Published online Dec 21, 2013. doi: 10.3748/wjg.v19.i47.9092
Revised: October 19, 2013
Accepted: November 3, 2013
Published online: December 21, 2013
Processing time: 173 Days and 18.7 Hours
AIM: To explore whether the antitumor effect of a vascular disrupting agent (VDA) would be enhanced by combining with an antiangiogenic agent, and whether such synergistic effects can be effectively evaluated with separate calculation of diffusion weighted magnetic resonance imaging (DW-MRI).
METHODS: Thirty-seven rats with implanted liver tumors were randomized into the following three groups: (1) ZD6126, a kind of VDA; (2) ZDTHA, ZD6126 in combination with an antiangiogenic, thalidomide; and (3) control. Morphological DW-MRI were performed and quantified before, 4 h and 2 d after treatment. The apparent diffusion coefficient (ADC) values were calculated separately for low b values (ADClow), high b values (ADChigh) and all b values (ADCall). The tissue perfusion contribution, ADCperf, was calculated as ADClow-ADChigh. Imaging findings were finally verified by histopathology.
RESULTS: The combination therapy with ZDTHA significantly delayed tumor growth due to synergistic effects by inducing cumulative tumor necrosis. In addition to delaying tumor growth, ZDTHA caused tumor necrosis in an additive manner, which was verified by HE staining. Although both ADChigh and ADCall in the ZD6126 and ZDTHA groups were significantly higher compared to those in the control group on day 2, the entire tumor ADChigh of ZDTHA was even higher than that of ZD6126, but the significant difference was not observed for ADCall between ZDTHA and ZD6126. This indicated that the perfusion insensitive ADChigh values calculated from high b value images performed significantly better than ADCall for the monitoring of tumor necrosis on day 2. The perfusion sensitive ADCperf derived from ADClow by excluding high b value effects could better reflect the reduction of blood flow due to the vessel shutdown induced by ZD6126, compared to the ADClow at 4 h. The ADCperf could provide valuable perfusion information from DW-MRI data.
CONCLUSION: The separate calculation of ADC is more useful than conventional averaged ADC in evaluating the efficacy of combination therapy with ZD6126 and thalidomide for solid tumors.
Core tip: The combination therapy with ZD6126 and thalidomide significantly delayed liver tumor growth due to synergistic effects by inducing cumulative tumor necrosis in rodents. The apparent diffusion coefficient (ADC)high performed significantly better than ADCall for the monitoring of tumor necrosis on day 2. The ADCperf could better reflect the reduction of blood flow due to the vessel shutdown induced by ZD6126, compared to the ADClow. The ADCperf could provide valuable perfusion information from diffusion weighted magnetic resonance imaging data.
- Citation: Chen F, Keyzer FD, Feng YB, Cona MM, Yu J, Marchal G, Oyen R, Ni YC. Separate calculation of DW-MRI in assessing therapeutic effect in liver tumors in rats. World J Gastroenterol 2013; 19(47): 9092-9103
- URL: https://www.wjgnet.com/1007-9327/full/v19/i47/9092.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i47.9092
Tumor vasculature has become an attractive target for therapy. One of such therapies is to use vascular disrupting agents (VDAs), which can selectively destroy existing tumor blood vessels by disrupting the microtubules of the cytoskeleton in endothelial cells; this leads to ischemic central necrosis of the tumor[1]. However, tumors can rapidly rebound from the residual viable rim when VDAs are used alone; this compromises the therapeutic utility of these agents[2]. Another therapy is to prevent new tumor blood vessel formation with antiangiogenic agents. Therefore, current efforts have gradually shifted from the single use of VDA to the combination of a VDA with an antiangiogenic agent[3,4]. As the latter may inhibit the growth of new tumor vessels, the combination of two approaches thus is likely to have synergistic therapeutic efficacy.
As an established non-invasive technique, in vivo magnetic resonance imaging (MRI) has played an important role in the evaluation of tumor response to treatment. Diffusion-weighted MRI (DW-MRI), due to its ability to detect molecular water motion at the cellular level, i.e., the measurement of tissue apparent diffusion coefficient (ADC), has become a favorite choice of measures in a variety of oncological studies and tissue viability assessments[5]. Technological innovations in recent years have enabled the increasing use of high-quality and quantitative DW-MRI in monitoring tumor treatment. However, it has been realized that the information acquired from conventional calculation of DW-MRI data actually represents the combined effects of tissue microcirculation perfusion and pure tissue diffusivity in each imaging voxel at DW-MRI. This may hinder the appropriate interpretation of the DW-MRI data[6]. Therefore, there is growing interest in applying more sophisticated approaches, such as separate ADC (calculating different ADC values based on various combinations of b values with a monoexponential fitting algorithm)[7-9] and intravoxel incoherent motion (IVIM)[10,11], to differentiate the fraction of microcirculation perfusion from pure diffusivity within the DW-MRI data.
The purpose of the present study was to test our hypotheses that the antitumor effect of a VDA, ZD6126, would be enhanced by combining with an antiangiogenic agent, thalidomide, and that the effect can be monitored and better elucidated with separate calculation of ADC values compared to conventional ADC value in a rat liver tumor model. To our knowledge, the application of separate calculation of ADC maps has not been reported in such a combined antitumor therapy.
A total of 37 rats were randomly assigned into the following 3 groups: (1) ZD6126 group (n = 14): ZD6126 (AstraZeneca, Cheshire, United Kingdom) was dissolved with 4 portions of 8.4% sodium carbonate and 1 portion of phosphate-buffered saline (PBS), pH 7.4. On day 0, one dose of 50 mg/kg ZD6126 was injected iv into each animal; (2) ZDTHA group (n = 13): Stock solutions of thalidomide (Pharmaceutical Factory, Changzhou, China) were prepared in DMSO (Sigma-Aldrich NV/SA, Bornem, Belgium) and injected ip at a dose of 200 mg/kg three times at a interval of one day during the entire experiment[12]; the first dose of thalidomide was injected 24 h prior to ZD6126 administration, and the second and third doses of thalidomide were given immediately and 2 d after ZD6126 administration, respectively; and (3) Control group (n = 10): Animals were iv and ip injected with the vehicles (solvents) of both agents at the same time points that the other groups were injected. For all groups, MRI was performed before, and 4 h and 2 d after the initial ZD6126 treatment. At the end of the experiment, all animals were sacrificed for histopathological examinations.
This study was approved by the institutional ethical committee for the use and care of laboratory animals. Adult WAG/Rij rats (Iffa Credo, Brussels, Belgium) with existing subcutaneous rhabdomyosarcomas were used as donors. The tumor tissues were excised and implanted into 37 normal adult WAG/Rij rats that weighed 225-275 g, as described previously[13].
All rats were initially anesthetized by inhalation of 2% isoflurane and maintained with 0.8% isoflurane for MRI. A clinical 1.5T MRI system (Sonata, Siemens, Erlangen, Germany) was used with a maximum gradient capability of 40 mT/m. The following sequences were acquired in the transverse plane for all rats, with a slice thickness of 2 mm and an inter-slice gap of 0.2 mm: (1) Fat saturated T2-weighted fast spin echo MRI (T2W-MRI) with a repetition/echo time (TR/TE) of 3860/106 ms, a turbo factor of 19, a field of view (FOV) of 140 mm × 70 mm, and an acquisition matrix of 256 × 256 (in-plane resolution: 0.5 mm × 0.3 mm). Three signals were acquired, in a scan time of 1 min 25 s; (2) Contrast-enhanced fat saturated T1-weighted fast spin echo MRI (CE-T1W-MRI) immediately after an iv bolus of 0.3 mmol/kg gadoterate meglumine (Dotarem®, Guerbet, France), with the following parameters: a TR/TE of 535/9.2 ms, a turbo factor of seven, a FOV of 140 mm × 70 mm, and an acquisition matrix of 256 × 256 (in-plane resolution: 0.5 mm × 0.3 mm). Four signals were acquired, in a scan time of 1 min 24 s; (3) DW-MRI with a 2-dimensional (2D), spin echo, echo-planar imaging sequence. We used a TR/TE of 1700/83 ms, a FOV of 140 mm × 82 mm, and an acquisition matrix of 192 × 91 (in-plane resolution: 0.7 mm × 0.9 mm). For the DW-MRI, six signals were acquired, including repeated measurements for 10 different b values (0, 50, 100, 150, 200, 250, 300, 500, 750, and 1000 s/mm2) in 3 directions (x, y, and z) and averaged for the calculation of the isotropic ADC value. A parallel imaging technique was applied to reduce susceptibility artifacts and examination times. The total examination time was 4 min 51 s.
All rats were euthanized for tissue processing and histology at the end of the experiment. First, animals were over-anesthetized by an intraperitoneal injection of pentobarbital (50 mg/kg) (Nembutal, Sanofi Sante Animale, Brussels, Belgium). Then, the livers were collected, fixed with formalin, embedded in paraffin, and sliced into transverse sections. The sections were 2 mm thick, and were positioned on the same planes used for the MRI scans, based on a grid (Agar Scientific, England). The tumor slices (5 μm thick) were stained with hematoxylin and eosin (HE).
An off-line LINUX workstation with dedicated software (Biomap, Novartis, Basel, Switzerland) was used for image analyses. Two experienced radiologists delineated the entire tumor with operator-defined regions of interest (ROI) in consensus to obtain robust measurements and to facilitate comparisons between different treatment approaches. All ROIs were larger than 10 pixels in size. For each imaging parameter, tumor and normal liver were measured with ROIs on all tumor-containing image slices, and mean values were obtained for each tumor and the liver, respectively. After that, the mean value and standard deviation for each parameter were calculated for each group at each time point for statistical analysis.
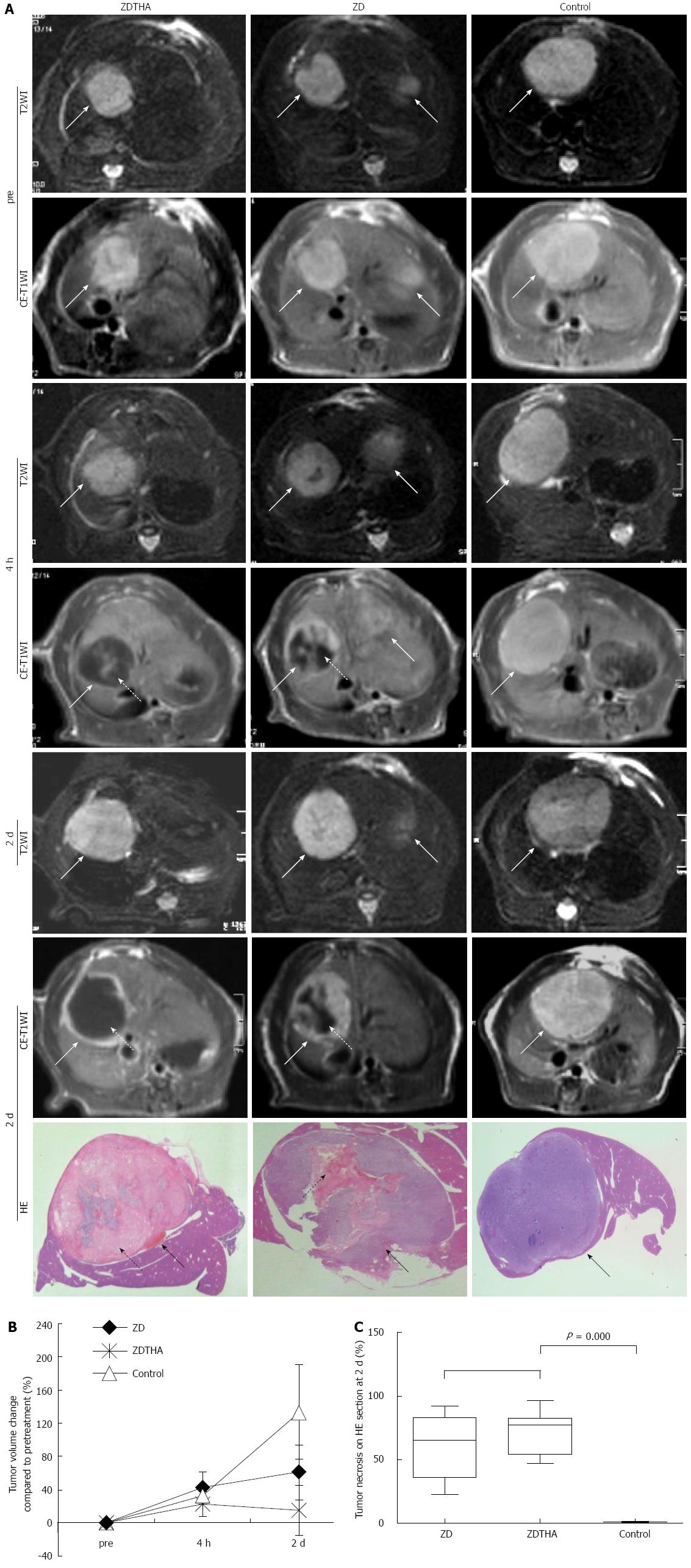
T2 weighted and contrast enhanced-T1 weighted MRI: The residual viable tumor or rim after treatment was visualized as contrast-enhanced, high signal region on the CE-T1W-MRI. The tumor necrotic areas were contoured on CE-T1W-MRI based on the unenhanced, low-signal areas within the tumors that were observed after injection of a contrast agent. Relative volumes (%) of tumor necrosis were calculated by normalizing them to the entire tumor volume. For each lesion, the tumor areas were delineated at T2W-MRI on all slices and automatically combined into the total tumor volume. The tumor volume change (%) was calculated using the following formula: [(volumepost - volumepre)/volumepre] × 100.
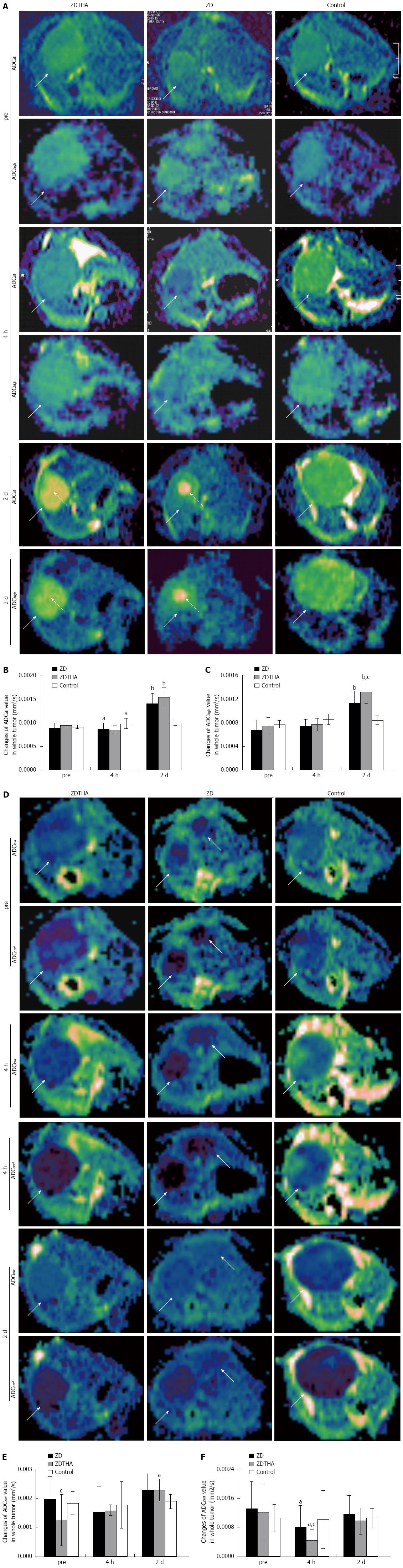
Separate calculation of tumor ADC: For calculating different ADC values, the first step was to measure the entire tumor signal intensity (SI) from original DW-MRI images of 10 b values, respectively. Briefly, for each tumor, freehand delineations were performed on all slices of the original DW-MRI at a b value of 1000 s/mm2. These delineations were merged to form one 3D volume of interest per lesion. The volume of interest was then automatically copied to all images with different b values and the average SI of each lesion per b value was determined. The second step was to obtain separate ADC values according to a monoexponential model using all 10 b values[14]. To differentiate the individual contributions of tissue microcapillary perfusion and pure tissue diffusivity, the ADC values of each tumor were obtained separately for low b values (b = 0, 50, and 100 s/mm2; ADClow) and high b values (b = 500, 750, and 1000 s/mm2; ADChigh) from the average SI per tumor and per b value. Each ADC value was calculated by using a least squares solution of the following system of equations: ADCall: Sk = S0×exp (-bk× ADClow), for k = 0, 50, 100; 150, 200, 250, 300, 500, 750, 1000; ADClow: Si = S0×exp (-bi× ADClow), for i = 0, 50, 100; ADChigh: Sj = S0×exp (-bj× ADChigh), for j = 500, 750, 1000; where Sk, Si, and Sj are the SI measured on the DW-MRI acquired with the corresponding b values bk, bi and bj, S0 represents the exact SI (without the influence of noise induced by the MR measurement) with b value equal to 0 s/mm2. ADClow is perfusion sensitive, while ADChigh is perfusion insensitive. Although ADClow is perfusion sensitive, it is also affected by diffusion effects in tissue[15]. Therefore, an approximate indicator, ADCperf, for the tissue perfusion contribution can be calculated as ADClow-ADChigh[7]. Imaging software (MeVisLab 2.2.1, MeVis Medical Solutions AG, Bremen, Germany) was used to generate the maps of ADCall, ADChigh, ADClow and ADCperf.
Microscopic image analyses were performed by a pathologist blinded to the experimental detail with magnifications ranging from × 50 to × 400. On HE stained macroscopic sections, image analysis software (ImageJ 1.34s, NIH, United States) was used to quantify the percentages of amorphous eosinophilic necrosis in the total tumor area.
Statistical analysis was carried out with the SPSS for windows software package (release 18.0, SPSS Inc., Chicago, United States). A general linear model, with repeated-measures, was used to compare changes in various parameters over time among groups. The nonparametric Kruskal-Wallis analysis of variance was performed for comparing parameters between groups at certain time points, followed by post-hoc group-wise comparisons using a Bonferroni correction for multiple tests. A P value < 0.05 was considered statistically significant.
A total of 37 rats (72 tumors) were included in the study. Four rats in the ZDTHA group were found to have minor hemorrhage around the eye socket and perianal area on day 1 after the first thalidomide treatment. This was probably due to a venous thromboembolism induced by thalidomide[16].
As shown on T2W-MRI images, ZD6126 and ZDTHA both induced a significant tumor volume growth delay from pretreatment to 2 d after administration, compared to the control group (P < 0.0001 for both). Furthermore, ZDTHA performed significantly better than ZD6126 in delaying tumor growth on day 2 after treatment (P < 0.0001) (Figure 1).
Perfusion insensitive ADChigh: Before treatment: there were no significant differences in ADChigh among the three groups (P > 0.05 for all). At 4 h, there was no significant change in the ADChigh (P > 0.05 for both) in both the ZDTHA and ZD6126 groups, compared to the control group. On day 2, the therapy-induced tumor necrosis caused a significant rise in the ADChigh in both ZDTHA and ZD6126 groups compared to the control group (P < 0.0001 and = 0.0004, respectively). Furthermore, the ADChigh was much higher in the ZDTHA group than in the ZD6126 group (P = 0.03) (Table 1 and Figure 2A-C).
| Group and time | ADCall (× 10-3 mm2/s) | ADChigh (× 10-3 mm2/s) | ADClow (× 10-3 mm2/s) | ADCperf (× 10-3 mm2/s) |
| ZD6126 | ||||
| Pre | 0.90 ± 0.10 | 0.68 ± 0.16 | 1.98 ± 0.75 | 1.30 ± 0.74 |
| 4 h | 0.86 ± 0.13 | 0.73 ± 0.12 | 1.54 ± 0.87 | 0.81 ± 0.70 |
| 2 d | 1.40 ± 0.22 | 1.13 ± 0.21 | 2.28 ± 0.54 | 1.15 ± 0.52 |
| ZDTHA | ||||
| Pre | 0.95 ± 0.08 | 0.70 ± 0.15 | 1.25 ± 0.87 | 1.22 ± 0.77 |
| 4 h | 0.85 ± 0.08 | 0.77 ± 0.11 | 1.21 ± 0.59 | 0.44 ± 0.50 |
| 2 d | 1.54 ± 0.21 | 1.31 ± 0.19 | 2.28 ± 0.37 | 0.97 ± 0.36 |
| Control | ||||
| Pre | 0.91 ± 0.04 | 0.77 ± 0.07 | 1.82 ± 0.39 | 1.06 ± 0.37 |
| 4 h | 0.98 ± 0.11 | 0.85 ± 0.09 | 1.76 ± 0.79 | 1.01 ± 0.80 |
| 2 d | 1.00 ± 0.06 | 0.95 ± 0.08 | 1.90 ± 0.24 | 1.06 ± 0.27 |
ADCall: At 4 h, the ADCall in both the ZDTHA and ZD6126 groups was significantly reduced compared to the control group (P = 0.01 and 0.02, respectively). In contrast, the ADCall in both the ZDTHA and ZD6126 groups showed a sharp increase on day 2 compared to the control group (P < 0.0001 for both). However, no difference in ADCall was found between the ZDTHA and ZD6126 groups (P = 0.08) (Table 1 and Figure 2A-C).
Comparison of ADChigh with ADCall: The performance of ADCall was different with that of ADChigh at the following time points. At 4 h, the ADCall in both the ZDTHA and ZD6126 groups showed a significant decrease compared to the control group; however, this was not observed for ADChigh in the same two groups. On day 2, the ADChigh of ZDTHA was significantly greater than that of ZD6126 (P = 0.03), but the significant difference was not observed for ADCall between the ZDTHA and ZD6126 groups (P = 0.08) (Table 1 and Figure 2A-C).
ADClow: The ADClow of ZDTHA was significantly lower than that of ZD6126 before treatment (P < 0.05), but it was not for the control group (P = 0.12). No significant differences in ADClow were observed among the three groups at 4 h (P > 0.05 for all). On day 2, the ADClow of ZDTHA was much higher compared to the control group (P = 0.02) (Table 1 and Figure 2D-F).
ADCperf: Compared to the control group, tumor ADCperf in both the ZD6126 and ZDTHA groups decreased dramatically at 4 h, most likely due to a rapid vascular shutdown induced by ZD6126 (P = 0.016 and 0.047, respectively). This was followed by a rapid rebound on day 2 in both the ZD6126 and ZDTHA groups (no longer significantly different compared to the control group, P = 0.979 and 0.525, respectively) (Figure 2D-F). A significant reduction in the tumor ADCperf of ZDTHA was noted at 4 h compared to the ZD6126 group (P = 0.025). The ADCperf of ZDTHA still showed a lower level compared to the ZD6126 at 2 d, although there was no significant difference (P = 0.44) (Table 1 and Figure 2D-F).
Two days after treatment, the percentages of necrosis compared to the total tumor areas on HE stained tumor sections were significantly higher in both the ZDTHA and ZD6126 groups compared to the control group (P = 0.000 for both). No significant difference was found in the necrotic areas of the ZDTHA and ZD6126 groups (P = 0.09) (Figure 1).
We have demonstrated three main findings in the present study. First, tumor growth was significantly delayed by both ZD6126 and ZDTHA treatments compared to the control group, and a significant delay could be observed only two days after application of a single dose of ZD6126. In addition, ZDTHA significantly delayed tumor growth than ZD6126, indicating a synergistic anticancer effect of ZD6126 and thalidomide. It has been known that tumors can rapidly regrow due to the residual viable rim when ZD6126 was used alone[2]. It has also been reported that thalidomide, which was reintroduced into clinical practice with its antiangiogenic properties, had little or no effect on full-grown tumors like those in our patients, when used alone[4]. Therefore, the synergistic effects of ZDTHA may have contributed to the enhanced antitumor effect in this study. In the combination therapy, ZD6126-induced tumor necrosis, which may then promote tumor angiogenesis, could provide the appropriate conditions for THA to indirectly inhibit angiogenesis[17], because thalidomide is effective only in the early stages of tumor formation[4]. Thalidomide indirectly inhibits angiogenesis via tumor necrosis factor and the prostaglandin E pathway[17]. Therefore, the proposed combination therapy with ZD6126 and thalidomide may have some potential applications for solid tumor treatment in clinic.
Second, ADChigh, a separate ADC value calculated from high b value images, performed significantly better than ADCall for the monitoring of tumor necrosis. In addition to delaying tumor growth, ZDTHA caused tumor necrosis in an additive manner, which was verified by HE staining. Our results showed that although both the ADChigh and ADCall of ZDTHA and ZD612 were significantly higher compared to those of the control group on day 2, the entire tumor ADChigh of ZDTHA was even higher than that of ZD6126, but the significant difference was not observed for ADCall between ZDTHA and ZD6126. This was due to that ADChigh was more sensitive to the diffusion change resulting from the therapeutic necrosis of the tumor on day 2. It has been reported that VDA can cause massive central necrosis 2 d after treatment[18]. Thalidomide can directly induce apoptosis or G1 phase arrest[19]. Consequently, tumor cells treated with ZD6126 and thalidomide underwent increased necrosis compared to the single use of ZD6126 at the end of the study; this synergistic effect on necrosis could be better reflected with ADChigh as shown in this study.
Third, ADCperf, a separate ADC value calculated as ADClow minus ADChigh, can provide valuable perfusion information from DWI data. Although the ADClow was calculated from low b value images and perfusion sensitive, it was still contaminated with diffusion effects in tissues[15]. Therefore, ADClow was not satisfactory in evaluating the tumor response to treatment as indicated in this study. However, ADCperf was calculated from ADClow by excluding high b value effects; it would be more perfusion sensitive. In this study, for instance, strikingly reduced perfusion in response to treatment was detected with ADCperf at 4 h, but not with ADClow for both the ZDTHA and ZD6126 groups compared to the control group. Furthermore, the reduction of ADCperf in ZDTHA was even lower; this indicated a more pronounced decrease in blood perfusion induced by ZDTHA. The ADCperf of ZDTHA still showed a lower level compared to ZD6126 on day 2, although there was no significant difference. This could be explained by the fact that besides the vascular shutdown effect of ZD6126, thalidomide may also induce a transient normalization of tumor vasculature via aggressive vascular pruning and improve pericyte coverage on vessels. As a result, tumor perfusion was reduced[20,21]. Our results indicated that ADCperf allowed the early monitoring of therapeutic effects, because it was more sensitive to the microcapillary perfusion and could detect perfusion in response to therapy before the appearance of tumor necrosis without the administration of contrast media.
Similarly, such a significant perfusion reduction at 4 h was also detected with ADCall in both the ZDTHA and ZD6126 groups, however, this was not observed for ADChigh, compared to the control group. The reason is that ADCall was derived from 10 b values including low and high b values; consequently it was affected by both diffusion and perfusion in the tumor. Even though, the perfusion change measured with ADCall was not as striking as that noted with ADCperf due to the influence of diffusion contribution. Despite the delayed growth and the massive central necrotic areas in both the ZDTHA and ZD6126 groups, tumors began to relapse evidenced by the recovery of tumor ADCperf and ADClow, as well as the enhanced rim visualized on CE-T1WI, due to residue viable tumor cells on day 2 after therapy. These results are consistent with previous findings[18,22]. However, ZDTHA demonstrated significantly less tumor relapse than ZD6126, suggesting the benefit of applying the combination therapy.
It remains controversial regarding the option of mono- or biexponential model in extracting diffusion and perfusion information from DWI data. Because each model has its own advantages and drawbacks[15,23]. As a pioneering work in the mid-1986s, Le Bihan et al[24,25] proposed the concept of IVIM to address the microscopic movements in image voxel in MRI. In biologic tissue, the motions include the molecular diffusion of water and the microcirculation of blood or capillary perfusion. With the biexponential model of IVIM, the fraction of capillary perfusion can be separated from diffusion. Therefore, there is growing studies using IVIM from DWI data[26-29]. However, the clinical benefit of the biexponential model as compared to the monoexponential model has not been comprehensively established[10,15]. Our study supports that the ADChigh values are similar to the diffusion coefficient derived from IVIM model. The separate calculations of ADCall, ADChigh, ADClow and ADCperf using a monoexponential fitting algorithm are relatively simple to estimate and are readily available for most users of clinical MR scanners. However, a lack of direct comparison of diffusion parameters derived from mono- and biexponential model may be a limitation of the present study.
In conclusion, we have demonstrated that ZDTHA combination treatment significantly delayed tumor growth due to synergistic effects by inducing cumulative tumor necrosis. The perfusion insensitive ADChigh values calculated from high b value images performed significantly better than ADCall values for the monitoring of tumor necrosis. The perfusion sensitive ADCperf values derived from ADClow by excluding high b value effects could provide valuable perfusion information from DWI data. Therefore, the in vivo separate calculations of ADC values derived from monoexponential model are more useful than conventional averaged ADC values in the successful evaluation of tumor therapeutic effects.
Diffusion weighted magnetic resonance imaging (DW-MRI), due to its ability to detect molecular water motion at the cellular level, i.e., the measurement of apparent diffusion coefficient (ADC), has become a favorite choice of measures in a variety of oncological studies and tissue viability assessments.
Technological innovations in recent years have enabled the increasing use of high-quality and quantitative DW-MRI in monitoring tumor treatment. However, it has been realized that the information acquired from conventional calculation of DW-MRI data actually represents the combined effects of tissue microcirculation perfusion and pure tissue diffusivity in each imaging voxel at DW-MRI. This may hinder the appropriate interpretation of the DW-MRI data. Therefore, there is growing interest in applying more sophisticated approaches, such as separate ADC (calculating different ADC values based on various combinations of b values with a monoexponential fitting algorithm) and intravoxel incoherent motion, to differentiate the fraction of microcirculation perfusion from pure diffusivity within the DW-MRI data.
The combination therapy with ZD6126 and thalidomide significantly delayed liver tumor growth due to synergistic effects by inducing cumulative tumor necrosis in rodents. The ADChigh performed significantly better than ADCall for the monitoring of tumor necrosis on day 2. The ADCperf could better reflect the reduction of blood flow due to the vessel shutdown induced by ZD6126, compared to the ADClow. The ADCperf could provide valuable perfusion information from diffusion weighted MRI data.
The separate calculation of ADC is more useful than conventional averaged ADC in evaluating the efficacy of combination therapy with ZD6126 and thalidomide for solid tumors.
DW-MRI is an in vivo imaging technique to detect molecular water motion at the cellular level by using the ADC parameter. Separate ADC measurement is to calculate the different ADC values based on various combinations of b values with a monoexponential fitting algorithm.
The authors present ADC is more useful than conventional averaged ADC in evaluating the efficacy of combination therapy with ZD6126 and thalidomide for solid tumors. It would be of interest to perform the same study in a vascular tumor such as infantile hemangioma or a malignant vascular tumor such as angiosarcoma or hemangiopericytoma.
P- Reviewers: Casal Moura M, Fernandez-Pineda I S- Editor: Zhai HH L- Editor: Wang TQ E- Editor: Ma S
| 1. | Dark GG, Hill SA, Prise VE, Tozer GM, Pettit GR, Chaplin DJ. Combretastatin A-4, an agent that displays potent and selective toxicity toward tumor vasculature. Cancer Res. 1997;57:1829-1834. [PubMed] |
| 2. | Tozer GM, Kanthou C, Baguley BC. Disrupting tumour blood vessels. Nat Rev Cancer. 2005;5:423-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 737] [Cited by in RCA: 743] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 3. | Kanthou C, Tozer GM. Microtubule depolymerizing vascular disrupting agents: novel therapeutic agents for oncology and other pathologies. Int J Exp Pathol. 2009;90:284-294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 161] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 4. | Siemann DW. The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by Tumor-Vascular Disrupting Agents. Cancer Treat Rev. 2011;37:63-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 483] [Cited by in RCA: 451] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 5. | Padhani AR, Miles KA. Multiparametric imaging of tumor response to therapy. Radiology. 2010;256:348-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 166] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 6. | Thoeny HC, De Keyzer F, Chen F, Vandecaveye V, Verbeken EK, Ahmed B, Sun X, Ni Y, Bosmans H, Hermans R. Diffusion-weighted magnetic resonance imaging allows noninvasive in vivo monitoring of the effects of combretastatin a-4 phosphate after repeated administration. Neoplasia. 2005;7:779-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Thoeny HC, De Keyzer F, Vandecaveye V, Chen F, Sun X, Bosmans H, Hermans R, Verbeken EK, Boesch C, Marchal G. Effect of vascular targeting agent in rat tumor model: dynamic contrast-enhanced versus diffusion-weighted MR imaging. Radiology. 2005;237:492-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 131] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 8. | Thoeny HC, De Keyzer F, Chen F, Ni Y, Landuyt W, Verbeken EK, Bosmans H, Marchal G, Hermans R. Diffusion-weighted MR imaging in monitoring the effect of a vascular targeting agent on rhabdomyosarcoma in rats. Radiology. 2005;234:756-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 123] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Sun X, Wang H, Chen F, De Keyzer F, Yu J, Jiang Y, Feng Y, Li J, Marchal G, Ni Y. Diffusion-weighted MRI of hepatic tumor in rats: comparison between in vivo and postmortem imaging acquisitions. J Magn Reson Imaging. 2009;29:621-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Chandarana H, Lee VS, Hecht E, Taouli B, Sigmund EE. Comparison of biexponential and monoexponential model of diffusion weighted imaging in evaluation of renal lesions: preliminary experience. Invest Radiol. 2011;46:285-291. [PubMed] |
| 11. | Yamada I, Aung W, Himeno Y, Nakagawa T, Shibuya H. Diffusion coefficients in abdominal organs and hepatic lesions: evaluation with intravoxel incoherent motion echo-planar MR imaging. Radiology. 1999;210:617-623. [PubMed] |
| 12. | Kivivuori SM, Riikonen P, Valanne L, Lönnqvist T, Saarinen-Pihkala UM. Antiangiogenic combination therapy after local radiotherapy with topotecan radiosensitizer improved quality of life for children with inoperable brainstem gliomas. Acta Paediatr. 2011;100:134-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Chen F, Sun X, De Keyzer F, Yu J, Peeters R, Coudyzer W, Vandecaveye V, Landuyt W, Bosmans H, Van Hecke P. Liver tumor model with implanted rhabdomyosarcoma in rats: MR imaging, microangiography, and histopathologic analysis. Radiology. 2006;239:554-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Chen F, De Keyzer F, Wang H, Vandecaveye V, Landuyt W, Bosmans H, Hermans R, Marchal G, Ni Y. Diffusion weighted imaging in small rodents using clinical MRI scanners. Methods. 2007;43:12-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Koh DM, Collins DJ, Orton MR. Intravoxel incoherent motion in body diffusion-weighted MRI: reality and challenges. AJR Am J Roentgenol. 2011;196:1351-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 428] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 16. | van Heeckeren WJ, Sanborn SL, Narayan A, Cooney MM, McCrae KR, Schmaier AH, Remick SC. Complications from vascular disrupting agents and angiogenesis inhibitors: aberrant control of hemostasis and thrombosis. Curr Opin Hematol. 2007;14:468-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Reck M, Gatzemeier U. Targeted therapies: Thalidomide in lung cancer therapy-what have we learned? Nat Rev Clin Oncol. 2010;7:134-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Wang H, Sun X, Chen F, De Keyzer F, Yu J, Landuyt W, Vandecaveye V, Peeters R, Bosmans H, Hermans R. Treatment of rodent liver tumor with combretastatin a4 phosphate: noninvasive therapeutic evaluation using multiparametric magnetic resonance imaging in correlation with microangiography and histology. Invest Radiol. 2009;44:44-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Figg WD, Li H, Sissung T, Retter A, Wu S, Gulley JL, Arlen P, Wright JJ, Parnes H, Fedenko K. Pre-clinical and clinical evaluation of estramustine, docetaxel and thalidomide combination in androgen-independent prostate cancer. BJU Int. 2007;99:1047-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Chen F, Feng Y, Zheng K, De Keyzer F, Li J, Feng Y, Cona MM, Wang H, Jiang Y, Yu J. Enhanced antitumor efficacy of a vascular disrupting agent combined with an antiangiogenic in a rat liver tumor model evaluated by multiparametric MRI. PLoS One. 2012;7:e41140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Stewart EE, Sun H, Chen X, Schafer PH, Chen Y, Garcia BM, Lee TY. Effect of an angiogenesis inhibitor on hepatic tumor perfusion and the implications for adjuvant cytotoxic therapy. Radiology. 2012;264:68-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Wang H, Li J, Chen F, De Keyzer F, Yu J, Feng Y, Nuyts J, Marchal G, Ni Y. Morphological, functional and metabolic imaging biomarkers: assessment of vascular-disrupting effect on rodent liver tumours. Eur Radiol. 2010;20:2013-2026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Rheinheimer S, Stieltjes B, Schneider F, Simon D, Pahernik S, Kauczor HU, Hallscheidt P. Investigation of renal lesions by diffusion-weighted magnetic resonance imaging applying intravoxel incoherent motion-derived parameters--initial experience. Eur J Radiol. 2012;81:e310-e316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 24. | Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161:401-407. [PubMed] |
| 25. | Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168:497-505. [PubMed] |
| 26. | Le Bihan D. Intravoxel incoherent motion perfusion MR imaging: a wake-up call. Radiology. 2008;249:748-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 245] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 27. | Takahara T, Kwee TC. Low b-value diffusion-weighted imaging: emerging applications in the body. J Magn Reson Imaging. 2012;35:1266-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Guiu B, Petit JM, Capitan V, Aho S, Masson D, Lefevre PH, Favelier S, Loffroy R, Vergès B, Hillon P. Intravoxel incoherent motion diffusion-weighted imaging in nonalcoholic fatty liver disease: a 3.0-T MR study. Radiology. 2012;265:96-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 133] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 29. | Pang Y, Turkbey B, Bernardo M, Kruecker J, Kadoury S, Merino MJ, Wood BJ, Pinto PA, Choyke PL. Intravoxel incoherent motion MR imaging for prostate cancer: an evaluation of perfusion fraction and diffusion coefficient derived from different b-value combinations. Magn Reson Med. 2013;69:553-562. [PubMed] |