Published online Feb 14, 2013. doi: 10.3748/wjg.v19.i6.846
Revised: September 13, 2012
Accepted: September 22, 2012
Published online: February 14, 2013
AIM: To investigate the effects of chronic obstruction on enteric reflexes evoked by electrical stimulation (EFS) or intraluminal distension of the rat hypertrophic ileum.
METHODS: Motor responses to EFS and to intraluminal distension were studied in the absence and in the presence of various inhibitors of enteric mediators. Ileum segments from operated (chronic ileal obstruction), sham-operated (control) and normal rats were horizontally mounted, connected to a pressure transducer and intraluminally perfused. The effects of selective serotonin receptor (5-HTR) blockers were investigated on distension-induced responses. The cellular localization of 5-HT3Rs was also examined in control and hypertrophic tissues through confocal microscopy.
RESULTS: In non-obstructed segments, EFS elicited tetrodotoxin (TTX)-sensitive responses with high amplitude contraction followed by weak relaxation. In hypertrophic tissues, EFS lowered the baseline pressure and evoked TTX-sensitive contractions significantly larger than normal (P < 0.01) or control (P < 0.05), and devoid of any relaxation phase (P < 0.01 vs normal). Incubation with atropine and guanethidine [non-adrenergic non-cholinergic (NANC) conditions] did not modify intestinal tone in normal and control preparations, but reversed the accommodation produced by EFS in hypertrophic tissues, and depressed the amplitude of contractions in all types of tissues. L-NAME and α-chymotrypsin blocked residual NANC motility in all tissues and augmented intraluminal pressure in hypertrophic segments (P < 0.05 vs NANC conditions). Intraluminal distension of the intestinal wall evoked non-propulsive cycles of contractions and relaxations in non-obstructed tissues. In all hypertrophic segments, strong propulsive strokes, markedly wider (P < 0.001), and larger than normal (P < 0.001) or control (P < 0.05) were elicited. Both motor patterns were blocked under NANC conditions and with simultaneous incubation with L-NAME and α-chymotrypsin. In all types of tissues, incubation with ketanserin or GR125487 did not modify distension-induced motility. In contrast, blockade of 5-HT3Rs by ondansetron concentration-dependently inhibited motor responses in normal and control tissues, but only slightly impaired enteric reflexes in the hypertrophic preparations. Finally, confocal microscopy did not reveal a different cellular distribution of 5-HT3Rs in control and hypertrophic ileum.
CONCLUSION: Accommodation and distension-induced peristalsis of rat hypertrophic ileum are controlled by cholinergic and peptidergic transmission and are negligibly affected by 5-HT3Rs, which modulate distension-induced motility in non-obstructed tissues.
- Citation: Bertoni S, Saccani F, Gatti R, Rapalli A, Flammini L, Ballabeni V, Barocelli E. Accommodation and peristalsis are functional responses to obstruction in rat hypertrophic ileum. World J Gastroenterol 2013; 19(6): 846-854
- URL: https://www.wjgnet.com/1007-9327/full/v19/i6/846.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i6.846
Small bowel obstruction is a common clinical problem resulting from a variety of causes: intraluminal (food bolus, gallstones), intramural (neoplasms, strictures) or extramural (adhesions, hernias) factors. It can be partial or complete and, if not properly diagnosed and treated, it may rapidly lead to death[1]. Experimentally, striking morphological and neurochemical changes, affecting all the layers of the gut wall, have been reported to occur orally to the site of a mechanical long-standing partial obstruction. Massive thickening of the muscle coats, neuronal hypertrophy, neoangiogenesis and neoformation of collagen[2], a loss of interstitial cells of Cajal[3,4] and an altered expression of enteric neuropeptides[3,5] are the most prominent alterations detected in the obstructed intestine. Functionally, along with pronounced changes in biomechanical and motility patterns, detected both in vitro[6,7] and in vivo[8], preservation of the peristaltic reflex in the isolated guinea pig ileum has also been described[9]. Indeed, peristaltic reflex, responsible for the mixing and aboral propulsion of luminal contents in vivo, is the result of a highly sophisticated and localized integration between neural and myogenic components; in vitro, it can be initiated by local mechanical stimulation of the mucosa or by muscle stretch, as elegantly shown by Trendelenburg’s pioneering work[10]. Hypertrophic growth, induced by mechanical obstruction of the rat ileum, affects the functional responses of both smooth muscle layers and of intrinsic innervation[7,11]. Therefore, the present work focused on the consequences of a partial long-standing stenosis on enteric reflexes evoked by electrical field stimulation (EFS) or by intraluminal distension of rat isolated terminal ileum. To this end, the changes in intraluminal pressure elicited by neurogenic stimulation of rat obstructed intestine were investigated in the absence and in the presence of different inhibitors of enteric neurotransmitters which control gut motility, and compared with those triggered in preparations obtained from normal or sham-operated (control) rats. Secondly, given the pivotal role played by endogenous serotonin (5-HT) in the initiation and propagation of enteric reflexes, such as the peristaltic reflex[12,13], special attention was directed at evaluation of its role in specific distension-induced motor patterns exhibited by non-hypertrophic and hypertrophic tissues by investigating the effects of different selective 5-HT receptor (5-HTR) antagonists. The cellular localization of 5-HT3R was also investigated through confocal microscopy.
Adult female Wistar rats (180-200 g), bred from a local colony, were housed in single cages with a 12 h/12 h light/dark cycle and received food and water ad libitum. The rats were randomly assigned to normal group (age-matched animals that did not undergo any type of surgery), a control group (age-matched sham-operated animals subjected to the same intestinal manipulation except for ileal obstruction) and an operated group (25 animals per group). Under general anesthesia (sodium pentobarbital 33 mg/kg ip), the rats were operated on according to Gabella’s method[14], as previously described[7]. Briefly, a polyethylene ring of diameter 1-2 mm larger than that of the intestine, was applied around the ileum, proximal to the ileo-cecal junction, keeping it initially free for turning around. Postoperatively, the animals were monitored daily with regard to weight, general well-being and distension of the abdomen. Fourteen days after ileal obstruction, when previous experiments had shown that intestinal hypertrophy is fully and homogeneously developed over 10 cm aboral to the stenosis[7], the rats were killed, and functional or immunohistochemical studies were performed on ileal tissue. The experimental protocol complied with the requirements of animal care and was approved by Ministero della Salute, Italy (DL 116/92).
Rats were killed by CO2 asphyxiation. Full-thickness 4-cm long segments of terminal ileum, immediately proximal to the site of occlusion excised from operated rats, and loops of the same part of the gut excised from normal or control rats, were cleared of their contents by flushing with Krebs-Henseleit solution (composition: NaCl 118.9 mmol/L, KCl 4.6 mmol/L, CaCl2 2.5 mmol/L, KH2PO4 1.2 mmol/L, NaHCO3 25 mmol/L, MgSO4 1.2 mmol/L, glucose 11 mmol/L). From each animal, only one preparation was obtained either immediately proximal to the point of obstruction, where hypertrophy was maximal and uniform, or from the corresponding portion of terminal ileum of normal and control rats. According to the method described by Costall et al[15], the segments were cannulated at the oral and anal ends, and were mounted in a Mayflower horizontal 20-mL tissue bath (Hugo Sachs Elektronik-Harvard Apparatus GmbH D-79232 March Hugstetten, Germany) filled with Krebs-Henseleit solution gassed with a mixture of 5%CO2/95%O2 at 37 °C, and stretched at their initial length of 4 cm. The inlet cannula was connected via tubing to a reservoir filled with nutritive solution, maintained at 37 °C and continuously oxygenated, and to a pressure transducer (TSD104A Biopac Systems, 2Biological Instruments, Besozzo, VA, Italy) connected to a MacLab digital data acquisition system to record changes in the intraluminal pressure. The outflow tube at the aboral end had two outlets: one at the level of the bath (A) while the other (B) could be raised above the level of the tissue. A continuous flow of fresh oxygenated solution was delivered intraluminally at a flow rate of 0.5 mL/min and extraluminally at a speed of 2 mL/min by means of the same peristaltic pump (Gilson Minipuls-3, Gilson Italia SRL, MI, Italy) to remove perfusate and extraluminal solution from the bath. The extraluminal flow was temporarily suspended during the period of incubation of drugs.
Electrical field stimulation: After a period of equilibration of 30 min, during which the fluid level in the outflow tube was equal to that in the bath (drain A open), the intraluminal pressure was augmented by closing drain A and by raising drain B 5 cm above the level of the tissue in the bath (5 cm hydrostatic resistance or low distension, corresponding to a pressure of 3.68 mmHg); 30 V square pulses of 1 ms duration were then delivered to the tissues at 3 Hz frequency through platinum wire electrodes positioned at the two opposite sides of ileum and connected to a generator that provided trains lasting 20 s at 120 s intervals. EFS-evoked changes in intraluminal pressure were registered in the absence (basal conditions) and in the presence of the muscarinic receptor antagonist atropine 1 μmol/L and sympatholytic agent guanethidine 4 μmol/L [non-adrenergic non-cholinergic (NANC) conditions]. The effects produced by the application of nitric oxide synthase (NOS) inhibitor NG-nitro-L-arginine methyl ester (L-NAME) (300 μmol/L) and by the protease α-chymotrypsin (10 IU/mL) on NANC responses evoked by EFS were investigated. Drugs were added to the extraluminal solution.
Distension-induced motility: In a second series of experiments, after a period of equilibration of 30 min, drain A was closed and drain B was elevated 8 cm above the level of the tissue in the bath (8 cm hydrostatic resistance or high distension, corresponding to a pressure of 5.88 mmHg). After another 30 min, once regular motility had been evoked by the increase in the intraluminal pressure, the effects of simultaneous application of atropine 1 μmol/L and guanethidine 4 μmol/L or of single concentrations of the 5-HT2AR antagonist ketanserin (1 μmol/L), the 5-HT3R antagonist ondansetron (1-10 μmol/L) and the 5-HT4R antagonist GR125487 (1 μmol/L) were studied over the next 30 min. Each preparation was challenged with a single application of each drug in order to minimize time-dependent variability. About 25% of non-obstructed tissues, which failed to develop a regular motility following distension, were discarded from further investigation in the experiment.
Tissue preparation and processing. Full-thickness 2-cm long segments of terminal ileum, excised as described above from hypertrophic and control animals, were cleared of their contents by flushing with phosphate buffered saline (PBS) 100 mmol/L at pH 7.4, opened along the mesenteric border, cleaned and pinned flat onto slabs of Sylgard 184 (Dow Corning), with the serosal side exposed. After 2 h fixation with 4% formaldehyde in 100 mmol/L PBS, whole-mount preparations containing the longitudinal muscle layer and the adherent myenteric ganglia were peeled off from the mucosa and the circular muscle under a dissection microscope using fine forceps, and were processed by the immunofluorescence method for double labeling using a free floating technique. After washing in 100 mmol/L PBS, whole mounts were incubated with a mixture of rabbit-anti-rat immunoglobulin G (IgG) primary antibody directed against 5-HT3R 1:500 (No. 95247, a generous gift from CURE/UCLA Digestive Disease Research Center, Los Angeles, CA, United States) and mouse-anti-rat clathrin IgG 1:100 (Becton Dickinson Italia SpA) for 48 h at 4 °C. This was followed by a 2-h incubation period at room temperature with a mixture of Alexafluor 488 donkey-anti-rabbit IgG 1:100 (Invitrogen Ltd, United Kingdom) and DyLight 549-conjugated AffiniPure donkey-anti-mouse IgG 1:200 (Jackson ImmunoResearch). Tissues were finally washed with 100 mmol/L PBS, mounted on slides and cover-slipped with ProLong Gold Antifade Reagent (Invitrogen Ltd, United Kingdom). Specificity controls were obtained by omitting primary or secondary antibodies from the incubation solution. Primary and secondary antibodies were diluted in Triton X-100 (0.5% in 100 mmol/L PBS, Sigma). Normal donkey serum (Jackson ImmunoResearch) was added to a final concentration of 10% to reduce the unspecific background staining.
Confocal microscopy specimens were analyzed with a confocal system LSM 510 Meta scan head integrated with the Axiovert 200 mol/L inverted microscope (Carl Zeiss, Jena, Germany). Specimens were observed through a 63 × 1.4 NA oil objective. Alexafluor and DyLight were excited with 488 nm argon and 543 nm He-Ne laser lines, respectively. Image acquisition was carried out in a multitrack mode, with the relevant beamsplitters; barrier filters were 505-530 band pass and 585 long pass for the above signals, respectively. A series of x-y sections was acquired with a z-step of 0.5 μm, to cover the whole height of the samples.
Data were expressed as mean ± SE of 5 distinct experiments. The following parameters were measured: changes in intestinal tone (baseline intraluminal pressure), index of accommodation capacity; amplitude (area under the pressure-time curve or area under curve for each wave of contraction/relaxation), height and frequency of electrically- or distension-induced contractions/relaxations over a 15 min period immediately prior to and after 15 min incubation with each drug tested. When peristalsis, identifiable by propagating and propulsive waves of contraction (visually confirmed as rings of contraction within 1 cm from the oral end of the loop moving towards the distal end), was induced by distension, a preparatory and an emptying phase could be detected. During the preparatory phase, the intraluminal pressure increased slowly until the threshold pressure was reached and the emptying phase, marked by an abrupt increase in the intraluminal pressure, was triggered (peristaltic stroke). The average compliance of the intestinal wall during the preparatory phase (volume infused in the preparatory phase/change in pressure in the preparatory phase) and the average power generated by the intestine during the peristaltic stroke (average pressure in emptying phase × volume expelled/duration of emptying phase) were measured according to Waterman et al[16] for 5-8 complete waves of contraction immediately prior to and after 15 min incubation with the drug. In order to calculate the average power, the volume of fluid expelled from the aboral end of the intestine by each peristaltic wave was measured with a measuring cylinder.
Data acquisition and analysis were carried out using MacLab digital data acquisition system and applying PowerLab Chart v4.1.1 software (PowerLab/4SP ADI Instruments, Ugo Basile, Comerio, VA, Italy). Statistical analysis was performed by two-way analysis of variance test followed by Bonferroni’s post-test unless otherwise indicated. A P value < 0.05 was considered significant, a P value < 0.01 highly significant and a P value < 0.001 extremely significant.
The following drugs were used: tetrodotoxin citrate (TTX), atropine sulfate, guanethidine sulfate, L-NAME, α-chymotrypsin, ketanserin tartrate, ondansetron hydrochloride dihydrate, purchased from Sigma Aldrich (St Louis, MO, United States), and GR125487 sulfamate, bought from Tocris Bioscience (Bristol, United Kingdom). All other reagents were of the highest grade commercially available. The stock solutions were prepared by dissolving all drugs in distilled water. The working solutions were prepared fresh on the day of the experiment by diluting the stock solutions with distilled water or nutritive solution. The volume of the drugs added to the organ bath was 1% of the final volume of the bath solution.
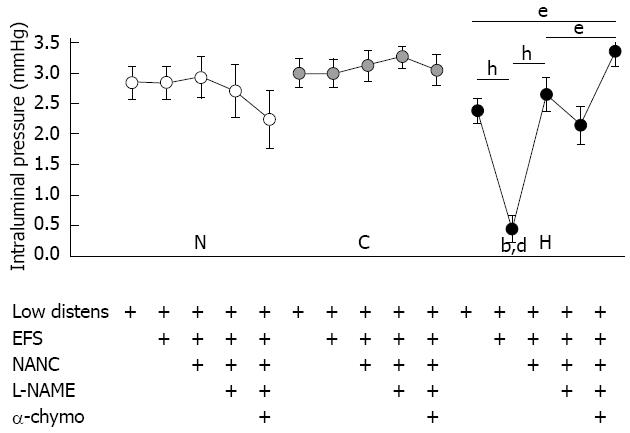
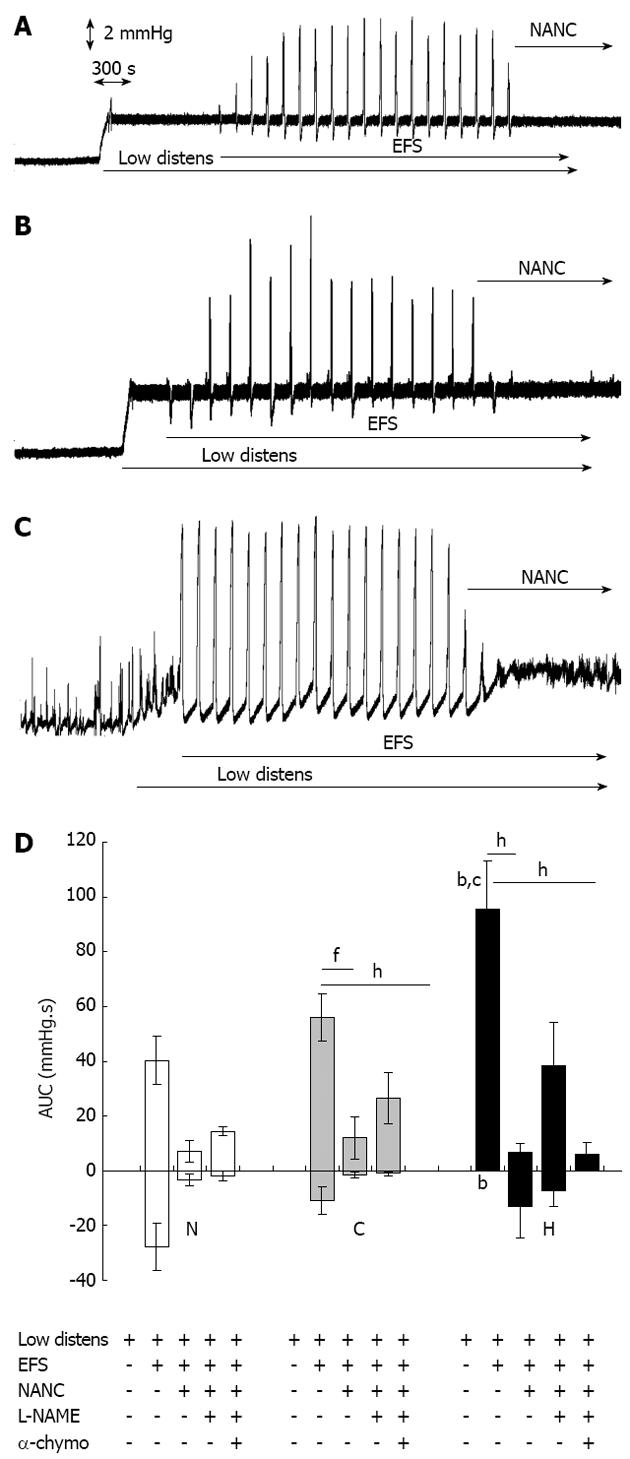
During the equilibration period, normal, control and hypertrophic tissues exhibited no or only sporadic spontaneous motility. The raising of the outflow block to 5 cm above the level of the tissue in the bath increased the intraluminal pressure by about 2.5-3.0 mmHg in all the tissues tested (Figure 1), but it did not evoke any wave of contraction or relaxation. In normal and sham-operated segments, EFS did not further modify the intestinal tone but elicited biphasic TTX-sensitive responses comprising a transient high amplitude contraction followed by a weak relaxation (Figure 2A, B and D). In hypertrophic tissues, EFS lowered the intraluminal pressure almost to the same level as in the equilibration phase (P < 0.001 vs low distension), in contrast to that observed in normal and control tissues (P < 0.001) (Figure 1). EFS also evoked TTX-sensitive phasic contractions of significantly larger amplitude in hypertrophic tissue (95.5 ± 17.1 mmHg.s) compared with that in normal tissue (40.3 ± 8.9 mmHg.s, P < 0.01) or control tissue (56.0 ± 8.7 mmHg.s, P < 0.05), and which were devoid of any relaxation phase (P < 0.01 vs normal, Figure 2C and D). Incubation with atropine 1 mmol/L and guanethidine 4 mmol/L (NANC conditions) did not modify the intestinal tone in normal and sham-operated preparations but reversed the accommodation produced by EFS in hypertrophic tissues, with intraluminal pressure slowly increasing to the same level reached after raising the outflow block (Figures 1 and 2A-C). Furthermore, the amplitude of contractions in all types of tissues was markedly depressed (Figure 2D). Subsequent exposure to the NOS inhibitor L-NAME 300 mmol/L moderately, although not significantly, increased the contractile responses compared with NANC conditions, while simultaneous incubation with the protease α-chymotrypsin 10 IU/mL blocked the residual NANC motor activity in all the tissues (Figure 2D) and significantly augmented intraluminal pressure in hypertrophic segments (P < 0.05 vs low distension and vs NANC conditions) (Figure 1).
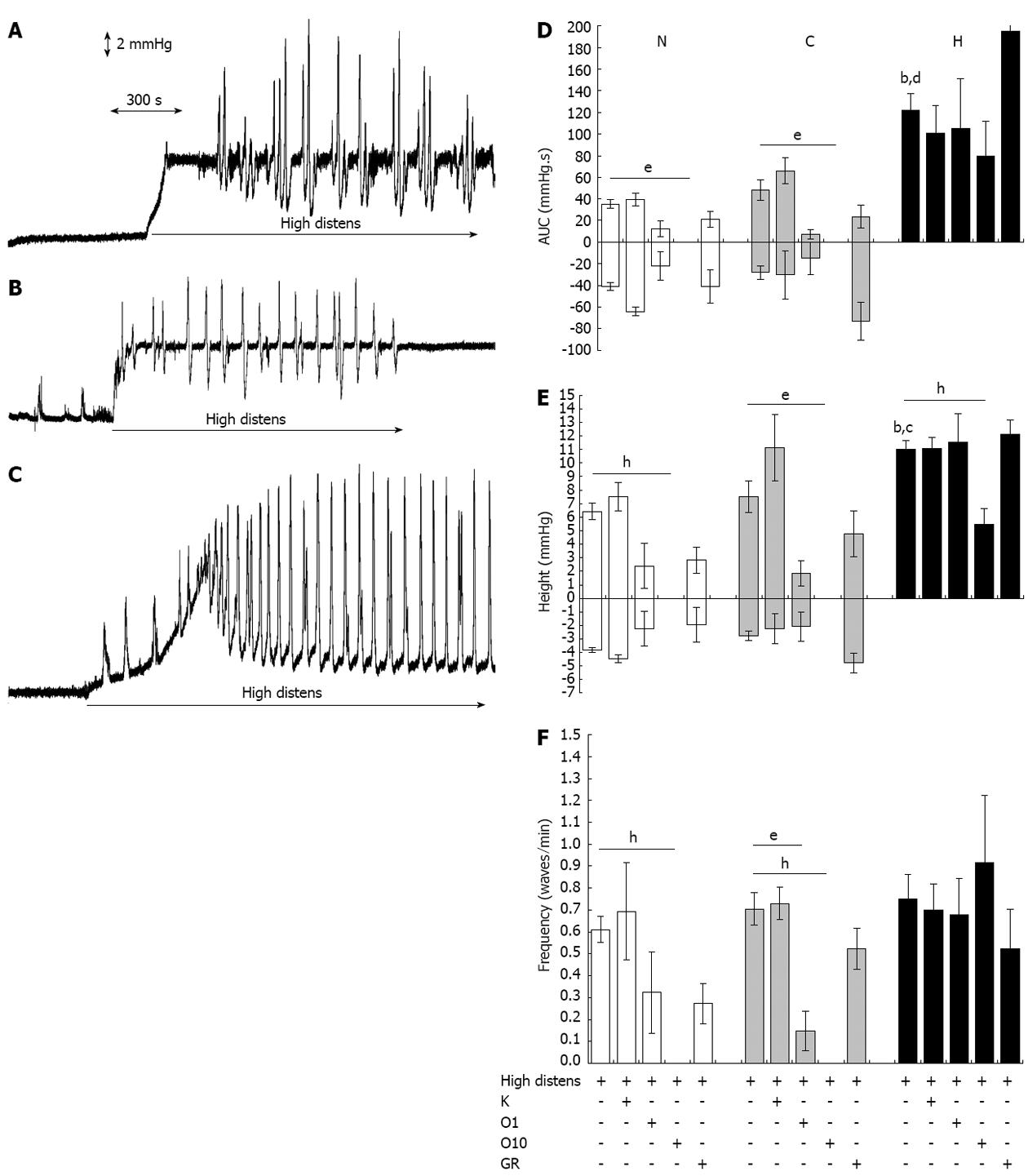
Distension of the intestinal wall, produced by elevating the outflow block to 8 cm above the level of the tissue in the bath, evoked distinct motor patterns in normal and control preparations compared with hypertrophic tissue. In normal and control tissues, intraluminal pressure increased steeply by about 6.5-7.0 mmHg, and non-propulsive, local cycles of contractions and relaxations were exhibited by 75% of the preparations (Figure 3A and B). In contrast, in all hypertrophic segments, intestinal tone first gradually and slowly increased up to a threshold pressure. At this point, rhythmic, anally propagating, strong propulsive strokes, markedly wider than normal and control tissues (P < 0.001) (Figure 3D) and markedly higher than normal (P < 0.001) or control tissue (P < 0.05) (Figure 3E) were elicited; simultaneously, basal intraluminal pressure progressively decreased to equilibration values (Figure 3C). Both motor patterns were blocked under NANC conditions and with simultaneous incubation with L-NAME 300 μmol/L and α-chymotrypsin 10 IU/mL (data not shown). In all types of tissues, incubation with the 5-HT2AR antagonist ketanserin 1 μmol/L did not modify any parameter of the distension-induced motility (Figure 3D-F). In contrast, blockade of 5-HT3R by ondansetron concentration-dependently reduced the amplitude, height and frequency of contractions and relaxations in normal and control tissues; in hypertrophic preparations, only the highest concentration of ondansetron tested (10 μmol/L) had an effect, with a decreased height of the peristaltic strokes without modification of their area or frequency (Figure 3D-F). Regarding antagonism of 5HT4R by GR125487 1 μmol/L, no significant changes were produced in the distension-induced motility of any tissue (Figure 3D-F).
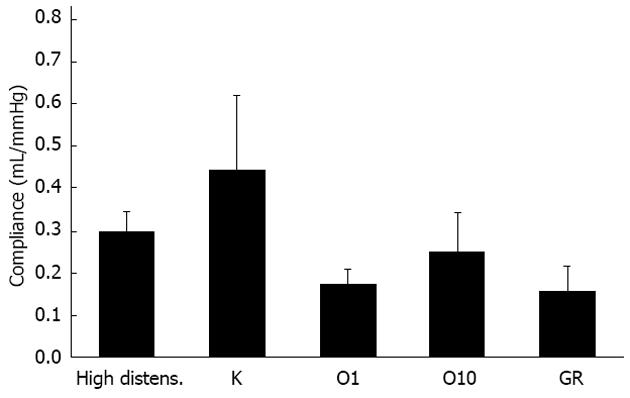
When the propulsive ability of hypertrophic tissues was taken into consideration, none of the 5-HTR antagonists tested modified the average compliance of the intestinal wall during the preparatory phase (Figure 4), or the efficacy of the propulsion of luminal fluid during the emptying phase, expressed by the average power (data not shown).
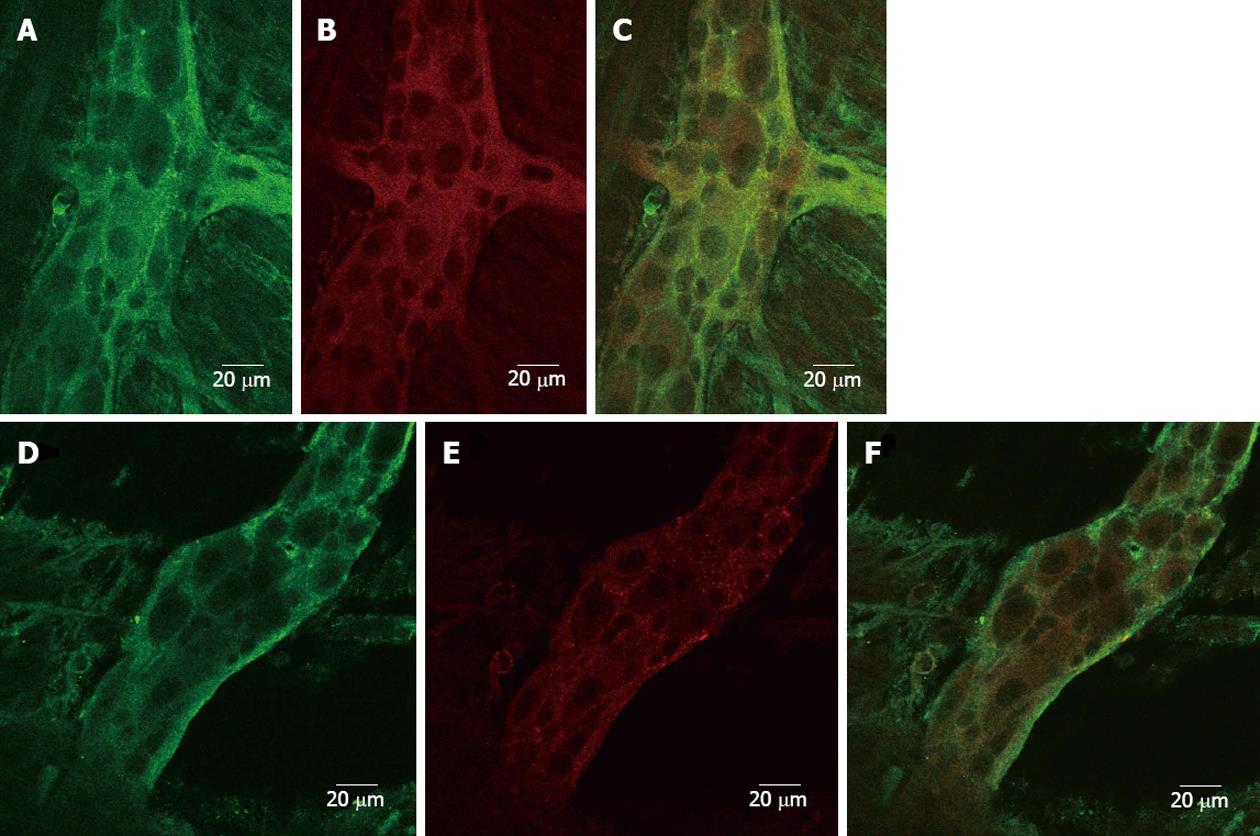
In both sham-operated and hypertrophic specimens, 5-HT3R immunoreactivity was detected in many neurons and fibers of the ileal myenteric plexus, appearing concentrated primarily near the neuronal plasma membrane; only slight staining was observed also in the cytoplasm (Figure 5A and D). Double-labeling with immunoreactivity for 5-HT3R and clathrin, a marker for early endosomes, whose distribution seemed mainly cytosolic (Figure 5B and E), did not show any clear significant co-localization, suggesting that in control and hypertrophic tissues the internalization of this ligand-gated ionotropic receptor appeared as an occasional event under basal conditions (Figure 5C and F).
The results of the present study indicate that hypertrophy of the intestinal wall induced by a partial chronic obstruction located at the terminal portion of rat ileum is associated with striking changes in the shape and nature of motor patterns elicited by EFS and by intraluminal distension. In particular, an increased accommodation capacity and little involvement of serotonergic neurotransmission in the control of enteric reflexes in hypertrophic tissues compared with normal and control reflexes can be hypothesized. First, when the motor responses evoked by electrical stimulation of intrinsic innervation are taken into consideration, it is worth noting that the typical biphasic profile, comprising a transient contraction followed by a weak relaxation in normal and control tissues, was converted into a high-amplitude monophasic contraction in hypertrophic tissue. This modification, similar to that already detected in electrically stimulated hypertrophic ileum when longitudinally mounted[7], can be accounted for by two different factors: a greater force of contraction due to increased muscle mass developed by hypertrophic growth; an increased accommodation capacity as shown by the quick drop in basal intraluminal pressure at the onset of EFS. The subsequent pharmacological investigation confirmed the pivotal role played by acetylcholine not only as an excitatory neurotransmitter in normal, control and especially hypertrophic tissues[7], but also as one of the primary transmitters of descending interneurons, corroborating the findings collected in guinea pig small intestine[17]. Indeed, incubation with atropine and guanethidine markedly reduced the contractile phase in all preparations and nearly abolished it in hypertrophic tissues. Furthermore, in obstructed segments, atropine and guanethidine impaired the accommodation reflex elicited by electrical stimulation, by increasing the intraluminal pressure to pre-stimulation values.
Along with acetylcholine, other mediators were involved in the regulation of intestinal motility both in physiological conditions and after chronic obstruction: only simultaneous incubation with the NOS inhibitor L-NAME and the protease α-chymotrypsin completely blocked neurogenic motor responses in all the preparations and further impaired the accommodation capacity in the obstructed tissue. It is worth noting that an increased sensitivity to relaxing mediators, including nitric oxide and vasoactive intestinal peptide (VIP), was exhibited by hypertrophic smooth muscle layers compared with non-obstructed ones[11], possibly concurring with the higher accommodation capacity shown by hypertrophic ileum in these conditions.
In the second part of the research, the increase in hydrostatic pressure on the intestinal wall and the resulting distension triggered atropine- and α-chymotrypsin-sensitive typical motor patterns in normal, control and hypertrophic tissues. In particular, in chronically obstructed segments, distension evoked wide, rhythmic and propulsive peristaltic waves propagating aborally, with shape similar to those elicited by electrical stimulation in the same type of tissues and totally different from the stationary, non-propulsive clusters of cycles arising in normal and control preparations in these experimental conditions. The precise mechanisms underlying this particular motor pattern are not known and the possible contribution given by each morphological and neurochemical alteration, already thoroughly described in the hypertrophic intestine[2-5], requires further investigation. The augmented total collagen content, possibly acting as a force transducer among the components of the contractile system[18], or the higher density of inhibitory neurons expressing VIP or pituitary adenylate cyclase-activating polypeptide[3], may be only two of the reasons for this motor behavior.
The collected results seem however to be consistent with the enhanced responsiveness to contractile agents and relaxing mediators which is seen in isolated hypertrophic smooth muscle layers[11], both aspects likely playing a critical role in the powerful peristaltic reflex. According to the original idea attributed to Bulbring et al[13], intraluminal distension initiates the peristaltic reflex through stimulation of intrinsic sensory primary afferent neurons by 5-HT released into the wall of the gut from enterochromaffin cells. Starting from this premise it seemed interesting to investigate the role played by endogenous 5-HT in the modulation of enteric motor reflexes through the application of selective 5HT2AR, 5-HT3R or 5-HT4R antagonists both in non-obstructed and in hypertrophic distended ileum. The findings provided by the present work suggest that in normal and control tissues, distension-induced motility is independent of the activation of smooth muscle 5-HT2AR, mediating rat ileum contraction[19], and only slightly affected by blockade of 5HT4R. These receptors are chiefly located on terminals of submucosal intrinsic primary afferent neurons, where, by promoting the release of acetylcholine and calcitonin gene-related peptide, they are reported to play a critical role in facilitating the peristaltic reflex along with the putative 5-HT1PR[20], towards which no selective antagonist is currently available. Also 5-HT3R are involved in peristalsis: they are mainly localized on extrinsic sensory nerves and on myenteric neurons, where they initiate giant migrating contractions[20], and may participate in the triggering and propagation of the peristaltic reflex[21,22]. The application of 5-HT3R antagonist concentration-dependently inhibited distension-induced motor responses in normal and control tissues, while, only slightly at the highest tested concentration did it impair the motor reflexes activated by wall distension in hypertrophic segments, which were refractory to the other 5-HTR antagonists. With regard to this behavior, it is tempting to speculate, and worth further investigation, that the mucosa, hypertrophied following chronic obstruction[23], could contain massive levels of 5-HT, stored in enterochromaffin cells. The mediator, once released by distension of the gut wall, may strongly stimulate 5-HT3R, possibly leading to two effects: the initiation of the peristaltic reflex and the internalization of the ligand-gated ion channel. Indeed, a long-term increase in 5-HT content in the intestinal mucosa has been demonstrated by Freeman et al[24] to result in a pronounced 5-HT3R internalization in myenteric neurons of rat ileum. The results obtained through confocal microscopy in the present study do not seem to support the hypothesis of a different cellular localization of 5-HT3 receptors in control and hypertrophic tissues: on the contrary, in the adopted experimental conditions, internalization of this receptor subtype appears equally unlikely in both kinds of preparations. This finding, together with the inability of ondansetron even at high concentrations to affect the peristaltic response, casts doubts over the actual involvement of 5-HT3R in the motor reflexes stimulated by mechanical distension in hypertrophic ileum. Given the complexity of factors possibly altering 5-HT availability and metabolism and, therefore, also affecting the pharmacological response to 5-HT3R antagonists, future studies will help to elucidate the role exerted by 5-HT in this experimental model and in intestinal secreto-motor disorders in general, where refractoriness to blockade of 5HT3R has sometimes emerged[25].
In conclusion, on the basis of the findings of this study, it is rational to hypothesize that the ability of hypertrophic intestine to elicit a powerful peristaltic reflex in vitro is an expression of the functional plasticity of neural and muscular intestinal tissue, a property aimed at preserving its physiological role in more demanding conditions, and allowing it to maintain chyme propulsion even in the presence of a point of abnormal resistance. This distinctive motor pattern appears to be primarily controlled by cholinergic and peptidergic mediators and only slightly affected by serotonergic transmission, which is critically involved, via 5-HT3R, in the modulation of non-propulsive motility triggered by mechanical distension in non-obstructed tissues.
The authors wish to thank Professor Catia Sternini (CURE/UCLA Digestive Disease Research Center, Los Angeles, CA, United States) for kindly providing rabbit-anti-rat IgG primary antibody directed against the 5-HT3R (No. 95247); Dr. Giuseppe Domenichini for his skilful technical assistance and the University of Parma (FIL 2009) for financial support.
Intestinal obstruction is a frequently encountered clinical problem, resulting from congenital malformations (as in Hirschsprung’s disease) or by an acquired obstruction, such as a surgical-induced stenosis or a neoplasm, possibly leading to death if not properly diagnosed and treated. Morphological, biomechanical and neurochemical changes have been thoroughly described orally to the site of an experimental chronic obstruction; however, functional investigations, which would help to gain a deeper insight into the regulatory mechanisms of motility in this pathological condition, are still limited.
Disruption of digestive motor activity, changes in slow wave activity but also preservation of the peristaltic reflex have been functionally described in different experimental models of intestinal stenosis. In rat ileum, hypertrophic growth, induced by mechanical obstruction, has been demonstrated to affect the responses of both smooth muscle layers and of intrinsic innervation, but no information is available on its effects on a highly sophisticated and integrated process between neural and myogenic components such as intestinal peristalsis.
To the best of knowledge, this is the first report showing that, following obstruction, rat isolated terminal ileum develops a propulsive activity triggered simply by intraluminal distension. Furthermore, the peristaltic activity exhibited by rat hypertrophic ileum appears only slightly affected by serotoninergic transmission, a property quite unexpected on the basis of the pivotal role played, in general, by endogenous serotonin in the initiation and propagation of enteric reflexes and, in particular, in the non-propulsive motility triggered by mechanical distension in non-obstructed tissues.
The results of this research improve the understanding of the changes in motility patterns and enteric reflexes triggered by intestinal obstruction, through a pharmacological investigation. Moreover, the observed refractoriness to 5-HT3 antagonists makes this system an interesting model to study conditions of variable sensitivity to blockade of 5-HT3 receptors, such as that described for diarrhea-predominant irritable bowel syndrome in female patients treated with alosetron.
Peristalsis: A coordinated motor behavior, which allows the intestine to propel its contents in an anal direction; Accommodation: Indicates the ability of the intestine to adapt itself to the distension pressure, and, conversely, is a reflection of the resistance of the intestinal wall to the infused fluid; 5-HT3 receptor: Ligand-gated ion channel receptor activated by serotonin or 5-HT, localized on extrinsic sensory nerves and on myenteric neurons, where they initiate giant migrating contractions and may participate in the triggering and propagation of the peristaltic reflex.
This is a nice study, the findings are interesting and potentially important for clinical medicine. The authors showed that accommodation capacity and distension-induced peristalsis of rat hypertrophic ileum are primarily controlled by cholinergic and peptidergic transmission and negligibly by 5-HT3 receptors.
P-Reviewers Bian ZX, Inui A S- Editor Gou SX L- Editor Cant MR E- Editor Li JY
| 1. | Schuffler MD, Sinanan MN. Intestinal obstruction and pseudo-obstruction. Gastrointestinal disease – Pathophysiology/Diagnosis/Management. Philadelphia: WB Saunders editors 1993; 898-916. |
| 2. | Gabella G. Hypertrophy of visceral smooth muscle. Anat Embryol (Berl). 1990;182:409-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Ekblad E, Sjuve R, Arner A, Sundler F. Enteric neuronal plasticity and a reduced number of interstitial cells of Cajal in hypertrophic rat ileum. Gut. 1998;42:836-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Chang IY, Glasgow NJ, Takayama I, Horiguchi K, Sanders KM, Ward SM. Loss of interstitial cells of Cajal and development of electrical dysfunction in murine small bowel obstruction. J Physiol. 2001;536:555-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 166] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 5. | Jew JY, Williams TH, Gabella G, Zhang MQ. The intestine as a model for neuronal plasticity. Arch Histol Cytol. 1989;52 Suppl:167-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Storkholm JH, Zhao J, Villadsen GE, Gregersen H. Spontaneous and bolus-induced motility in the chronically obstructed guinea-pig small intestine in vitro. Dig Dis Sci. 2008;53:413-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Bertoni S, Gabella G, Ghizzardi P, Ballabeni V, Impicciatore M, Lagrasta C, Arcari ML, Barocelli E. Motor responses of rat hypertrophic intestine following chronic obstruction. Neurogastroenterol Motil. 2004;16:365-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Coelho JC, Gouma DJ, Moody FG, Li YF, Senninger N. Gastrointestinal motility following small bowel obstruction in the opossum. J Surg Res. 1986;41:274-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Schulze-Delrieu K, Brown B, Herman B, Brown CK, Lawrence D, Shirazi S, Palmieri T, Raab J. Preservation of peristaltic reflex in hypertrophied ileum of guinea pig. Am J Physiol. 1995;269:G49-G59. [PubMed] |
| 10. | Trendelenburg P. Physiological and pharmacological investigations of small intestinal peristalsis. Translation of the article “Physiologische und pharmakologische Versuche über die Dünndarmperistaltik”, Arch. Exp. Pathol. Pharmakol. 81, 55-129, 1917. Naunyn Schmiedebergs Arch Pharmacol. 2006;373:101-133. [PubMed] |
| 11. | Bertoni S, Ballabeni V, Flammini L, Gobbetti T, Impicciatore M, Barocelli E. Intestinal chronic obstruction affects motor responsiveness of rat hypertrophic longitudinal and circular muscles. Neurogastroenterol Motil. 2008;20:1234-1242. [PubMed] [DOI] [Full Text] |
| 12. | Bulbring E, Crema A. The release of 5-hydroxytryptamine in relation to pressure exerted on the intestinal mucosa. J Physiol. 1959;146:18-28. [PubMed] |
| 13. | Bulbring E, Lin RC. The effect of intraluminal application of 5-hydroxytryptamine and 5-hydroxytryptophan on peristalsis; the local production of 5-HT and its release in relation to intraluminal pressure and propulsive activity. J Physiol. 1958;140:381-407. [PubMed] |
| 14. | Gabella G. Hypertrophy of intestinal smooth muscle. Cell Tissue Res. 1975;163:199-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 94] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Costall B, Naylor RJ, Tuladhar BR. 5-HT4 receptor mediated facilitation of the emptying phase of the peristaltic reflex in the guinea-pig isolated ileum. Br J Pharmacol. 1993;110:1572-1578. [PubMed] |
| 16. | Waterman SA, Costa M, Tonini M. Modulation of peristalsis in the guinea-pig isolated small intestine by exogenous and endogenous opioids. Br J Pharmacol. 1992;106:1004-1010. [PubMed] |
| 17. | Kunze WA, Furness JB. The enteric nervous system and regulation of intestinal motility. Annu Rev Physiol. 1999;61:117-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 265] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 18. | Storkholm JH, Zhao J, Villadsen GE, Hager H, Jensen SL, Gregersen H. Biomechanical remodeling of the chronically obstructed Guinea pig small intestine. Dig Dis Sci. 2007;52:336-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Briejer MR, Mathis C, Schuurkes JA. 5-HT receptor types in the rat ileum longitudinal muscle: focus on 5-HT2 receptors mediating contraction. Neurogastroenterol Motil. 1997;9:231-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Gershon MD. Review article: serotonin receptors and transporters -- roles in normal and abnormal gastrointestinal motility. Aliment Pharmacol Ther. 2004;20 Suppl 7:3-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 366] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 21. | Monro RL, Bertrand PP, Bornstein JC. ATP and 5-HT are the principal neurotransmitters in the descending excitatory reflex pathway of the guinea-pig ileum. Neurogastroenterol Motil. 2002;14:255-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Kadowaki M, Wade PR, Gershon MD. Participation of 5-HT3, 5-HT4, and nicotinic receptors in the peristaltic reflex of guinea pig distal colon. Am J Physiol. 1996;271:G849-G857. [PubMed] |
| 23. | Bertoni S, Gabella G. Hypertrophy of mucosa and serosa in the obstructed intestine of rats. J Anat. 2001;199:725-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Freeman SL, Glatzle J, Robin CS, Valdellon M, Sternini C, Sharp JW, Raybould HE. Ligand-induced 5-HT3 receptor internalization in enteric neurons in rat ileum. Gastroenterology. 2006;131:97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Camilleri M, Atanasova E, Carlson PJ, Ahmad U, Kim HJ, Viramontes BE, McKinzie S, Urrutia R. Serotonin-transporter polymorphism pharmacogenetics in diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2002;123:425-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 200] [Article Influence: 8.7] [Reference Citation Analysis (0)] |