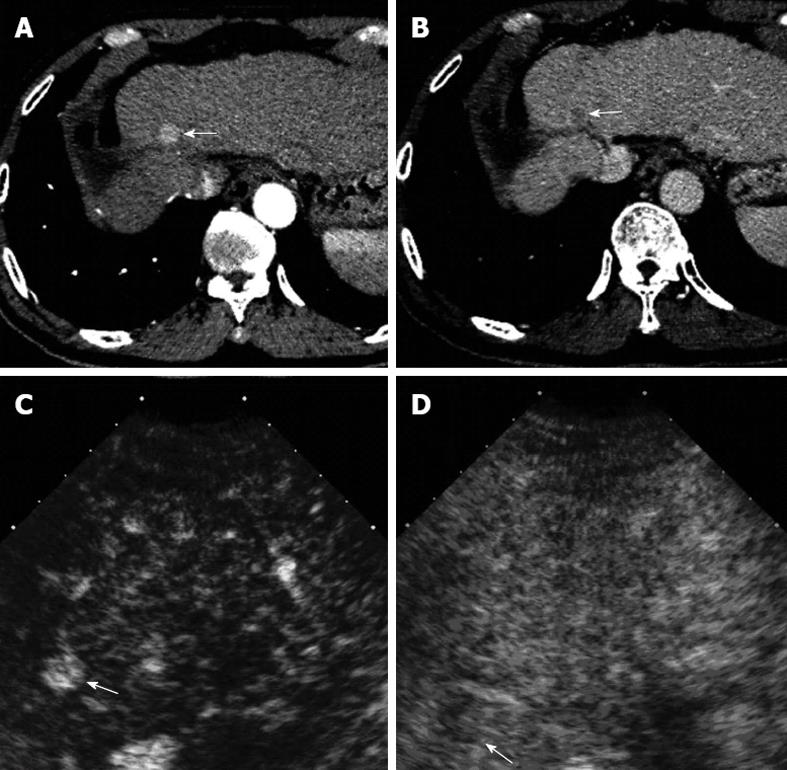
A total of 183 new intrahepatic recurrences were detected on CECT and all showed hyper-enhancement in the arterial phase and wash-out in the portal-venous phases (Figure 3A and B). Conversely, only 131 recurrent lesions were detected on CEUS (Figure 3C and D). Among them, 107 lesions were in the arterial phase with hyper-enhancement (n = 104) or iso-enhancement (n = 3) and the remaining 24 lesions were missed during the arterial phase. And 124 lesions showed wash-out in the portal-late phases on CEUS and the remaining 7 lesions showed iso-enhancement.
There was significant difference between the follow-up CECT and CEUS in detecting new intrahepatic recurrence when comparing the number of the detected lesions (P = 0.02, Table 3) or the number of the patients with detected lesions (P < 0.01, Table 3). With CECT as the reference standard, the sensitivity, specificity, PPV, NPV and overall accuracy of CEUS in detecting recurrence were 77.7% (73/94), 92.0% (69/75), 92.4% (73/79), 76.7% (69/90), 84.0% (142/169), respectively. The numbers of new intrahepatic recurrence in each patient detected by each follow-up CEUS and CECT are shown in Table 4.
| CEUS | CECT | ||
| Yes | No | Total | |
| New intrahepatic recurrence | |||
| Yes | 118 | 13 | 131 |
| No | 65 | 0 | 65 |
| Total | 183 | 13 | 196 |
| Lesion | |||
| Yes | 73 | 6 | 79 |
| No | 21 | 69 | 90 |
| Total | 94 | 75 | 169 |
| CEUS | CECT | ||||||
| N | 0 | 1 | 2 | 3 | 4 | 5 | Total |
| 0 | 69 (0/0) | 13 (0/13) | 7 (0/14) | 1 (0/3) | 0 (0/0) | 0 (0/0) | 90 (0/30) |
| 1 | 4 (4/0) | 25 (25/25) | 9 (9/18) | 8 (8/24) | 1 (1/4) | 0 (0/0) | 47 (47/71) |
| 2 | 2 (4/0) | 5 (10/5) | 8 (16/16) | 3 (6/9) | 0 (0/0) | 0 (0/0) | 18 (36/30) |
| 3 | 0 (0/0) | 0 (0/0) | 0 (0/0 ) | 7 (21/21) | 1 (3/4) | 1 (3/5) | 9 (27/30) |
| 4 | 0 (0/0) | 0 (0/0) | 0 (0/0) | 0 (0/0) | 3 (12/12) | 1 (4/5) | 4 (16/17) |
| 5 | 0 (0/0) | 0 (0/0) | 0 (0/0) | 0 (0/0) | 0 (0/0) | 1 (5/5) | 1 (5/5) |
| Total | 75 (8/0) | 43 (35/43) | 24 (25/48) | 19 (35/57) | 5 (16/20) | 3 (12/15) | 169 (131/183) |
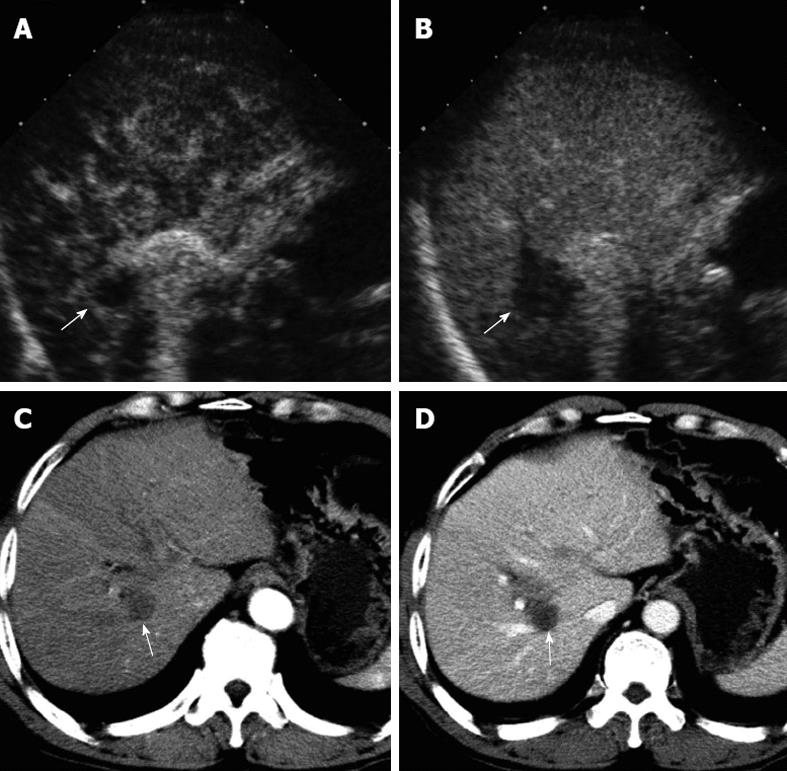
The numbers of false positive and false negative LTPs detected on CEUS were 6 and 13, respectively. Compared with CECT, among the 6 false positive LTPs, 5 misinterpreted hepatic blood vessels (Figure 4) and 1 new intrahepatic recurrence were misdiagnosed as LTPs. The main reasons for false negative LTPs (Figure 5) on CEUS were as follows: near liver dome and obscuration by lung gas (n = 5); deep location (n = 1; depth > 10 cm); obscuration by gastrointestinal tract gas (n = 2); obscuration by enhanced portal vein (n = 1); small lesion (n = 3, all < 0.7 cm in diameter); misdiagnosis as new intrahepatic recurrence (n = 1).
Compared with CECT, the numbers of false positive and false negative intrahepatic recurrences detected on CEUS were 13 and 65, respectively. The causes of false positive recurrences were as follows: 3 regenerative nodules, 9 misinterpreted hepatic blood vessels and 1 LTP were misdiagnosed as new intrahepatic recurrence. Among the 65 false negative recurrences, 4 regenerative nodules and 1 LTP were misdiagnosed as recurrence, and the remaining 60 new intrahepatic recurrences were missed on CEUS. Compared with CECT, the reasons for the missed new intrahepatic recurrences were as follows: multiple lesions (n = 7, > 2 in number), obscuration by gastrointestinal tract gas (n = 10); deep location (n = 4, > 10 cm in depth), near liver dome and obscuration by lung gas (n = 19), small lesion (n = 5, < 1 cm in diameter) and unknown causes (n = 15).
The treatment efficacy assessment after percutaneous ablation therapy for HCC mainly involves short-term local treatment response evaluation and long-term follow-up assessment. The short-term local treatment response evaluation is difficult as microscopic residual viable HCC is hardly detected by current imaging techniques. Therefore, follow-up scheme after ablation therapies is important. Early detection of LTP or new recurrence during follow-up after percutaneous ablation for HCC is critical and will facilitate retreatment at an early stage[3]. The short-term local treatment response evaluation is usually carried out within 1 mo after ablation therapy[2,8,28]. Contrast-enhanced imaging studies are the most widely accepted modalities to assess the local treatment response[10,12,28]. In contrast to the follow-up assessment, local treatment response focused on the specified known lesion, whereas not the whole liver. Many studies have shown that in local treatment response evaluation, CEUS is comparable with CECT or CEMRI[11,13,20].
However, up till now, no studies have been performed to evaluate the efficacy of CEUS in the follow-up assessment. This issue is very important since some centers may only use CEUS for follow-up because of the convenience of CEUS and the unwareness of the limitations of CEUS. In this study, the efficacy of CEUS in follow-up was firstly evaluated, in comparison with the widely accepted modality of CECT. The long-term follow-up assessment (up to 31 mo; mean ± SD, 6.7 ± 6.4 mo) provided a sufficient surveillance for HCC progression after ablation and the short interval (3.1 ± 4.3 d) between the paired CEUS and CECT examination was able to guarantee that the lesions were observed under the same status of vascularity for comparison between CEUS and CECT.
Many studies have confirmed the accuracy of CEUS in local treatment response evaluation, with the CECT or CEMRI as the reference standard. Among these studies, a prospective multicenter study showed that the sensitivity and the accuracy were as high as 97.0% and 94.2%, respectively[11,20]. The accuracy (92.9%) of follow-up CEUS in detecting LTP in this study was comparable to that in local treatment response evaluation, so were the specificity (97.3%), PPV (81.8%) and NPV (94.4%). However, the relatively low sensitivity (67.5%) showed that CEUS was not comparable to CECT (P < 0.001). The low sensitivity was partly due to short arterial phase duration of CEUS and the intrinsic shortcomings of US technique such as inability to detect the lesions in the dome or small lesions, and obscuration by gas from gastrointestinal tract or lung.
It is unknown whether CEUS is competent for the detection of new intrahepatic recurrence after HCC ablation as compared with CECT. According to the published literatures, although CEUS is comparable with CECT/MRI in the detection of liver metastasis, CEUS is incompetent to CECT for HCC surveillance, owing to the insufficient access to the lesion near the liver dome, short duration in arterial phase and the variable appearances in late phase[15,29-31]. Correas et al[32] found that CEUS had a sensitivity of 78% and an accuracy of 70% for detection of liver metastases by scanning entire liver parenchyma, similar to the 77.7% and 84% for detection of new intrahepatic recurrences in our study. In this study, CECT was significantly superior to CEUS for the detection of new intrahepatic recurrent foci and CEUS was unable to detect 65 (35.5%) of 183 lesions. The possible reasons might be as follows: small lesion, unfavorable location (i.e., deep location, near liver dome, near gastrointestinal tract or large hepatic blood vessel), atypical enhancement pattern, and background of fatty or cirrhotic liver[19,28,33,34].
During the routine CEUS procedure, the hepatic arterial phase starts from 10-20 s after injection of UCAs, and lasts approximately 10-15 s. Furthermore, the arterial phase presents the optimal contrast enhancement for detecting LTPs and recurrence[15]. However, the short duration of the arterial phase makes it hard to scan the whole liver and screen all suspected lesions, while CECT can scan the entire liver in a few seconds[35]. In this study, a total of 24 (18.3%, 24/131) new intrahepatic recurrences were missed in the arterial phase.
In the portal-late phases, though CEUS can guarantee sufficient duration for whole liver scan, some LTPs and new intrahepatic recurrences usually show iso-enhancement [5 (15.2%, 5/33) and 7 (5.3%, 7/131) in this study, respectively], making them indistinguishable from the surrounding liver parenchyma[28,33,36].
Besides the above-mentioned limitations of CEUS, there were some factors related to the false negative results on CEUS, e.g., near liver dome and obscuration by lung gas (5 LTPs and 19 recurrences) and obscuration by gastrointestinal tract gas (2 LTPs and 10 recurrences), which usually were displayed on CECT whereas not on CEUS. On the other hand, deep location (depth > 10 cm; 1 LTP and 4 recurrences) and small size (< 1.0 cm in diameter; 1 LTP and 5 recurrences) were not easy to be detected on CEUS due to acoustic attenuation and inconspicuous enhancement. Additionally, in this study 15 recurrences were missed on CEUS and it was unable to figure out definite reason in comparison with CECT.
It was notable that the misinterpreted abnormal hepatic blood vessels due to the presence of hepatic blood vessel enhancement in the vicinity of suspicious lesion (Figure 4) was the main reason of the false positive results on CEUS, which mainly involved arteriovenous shunting or marginally enhanced artifact of large vessel and may be caused by the ablation or severe liver cirrhosis. The misinterpreted hepatic blood vessels usually showed hyper-enhancement in the arterial phase and iso-enhancement in the portal-late phase on CEUS[13,34]. In this study, a total of 14 misinterpreted hepatic blood vessels were misdiagnosed as false positive LTPs (83.3%, 5/6) or new intrahepatic recurrence (69.2%, 9/13) by CEUS.
There are some limitations in this study: firstly, although the study was designed prospectively, not all the HCC patients who underwent ablation therapy received follow-up assessment by this paired CEUS and CECT examinations due to the low compliance of the patients, which may lead to bias of patient selection. Secondly, CECT was used as the reference standard in this study and not all the detected LTPs and new intrahepatic recurrences were confirmed by pathology. However, it was hard to obtain pathological results for all the lesions found in the follow-up due to the ethical concern and under current situation CECT is still acceptable to be used as the standard. Thirdly, we did not evaluate the role of CEUS using different ablation techniques. There might be some differences of the incidence of LTP or new intrahepatic recurrence between different ablation techniques such as RFA and EA, etc., because they have different efficacies in treating HCC. However, the role of the study was not to evaluate the treatment efficacy of different ablation therapies, but to evaluate the ability of CEUS in treatment response assessment. In theory, the viable tumor tissue will show arterial hypervascularity on CEUS, whether it is residual tumor tissue or the recurrent tumor, and whether it is after RFA or after EA. In addition, the number of patients undergoing EA was small. Finally, further prospective study with a large number of cases is necessary to confirm the results of the present study and to evaluate the real value of CEUS in the follow-up.
In conclusion, the sensitivity of CEUS in detecting LTP and new intrahepatic recurrence after percutaneous ablation therapy is relatively low in comparison with CECT. CEUS cannot replace CECT in the follow-up assessment after percutaneous ablation for HCC.
For the patients with hepatocellular carcinoma (HCC) after percutaneous ablation therapy, the regular follow-up after ablation can detect local tumor progression (LTP) and new recurrence as early as possible, so as to facilitate further treatment in time, and therefore can benefit the survival. Thus the follow-up assessment, similar to surveillance and diagnosis, plays a key role in the management of HCC. However, at present, only contrast-enhanced computed tomography (CECT) and contrast-enhanced magnetic resonance imaging (CEMRI), are recommended as accurate and reliable imaging tools and applied to the follow-up assessment. Unfortunately, some factors, such as high cost, radiation and side effect of agents, limit its application.
Contrast-enhanced ultrasound (CEUS) is a new imaging technique developed in recent decade. A lot of previous studies have demonstrated that CEUS is comparable to CECT and CEMRI in the area of characterization and treatment response assessment of HCC. Regarding the role of CEUS in follow-up assessment for HCC after ablation, most studies just focus on targeted lesion assessment and seldom investigate its capability of detecting LTP and new intrahepatic recurrence by scanning whole liver. Whether CEUS can be competent to this follow-up assessment is still controversial.
In most of the previous studies, CEUS has a good performance for treatment response assessment after HCC ablation, while just for the specific lesions. In this study, the authors aimed to investigate the ability of CEUS by scanning whole liver for detecting the LTPs and new intrahepatic recurrences, most of which are unknown in number, size and location, etc. It is concluded that CEUS is inferior to CECT in the follow-up assessment of HCC after ablation, which is mainly due to the innate defect of CEUS, such as limited acoustic penetration, display scope and relatively short duration of artery phase. Additionally, the incompetence of CEUS in the follow-up assessment might result from some traits of LTPs and new intrahepatic recurrences after HCC ablation, such as its small size, deep location, and atypical enhancement patterns.
The study results suggest that in follow-up assessment after HCC ablation CEUS can not take place of CECT and CEMRI for whole liver scanning, but can act as an adjuvant imaging tool for assessing the specific lesions.
Treatment response assessment is performed in a month after HCC ablation by using CECT or CEMRI to assess the efficacy of ablation. Follow-up assessment: after complete ablation of HCC is confirmed by treatment response assessment, the patients will be follow-up regularly for monitoring the progression and recurrence.
In this prospective study, the authors investigated extensively the role of CEUS in the follow-up of HCC patients undergoing radiofrequency ablations with CECT as the reference standard. The conclusion drawn by the authors is that the ability of CEUS in detecting LTP and new intrahepatic recurrence after percutaneous ablation therapy is inferior to CECT. This is the first study to evaluate the role of CEUS in the follow-up assessment after percutaneous ablation therapy for HCC and the results are relevant and objective. The conclusions are very important, which indicate that people should not overestimate the role of CEUS in the follow-up even though it is meaningful in local treatment evaluation.
P- Reviewers Chen F, Xu HX S- Editor Song XX L- Editor Ma JY E- Editor Li JY
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25538] [Article Influence: 1824.1] [Reference Citation Analysis (7)] |
| 2. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6569] [Article Influence: 469.2] [Reference Citation Analysis (1)] |
| 3. | Kim SK, Lim HK, Kim YH, Lee WJ, Lee SJ, Kim SH, Lim JH, Kim SA. Hepatocellular carcinoma treated with radio-frequency ablation: spectrum of imaging findings. Radiographics. 2003;23:107-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 89] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Xu HX, Wang Y, Lu MD, Liu LN. Percutaneous ultrasound-guided thermal ablation for intrahepatic cholangiocarcinoma. Br J Radiol. 2012;85:1078-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48 Suppl 1:S20-S37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 639] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 6. | Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, Rossi S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 825] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 7. | Tiong L, Maddern GJ. Systematic review and meta-analysis of survival and disease recurrence after radiofrequency ablation for hepatocellular carcinoma. Br J Surg. 2011;98:1210-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 202] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 8. | Salvaggio G, Campisi A, Lo Greco V, Cannella I, Meloni MF, Caruso G. Evaluation of posttreatment response of hepatocellular carcinoma: comparison of ultrasonography with second-generation ultrasound contrast agent and multidetector CT. Abdom Imaging. 2010;35:447-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Solbiati L, Ierace T, Tonolini M, Cova L. Guidance and monitoring of radiofrequency liver tumor ablation with contrast-enhanced ultrasound. Eur J Radiol. 2004;51 Suppl:S19-S23. [PubMed] |
| 10. | Sparchez Z, Radu P, Anton O, Socaciu M, Badea R. Contrast enhanced ultrasound in assessing therapeutic response in ablative treatments of hepatocellular carcinoma. J Gastrointestin Liver Dis. 2009;18:243-248. [PubMed] |
| 11. | Lu MD, Yu XL, Li AH, Jiang TA, Chen MH, Zhao BZ, Zhou XD, Wang JR. Comparison of contrast enhanced ultrasound and contrast enhanced CT or MRI in monitoring percutaneous thermal ablation procedure in patients with hepatocellular carcinoma: a multi-center study in China. Ultrasound Med Biol. 2007;33:1736-1749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Ricci P, Cantisani V, Drudi F, Pagliara E, Bezzi M, Meloni F, Calliada F, Erturk SM, D’Andrea V, D’Ambrosio U. Is contrast-enhanced US alternative to spiral CT in the assessment of treatment outcome of radiofrequency ablation in hepatocellular carcinoma? Ultraschall Med. 2009;30:252-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Vilana R, Bianchi L, Varela M, Nicolau C, Sánchez M, Ayuso C, García M, Sala M, Llovet JM, Bruix J. Is microbubble-enhanced ultrasonography sufficient for assessment of response to percutaneous treatment in patients with early hepatocellular carcinoma? Eur Radiol. 2006;16:2454-2462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Xu HX, Lu MD, Liu LN, Zhang YF, Guo LH, Xu JM, Liu C. Discrimination between neoplastic and non-neoplastic lesions in cirrhotic liver using contrast-enhanced ultrasound. Br J Radiol. 2012;85:1376-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Claudon M, Cosgrove D, Albrecht T, Bolondi L, Bosio M, Calliada F, Correas JM, Darge K, Dietrich C, D’Onofrio M. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) - update 2008. Ultraschall Med. 2008;29:28-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 498] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 16. | Xu HX, Chen LD, Xie XY, Xie XH, Xu ZF, Liu GJ, Lin MX, Wang Z, Lu MD. Enhancement pattern of hilar cholangiocarcinoma: contrast-enhanced ultrasound versus contrast-enhanced computed tomography. Eur J Radiol. 2010;75:197-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Xu HX, Xie XY, Lu MD, Liu GJ, Xu ZF, Zheng YL, Liang JY, Chen LD. Contrast-enhanced sonography in the diagnosis of small hepatocellular carcinoma < or = 2 cm. J Clin Ultrasound. 2008;36:257-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Liu GJ, Xu HX, Lu MD, Xie XY, Xu ZF, Zheng YL, Liang JY. Correlation between enhancement pattern of hepatocellular carcinoma on real-time contrast-enhanced ultrasound and tumour cellular differentiation on histopathology. Br J Radiol. 2007;80:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Liu GJ, Xu HX, Lu MD, Xie XY, Xu ZF, Zheng YL, Liang JY. Enhancement pattern of hepatocellular carcinoma: comparison of real-time contrast-enhanced ultrasound and contrast-enhanced computed tomography. Clin Imaging. 2006;30:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Kim CK, Choi D, Lim HK, Kim SH, Lee WJ, Kim MJ, Lee JY, Jeon YH, Lee J, Lee SJ. Therapeutic response assessment of percutaneous radiofrequency ablation for hepatocellular carcinoma: utility of contrast-enhanced agent detection imaging. Eur J Radiol. 2005;56:66-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Xu HX, Lu MD. Comparison between radiofrequency ablation and percutaneous microwave coagulation therapy for small hepatocellular carcinomas — a reply. Clin Radiol. 2006;61:801-802. [RCA] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Lu MD, Yin XY, Xie XY, Xu HX, Xu ZF, Liu GJ, Kuang M, Zheng YL. Percutaneous thermal ablation for recurrent hepatocellular carcinoma after hepatectomy. Br J Surg. 2005;92:1393-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Wong SN, Lin CJ, Lin CC, Chen WT, Cua IH, Lin SM. Combined percutaneous radiofrequency ablation and ethanol injection for hepatocellular carcinoma in high-risk locations. AJR Am J Roentgenol. 2008;190:W187-W195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Luo BM, Wen YL, Yang HY, Zhi H, Xiao XY, Ou B, Pan JS, Ma JH. Percutaneous ethanol injection, radiofrequency and their combination in treatment of hepatocellular carcinoma. World J Gastroenterol. 2005;11:6277-6280. [PubMed] |
| 25. | Mahnken AH, Bruners P, Günther RW. Local ablative therapies in HCC: percutaneous ethanol injection and radiofrequency ablation. Dig Dis. 2009;27:148-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Kuang M, Lu MD, Xie XY, Xu HX, Mo LQ, Liu GJ, Xu ZF, Zheng YL, Liang JY. Liver cancer: increased microwave delivery to ablation zone with cooled-shaft antenna--experimental and clinical studies. Radiology. 2007;242:914-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 27. | Kuang M, Lu MD, Xie XY, Xu HX, Xu ZF, Liu GJ, Yin XY, Huang JF, Lencioni R. Ethanol ablation of hepatocellular carcinoma Up to 5.0 cm by using a multipronged injection needle with high-dose strategy. Radiology. 2009;253:552-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Bartolotta TV, Taibbi A, Midiri M, De Maria M. Hepatocellular cancer response to radiofrequency tumor ablation: contrast-enhanced ultrasound. Abdom Imaging. 2008;33:501-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Jang HJ, Yu H, Kim TK. Contrast-enhanced ultrasound in the detection and characterization of liver tumors. Cancer Imaging. 2009;9:96-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 30. | Hoeffel C, Job L, Ladam-Marcus V, Vitry F, Cadiot G, Marcus C. Detection of hepatic metastases from carcinoid tumor: prospective evaluation of contrast-enhanced ultrasonography. Dig Dis Sci. 2009;54:2040-2046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Larsen LP, Rosenkilde M, Christensen H, Bang N, Bolvig L, Christiansen T, Laurberg S. Can contrast-enhanced ultrasonography replace multidetector-computed tomography in the detection of liver metastases from colorectal cancer? Eur J Radiol. 2009;69:308-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Correas JM, Low G, Needleman L, Robbin ML, Cosgrove D, Sidhu PS, Harvey CJ, Albrecht T, Jakobsen JA, Brabrand K. Contrast enhanced ultrasound in the detection of liver metastases: a prospective multi-centre dose testing study using a perfluorobutane microbubble contrast agent (NC100100). Eur Radiol. 2011;21:1739-1746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Nicolau C, Catalá V, Vilana R, Gilabert R, Bianchi L, Solé M, Pagés M, Brú C. Evaluation of hepatocellular carcinoma using SonoVue, a second generation ultrasound contrast agent: correlation with cellular differentiation. Eur Radiol. 2004;14:1092-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 149] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 34. | Pompili M, Riccardi L, Covino M, Barbaro B, Di Stasi C, Orefice R, Gasbarrini G, Rapaccini GL. Contrast-enhanced gray-scale harmonic ultrasound in the efficacy assessment of ablation treatments for hepatocellular carcinoma. Liver Int. 2005;25:954-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Lencioni R, Piscaglia F, Bolondi L. Contrast-enhanced ultrasound in the diagnosis of hepatocellular carcinoma. J Hepatol. 2008;48:848-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 36. | Bolondi L, Gaiani S, Celli N, Golfieri R, Grigioni WF, Leoni S, Venturi AM, Piscaglia F. Characterization of small nodules in cirrhosis by assessment of vascularity: the problem of hypovascular hepatocellular carcinoma. Hepatology. 2005;42:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 309] [Article Influence: 15.5] [Reference Citation Analysis (0)] |