Published online Dec 21, 2013. doi: 10.3748/wjg.v19.i47.8831
Revised: November 2, 2013
Accepted: November 18, 2013
Published online: December 21, 2013
Processing time: 111 Days and 19.2 Hours
Hepatocellular carcinoma (HCC) represents a global health problem. Infections with hepatitis B or C virus, non-alcoholic steatohepatitis disease, alcohol abuse, or dietary exposure to aflatoxin are the major risk factors to the development of this tumor. Regardless of the carcinogenic insult, HCC usually develops in a context of cirrhosis due to chronic inflammation and advanced fibrosis. Galectins are a family of evolutionarily-conserved proteins defined by at least one carbohydrate recognition domain with affinity for β-galactosides and conserved sequence motifs. Here, we summarize the current literature implicating galectins in the pathogenesis of HCC. Expression of “proto-type” galectin-1, “chimera-type” galectin-3 and “tandem repeat-type” galectin-4 is up-regulated in HCC cells compared to their normal counterparts. On the other hand, the “tandem-repeat-type” lectins galectin-8 and galectin-9 are down-regulated in tumor hepatocytes. The abnormal expression of these galectins correlates with tumor growth, HCC cell migration and invasion, tumor aggressiveness, metastasis, postoperative recurrence and poor prognosis. Moreover, these galectins have important roles in other pathological conditions of the liver, where chronic inflammation and/or fibrosis take place. Galectin-based therapies have been proposed to attenuate liver pathologies. Further functional studies are required to delineate the precise molecular mechanisms through which galectins contribute to HCC.
Core tip: Galectins, a family of glycan-binding proteins, are involved in the pathogenesis of hepatocellular carcinoma (HCC). Up-regulation of galectin-1, galectin-3 and galectin-4 is observed in HCC cells, whereas galectin-8 and galectin-9 appear to be down-regulated in tumor hepatocytes. This altered expression correlates with tumor growth, HCC cell migration and invasion, tumor aggressiveness, metastasis, postoperative recurrence and poor prognosis. These galectins are also implicated in inflammation- and fibrosis-related liver pathologies.
- Citation: Bacigalupo ML, Manzi M, Rabinovich GA, Troncoso MF. Hierarchical and selective roles of galectins in hepatocarcinogenesis, liver fibrosis and inflammation of hepatocellular carcinoma. World J Gastroenterol 2013; 19(47): 8831-8849
- URL: https://www.wjgnet.com/1007-9327/full/v19/i47/8831.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i47.8831
Hepatocellular carcinoma (HCC) represents a global health problem. It is the fifth most common solid tumor and the third cause of cancer-related mortality per year[1]. HCC is most prevalent in Eastern Asia and sub-Saharan Africa; whereas the incidence in Europe and North America is considerably lower[2-4]. The etiology of HCC includes major risk factors such as infection with Hepatitis B or C virus (HBV, HCV), alcohol abuse or dietary exposure to aflatoxin[5-7]. Regardless of the carcinogenic insult, HCC usually develops in patients with cirrhosis due to chronic inflammation and advanced fibrosis[8]. Non-alcoholic steatohepatitis (NASH), a metabolic disorder resulting from insulin resistance syndrome that underlies fibrosis and cirrhosis, is emerging as another important risk factor for HCC[9,10].
During the past decade the management of HCC has significantly improved[11]. New advances in the field have led to a better knowledge and an earlier detection of this disease. Additionally, current therapies such as, resection, transplantation, ablation and chemoembolization, have provided benefit to patients diagnosed at early HCC stages improving and extending their survival[12-14]. However, most patients are diagnosed at advanced stages and therefore, they are not amenable to surgical treatment. Even after resection or transplantation, the prognosis remains unsatisfactory due to recurrence, metastasis and the development of new primary tumors[15-17].
Recent progress toward a better understanding of the molecular biology of HCC has allowed the development of molecular targeted therapies and has shed light on new systemic therapies for HCC. Several intracellular signaling pathways involved in abnormal proliferation, survival, differentiation, invasion and metastasis have been found to be dysregulated in HCC. Clinical trials are currently testing the potential use of inhibitors of the Ras/Raf/mitogen-activated protein kinase/extracellular signal-regulated kinase (ERK), phosphatase and tensin homolog deleted on chromosome 10/phosphoinositide 3-kinase (PI3K)/Akt/mammalian target of rapamycin, transforming growth factor β (TGF-β), Wnt/β-catenin and epidermal growth factor receptor (EGFR) pathways, among others[18-20]. Sorafenib, a receptor tyrosine kinase inhibitor targeting vascular endothelial growth factor, platelet-derived growth factor and Raf signaling pathways prolongs survival in patients with advanced unresectable HCC[21,22]. Simultaneously, new immunotherapy strategies are being developed for the treatment of HCC, which could be administered in combination with conventional therapies in order to obtain a more favorable clinical outcome[23]. Undoubtedly, the approval of oral administration of sorafenib highlights the the importance of elucidating the molecular mechanisms underlying HCC progression for the development of novel therapies.
Recently, there has been increasing evidence highlighting the involvement of galectins, a family of glycan-binding proteins, in the pathogenesis of HCC. In this review, we present emerging data showing that expression of some members of this family is altered in HCC cell lines and tissues compared to normal liver. These observations led to the proposition that galectins are potential prognostic biomarkers and therapeutic targets in HCC. We will discuss the possible roles of these proteins in HCC tumor transformation, progression, aggressiveness and metastasis. Moreover, we will highlight the involvement of galectins in other pathological settings of the liver, where chronic inflammation and/or fibrosis take place.
Galectins are a family of evolutionary conserved glycan-binding proteins or lectins that recognize multiple N-acetyllactosamine (Galβ1,4GlcNAc) units on cell surface glycoconjugates. These animal proteins are defined by at least one carbohydrate recognition domain (CRD) with affinity for β-galactosides and conserved sequence motifs[24]. To date, fifteen galectins have been described in mammals and according to their structural characteristics they are classified into three groups: “proto-type” galectins (galectin-1, galectin-2, galectin-5, galectin-7, galectin-10, galectin-11, galectin-13, galectin-14 and galectin-15) contain one CRD and can dimerize; “tandem repeat-type” galectins (galectin-4, galectin-6, galectin-8, galectin-9 and galectin-12) contain two distinct CRD in tandem, connected by a linker peptide; and “chimera-type” galectin-3 which consists of unusual proline- and glycine-rich short stretches fused onto the CRD[25,26].
Some galectins (e.g., galectin-1, galectin-3 and galectin-9) are widely expressed among different tissues including, immune cells, endothelial and epithelial cells, and sensory neurons (reviewed by[27-29]); whereas other family members have a more restricted tissue localization and compartmentalization (e.g., galectin-7 is preferentially found in the skin, galectin-12 is abundantly expressed in adipose tissue, galectin-5 is restricted to rat reticulocytes, and galectin-10 is strongly represented in human but not mouse eosinophils)[27].
These lectins do not possess a signal peptide for export through the classical secretory pathway (Golgi-endoplasmic reticulum); however they are secreted to the extracellular milieu via a non-conventional poorly understood secretory pathway[30-32]. For instance, nonclassical secretion of galectin-1 has been observed in skeletal muscle during in vivo development and in cultured myoblasts during differentiation[33]. Besides, secretion of galectin-3 from macrophages, renal and polarized intestinal epithelial cells has been detected[34,35]. There is also evidence for secretion of galectin-9 in activated Jurkat T cells[36] and CD4 T cells expressing galectin-9 on the cell surface upon T cell receptor stimulation[37].
Through its binding to N-acetyllactosamine sequences, galectins form multivalent complexes with cell surface glycoconjugates and thus, transmit signals inside the cell[38-40]. Remarkably, it has also been demonstrated that galectin-1 can be internalized by Jurkat T cells in a carbohydrate-dependent mechanism, following dual pathways involving clathrin-coated vesicles and raft-dependent endocytosis[41]. Within the intracellular milleu, galectins bind to their ligands preferentially through protein-protein interactions, and regulate intracellular processes, including mRNA splicing, cell cycle progression, apoptosis, and cell proliferation[42].
Galectins have emerged as pivotal regulators of cellular physiology. Over the past decade, multiple biological functions have been reported for this protein family including roles in cell adhesion, migration, cytokine synthesis, and survival[43,44]. In fact, different members of the family have shown critical roles as mediators of acute and chronic inflammation[45,46]. Galectins are often aberrantly expressed in many different tumor types including astrocytoma, melanoma and prostate, thyroid, colon, head and neck, bladder, kidney, stomach, lung, bladder, uterine, breast and ovary carcinomas[27,47,48]. Moreover, mounting evidence indicates that these proteins play fundamental roles in cancer biology including tumor transformation, tumor growth, angiogenesis, migration, metastasis and tumor-immune escape[49-52]. Given these pleiotropic activities in the tumor microenvironment, galectins are being increasingly recognized as molecular targets for innovative cancer therapy[26,52-56].
In this review, we summarize the current data implicating galectins in HCC. Particularly, we focus our discussion on selected members of the family, including galectin-1, galectin-3, galectin-4, galectin-8 and galectin-9, which roles in HCC biology have been demonstrated.
The first protein discovered within the galectin family was galectin-1. This galectin possesses one CRD and can form homodimers via non-covalent binding, which confers the ability to cross-link specific glycoconjugates[26,28]. Galectin-1 displays features of typical cytoplasmic proteins; it has been described in nucleus and cytoplasm and can translocate to the intracellular face of cellular membranes. Although galectin-1 lacks a recognizable secretion signal sequence, it is secreted through a non-conventional secretory pathway[31,32]; thus being detected on the extracellular side of cellular membranes as well as in the extracellular matrices (ECM) of various normal and neoplastic tissues[57].
While the role of galectin-1 within the intracellular milieu is often independent of its lectin activity, its extracellular functions are mostly dependent on the binding to N-acetyllactosamine units on cell surface glycoconjugates[28]. Intracellularly, galectin-1 is engaged in fundamental processes such as pre-mRNA splicing; and also it interacts with oncogenic H-RAS and contributes to its membrane anchorage, evidencing a key role for this galectin in driving tumor transformation (reviewed by[49,58]). In the extracellular space, galectin-1 binds to glycoconjugates on the cell surface, including different members of the integrin family and glycoproteins of the ECM such as laminin and fibronectin[59,60]. It is likely that the local abundance of galectin-1 in the tumor microenvironment may play a critical role during attachment or detachment of cancer cells throughout cancer progression[43]. Furthermore, galectin-1 promotes cell migration, a function that correlates with the ability of this protein to influence tumor progression, invasion and angiogenesis. However, the biological roles of galectin-1 appear to be tissue-specific as it also decreases cell migration of most immune cells providing a rational basis for its anti-inflammatory properties[43,45,55,61].
Expression of galectin-1 has been well documented in many different tumor types including astrocytoma, melanoma and prostate, thyroid, colon, bladder and ovary carcinomas[57,62]. Moreover, preferential accumulation of galectin-1 in the peritumoral stroma has been described for thyroid, head and neck, colon, ovary and prostate carcinoma[57]. Functions of galectin-1 during tumor progression have been largely documented in the literature. High levels of galectin-1 correlate with aggressiveness of tumors[63-67], and the acquisition of a metastatic phenotype[68-71]. This lectin plays a fundamental role in tumor angiogenesis by modulating endothelial cell biology[72,73] and its expression is induced by hypoxia[74,75]. Importantly, galectin-1 has been proposed to be a major immunosuppressive factor which contributes to tumor immunoevasive programs[76,77]. In fact, galectin-1 expression by tumor cells or by their surrounding stroma can regulate the function, fate and viability of infiltrating tumor-specific T cells[78].
Galectin-1 gene (LGALS1) regulation was extensively studied using the well characterized system hepatoma x fibroblast hybrids. Activation of gene expression was achieved by treatment of galectin-1-non-expressing cells with the DNA demethylating agent azacytidine. The methylation status of the galectin-1 gene promoter was identified as a central mechanism that controls gene expression in normal tissues and also in transformed cells and tumors[27].
While in normal liver galectin-1 is expressed at low constitutive levels, in HCC its expression is dramatically up-regulated[79-82]. Gene expression profiling of normal and HCC human tissues using cDNA microarrays allowed the identification of LGALS1 as one of the hallmark genes that are over-expressed in HCC, a phenomenon which was further confirmed by RT-PCR[79].
Kondoh et al[80] elucidated the molecular mechanism governing LGALS1 gene expression in liver malignancy. This group investigated the methylation states of the galectin-1 gene promoter in human HCC and adjacent non-tumor liver tissue, and in different HCC cell lines. Analysis of the methylation profile revealed that certain CpG dinucleotides surrounding the transcription start site of LGALS1 promoter were frequently methylated in non-tumor liver, whereas these sequences were hypomethylated in HCC tissues. Interestingly, using a mobility shift assay with nuclear extracts from three HCC cell lines (HLF, HuH7, and HepG2) as well as human embryonic primary liver (PL) cells, the authors showed specific interaction of a methylation-sensitive factor to the upstream and downstream regulatory elements which appear to be essential for the activation of the LGALS1 gene in HCC cells[80]. Northern blot analysis demonstrated that galectin-1 mRNA was up-regulated in primary HCC in comparison to adjacent non-tumor liver tissues and human normal liver tissues. In fact, galectin-1 mRNA level was higher in the HuH-7 and HLF HCC cell lines as compared to HepG2 and PL cells[80].
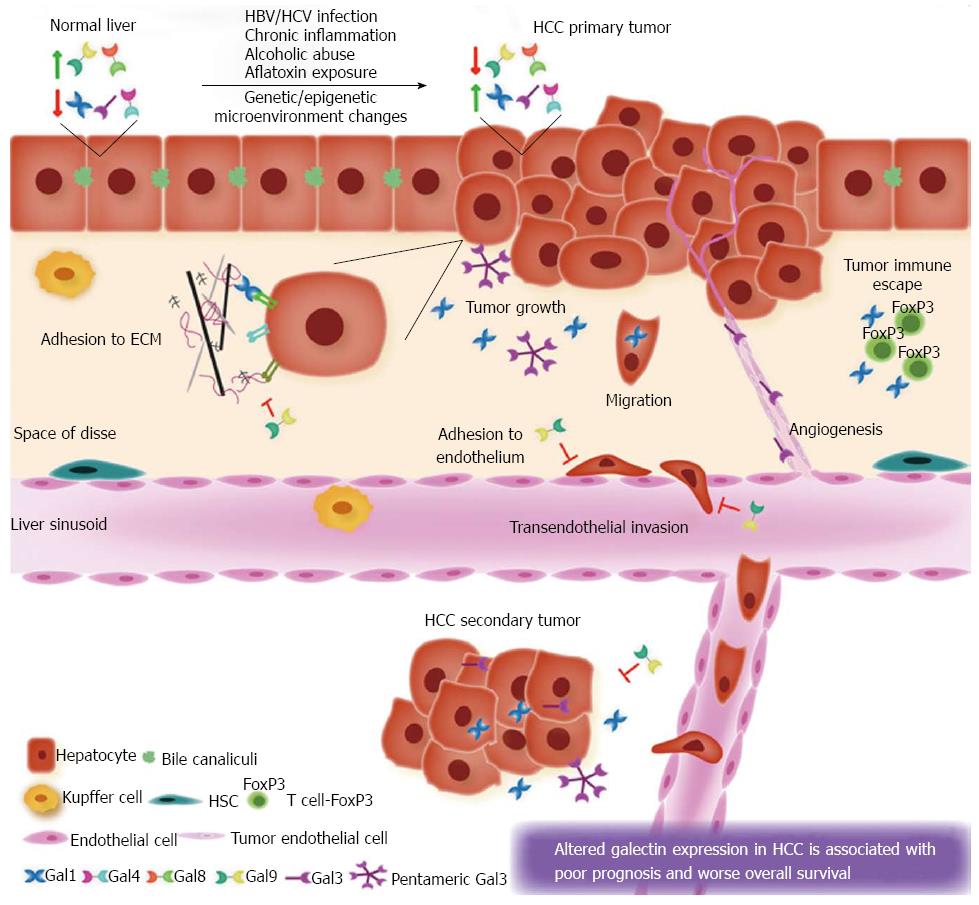
Although over-expression of galectin-1 was observed in HCC in vivo as well as in vitro, the precise function of this endogenous lectin in liver pathophysiology remained uncertain for many years. However, emerging findings shed light to the role to the leading role of galectin-1 in HCC development and progression. Spano et al[81] reported that galectin-1 expression was significantly increased in HCC samples from patients with metastatic disease compared to those harboring a non-metastatic primary tumor. However, no significant associations were found with other parameters, although a trend toward an association between increased galectin-1 expression in HCC and vascular invasion was observed. Moreover, galectin-1 expression profile was also examined in human HuH-7 and JHH-6 HCC cells and human normal liver, cirrhotic tissue and HCC specimens using tissue microarrays. In all cases, increased expression of the LGALS1 gene was confirmed in HCC. Furthermore, immunohistochemical analysis revealed a preferential accumulation of galectin-1 in the delicate stroma tissue surrounding tumor hepatocytes of HCC tumors. The authors hypothesized that neoplastic hepatocytes secrete galectin-1 which is then accumulated in the stroma surrounding HCC (Figure 1 and Table 1).
| Galectin member | Expression | Function and/or effect | Model | Ref. |
| Galectin-1 | Up-regulated (mRNA and protein) in HCC, secreted by tumor hepatocytes and accumulated in stroma surrounding HCC | Correlates with tumor aggressiveness, metastases and enhanced risk of post-operative recurrence | Human HCC tissues | [79-82] |
| Favors HCC cell adhesion to ECM, cell migration and invasion | Human HCC cell lines | [81,85] | ||
| Increases tumor growth and metastasis in draining-tumor lymph nodes | Nude mice injected with galectin-1 over-expressing HepG2 cells | [85] | ||
| Possible role in the suppression of antitumor immune responses | Human HCC tissues | [82] | ||
| Galectin-3 | Up-regulated (mRNA and protein) in HCC. Transactivation of murine LGALS3 promoter can occur by HBV-X protein. | Human HCC tissues and cell lines | [79,124,125] | |
| High nuclear expression | Correlates with histological differentiation and vascular invasion | Human HCC tissues | [126] | |
| Up-regulated in HCC-associated capillary endothelial cells | Probably promotes angiogenesis | Tumor-associated endothelial cells isolated from rats | [128] | |
| Galectin-4 | Higher expression in HCC than normal tissues | Human HCC tissues and cell lines | [154] | |
| Galectin-8 | Diminished expression in hepatoblastoma and hepatocarcinoma | Human HCC tissues | [159] | |
| Galectin-9 | Downregulated in HCC | Galectin-9 suppression promotes cell proliferation and adhesion to ECM, tumor cell-endothelial cell adhesion and trans-endothelial invasion of HepG2 cells. | Human cell lines | [181] |
| Downregulation of galectin-9 represents a risk factor for patient survival, correlates with tumor histopathological grade, vascular invasion and metastasis | Human HCC tissues | [181] |
The correlation between increased expression of galectin-1 in HCC and the presence of metastasis was validated by in vitro functional studies. Expression of LGALS1 gene and secretion of galectin-1 protein were substantially up-regulated in JHH-6 (undifferentiated cells) and HuH-7 (differentiated cells). Notably, galectin-1 over-expression increased the migratory and invasive capacities of HuH-7 cells, and both processes were mediated by the stimulation of the Sky receptor tyrosine kinase (RTK) phosphorylation. Thus, similar to breast cancer[68], neuroblastoma[83], oral squamous cell carcinoma and lung adenocarcinoma[84], galectin-1 expression correlates with HCC tumor aggressiveness (Figure 1 and Table 1).
Under this scenario, we have focused our attention on the role of galectin-1 and its contribution to HCC development. In this regard, we examined the involvement of this galectin in HepG2 HCC cell adhesion and tumor growth[85]. We found that galectin-1 acts as a glycan-dependent matricellular modulator of HepG2 cell adhesion. We observed that galectin-1 favored cell adhesion to laminin, a polylactosamine-enriched glycoprotein and a major component of the ECM and basement membranes. Moreover, we demonstrated that the pro-adhesive effects of galectin-1 are specifically mediated by α1, α2, α3, αv, and β1 integrins and involve PI3K and/or ERK1/2 signaling pathways. Besides, galectin-1 over-expressing HepG2 cells showed an increased secretion of this lectin to the extracellular compartment and remarkably, we also found that exogenously added recombinant galectin-1 was internalized by HepG2 cells[85]. Hence, in accordance with Spano et al[81], galectin-1 secreted from HCC cells might exert its biological functions either by engaging cell surface receptors and transmitting signals inside the cell or through receptor-mediated internalization and endocytosis. However, because intracellular functions have also been described for this protein[42] a cell surface-independent mechanism responsible for galectin-1 functions cannot be excluded. We also found that galectin-1 up-regulation in the tumor microenvironment favored HCC growth in vivo and promoted a considerable increase in tumor metastasis. This effect was evident in draining-tumor lymph nodes of mice injected with galectin-1 over-expressing HepG2 cells[85]. Collectively, these results suggested the involvement of galectin-1 in neoplastic and inflammatory processes of the liver (Figure 1 and Table 1).
Compelling evidence indicates that high expression of galectin-1 predicts poor patient outcome in a variety of tumors. However, the prognostic value of this endogenous lectin in HCC patients remained elusive for many years. Recently, Wu et al[82] reported that elevated galectin-1 expression in HCC is significantly associated with tumor aggressiveness (vascular invasion, incomplete encapsulation, poor differentiation, and large tumor size) and enhanced risk of post-operative recurrence. Additionally, galectin-1 expression in HCC was also associated with early tumor recurrence (≤ 24 mo) and dissemination of primary tumor cells. Furthermore, a positive correlation was observed between galectin-1 expression and tumor-infiltrating FoxP3+ regulatory T cells (Tregs) in HCC samples from a large, random HCC cohort. In line with this evidence, it has been demonstrated that galectin-1 is a key regulator of murine CD4+CD25+ regulatory Tregs[86] which play an essential role in suppression of anticancer immunity[87]. Taken this information into account it is possible to speculate that interaction between galectin-1 and Treg cells might play a role in the suppression of antitumor immune responses against HCC (Figure 1 and Table 1).
The immunomodulatory activities of galectin-1 in the liver were also investigated in a model of hepatitis induced by injection of concanavalin A (Con A) into mice, which leads to a dose-dependent injury in the liver[88]. T-cell activation is a crucial event in this model as shown by resistance to this inflammatory disease of mice lacking T and B lymphocytes. Furthermore, pretreatment with anti-interferon γ (IFN-γ) or anti-tumor necrosis factor (TNF) monoclonal antibodies conferred protection against Con A-induced liver injury, indicating that Th1-dependent cytokines are involved in this inflammatory disease. Interestingly, it has been demonstrated that galectin-1 exerts a protective role on Con A-induced autoimmune hepatitis in mice (Table 2)[89].
| Galectin member | Experimental model | Role | Effects | Ref. | |
| Galectin-1 | Hepatitis induced by injection of Con A | Protective | Prevents both liver injury and T-helper cell liver infiltration, induces apoptosis of Con A-activated T cells, suppresses plasma levels of TNF and IFN-γ | [89] | |
| Inflammation-induced chronic cholestatic hepatitis at an early age, and HCC at later age (Mdr2-KO mice) | Protective | Galectin-1 is up-regulated in Mdr2-KO/B6 strain at early age | [91] | ||
| Galectin-1-KO mice in the context of Con A-induced autoimmune hepatitis | Protective | Con A up-regulates galectin-1 in galectin-1-KO/B6 and Mdr2-KO/FVB strains. Endogenous galectin-1 selectively protects liver in the B6, but not in the FVB genetic background. It probably determines strain-specific differences in the course of chronic hepatitis and HCC development in the Mdr2-KO model | [91] | ||
| Galectin-3 | NASH model | Galectin-3-KO mice | Protective | Develops NAFLD/NASH spontaneously with aging | [140,141] |
| CDAA diet-induced NAFLD/NASH in galectin-3-KO mice | Protective | Galectin-3 deficiency causes more severe hepatic injury and alterations in the expression of genes associated with carcinogenesis and lipid metabolism | [142] | ||
| Atherogenic diet-induced NASH in galectin-3-KO mice | Promotes disease severity | Attenuates NASH: inhibits HSC-driven fibrosis, reduces inflammatory-cell infiltration and hepatocyte apoptosis, acts as a major scavenger receptor involved in ALE/AGE uptake by the liver | [143] | ||
| Human liver tissues | Protective | Negative expression of galectin-3 in normal hepatocytes, strong staining for galectin-3 in hepatocytes from patients with steatosis hepatitis, hepatitis, cholestasis and cirrhosis | [145] | ||
| Acute liver failure induced by APAP- hepatotoxicity in galectin-3-KO mice | Perpetuates liver injury | In wild type mice, galectin-3 is up-regulated in liver infiltrating macrophages. In galectin-3 deficient mice the pro-inflammatory M1-type macrophages subpopulation, the classical macrophage activation markers iNOS, TNF and IL-12 and pro-inflammatory chemokines are reduced | [147,148] | ||
| Hepatitis induced by injection of Con A in galectin-3-KO mice | Pro-inflammatory | Galectin-3 deficiency reduces the number of T lymphocytes, B lymphocytes, dendritic cells, NK and NKT cells and enhances apoptosis of mononuclear cells | [149] | ||
| Con A-induced liver injury in wild type mice pretreated with a selective inhibitor of galectin-3 (TD139) | Pro-inflammatory | TD139 attenuates liver injury, reduces the number of CD4+ and CD8+ T cells, favors the influx of IL-10-producing CD4+ T cells in the liver, decreases serum levels of IFN-γ, IL-17 and IL-4 | [149] | ||
| Galectin-9 | Blockade of the TIM-3/galectin-9 pathway using an anti-TIM-3 or anti-galectin-9 mAb in a context of liver IRI | Protective | Blockade of the TIM-3/galectin-9 pathway increases hepatocellular damage, local neutrophil infiltration, T cell and macrophage accumulation and liver cell apoptosis. Increases IFN-γ production by Con A-stimulated spleen T cells and augmented TNF and IL-6 production by Con A-stimulated macrophages/T cells | [190] | |
| Single injection of galectin-9 in the murine model of liver injury induced by Con A | Protective | Eliminates activated CD4+ effector T cells, prevents the synthesis and/or release of proinflammatory cytokine | [191] | ||
| Mouse model of diet-induced NAFLD treated with galectin-9 | Limits the inflammatory response | Induces apoptosis of NKT cells, also interacts with TIM-3-expressing Kupffer cells to induce secretion of IL-15, thus promoting NKT cell proliferation | [195] | ||
Recently, the protective role of galectin-1 in the liver inflammatory response was investigated using the Mdr2-knockout (Mdr2-KO) mice as a model of inflammation-induced chronic cholestatic hepatitis at an early age, and HCC at a later age, which together mimic the evolution of human disease[90]. Potikha et al[91] demonstrated that HCC development was retarded in Mdr2-KO/B6 strain compared to Mdr2-KO/FVB mice. Interestingly, up-regulation of galectin-1 transcript in the liver of Mdr2-KO/B6 mice was observed[91]. To highlight the relevance of the endogenous protein galectin-1-KO/B6 mice were used in the context of Con A-induced autoimmune hepatitis. The results demonstrated that endogenous galectin-1 selectively protects against Con A-induced liver injury in B6 mice (Table 2)[91].
Collectively, these data indicated that galectin-1 has an important role in HCC tumor growth, aggressiveness and metastasis (Figure 1 and Table 1). Moreover, they suggest that galectin-1 may act as a protective anti-inflammatory agent at early stages of the chronic liver pathology during inflammation-induced hepatocarcinogenesis, but as a pro-tumorigenic agent at late stages of the disease.
Hepatic fibrosis is the physiological result of the wound-healing response of the liver to repeated injury. This process is associated with an inflammatory response and a limited deposition of ECM. If the hepatic injury persists (e.g., chronic viral hepatitis), and eventually the liver regeneration fails, hepatocytes are substituted with abundant ECM, including fibrillar collagen[92]. Kristense et al[93] conducted a proteome analysis on cellular and secreted proteins of normal (quiescent) and activated rat hepatic stellate cells (HSCs), the main ECM-producing liver cells. These researchers found that galectin-1 was up-regulated in both in vivo and in vitro activated HSCs, and in fibrotic liver tissues[93]. When the biological role of galectin-1 was investigated in HSCs, it was found that this lectin stimulated the proliferation rate and migratory activity of cultured HSCs through carbohydrate-dependent mechanisms (Table 3)[94]. These data clearly indicated that galectin-1 has an important role in the development of liver fibrosis.
| Galectin Member | Expression | Function and/or effect | Model | Ref. |
| Galectin-1 | Over-expressed in activated HSCs | Induces proliferation of HSCs via ERK 1/2 through CRD domain | HSCs activated in vitro (cultured on plastic for several days) and in vivo (isolated form rats treated with CCl4 or with bile duct ligation) | [93,94] |
| Positive in ICC cells, intracellular expression and secretion | Correlates with histologic dedifferentiation, vascular invasion, and lymph node metastasis | ICC tissue samples, CCKS1 cholangiocarcinoma cell line | [96] | |
| Galectin-3 | Over-expressed in activated HSCs | Induces proliferation via ERK 1/2 involving PKA and PKC pathwaysDependent on CRD domain | HSCs activated in vitro (cultured on plastic for several days) and in vivo (isolated form rats treated with bile duct ligation) | [94] |
| Intracellular Gal3 is required for activation of HSCs via TGF-β | HSCs activated in vivo (isolated form rats treated with CCl4) | [132] | ||
| Extracellular Gal3 required for activation of HSCs. Integrin and CRD dependent effect | HSCs activated in vivo (isolated from rats with bile duct ligation) | [134] | ||
| NFκβ induces expression and secretion of Gal3 in activated HSCs | ||||
| Up-regulated in injured/cirrhotic hepatocytes | Poor liver function | Human fibrotic liver samples and extracts from rats treated with CCl4 | [124,133,136] | |
| Related to the preneoplastic and early neoplastic stages of ICC | ICC tissue samples | [96,137] | ||
| Positive in ICC cells | Intracellular expression is associated withanti-apoptotic activity and resistance to chemotherapeutic agents | ICC cell lines | [138] |
By immunohistochemistry, galectin-1 expression was also assessed in the intrahepatic biliary tree. The intrahepatic biliary epithelial cells or cholangiocytes are involved in modifying the bile of canalicular origin. Cholangiocarcinoma occurs frequently associated with inflammation and fibrosis of bile ducts, and is caused by multiple factors including autoimmune, bacterial, congenital, drug, or viral agents[95]. In normal livers, Shimonishi et al[96] observed that intrahepatic bile ducts and hepatocytes did not express galectin-1. Remarkably, 73 % of the intrahepatic cholangiocarcinoma (ICC) samples analyzed were positive for galectin-1[96]. Expression of this lectin significantly correlated with histologic dedifferentiation of ICC, vascular invasion, and lymph node metastasis of ICC[96]. These results suggest that galectin-1 over-expression in ICC cells is associated with neoplastic progression and tumor cell proliferation (Table 3).
These results highlight an important role of galectin-1 in chronically injured liver and its involvement in inflammation and fibrosis of bile ducts, thus providing the basis for the development of effective therapies based on the modulation of galectin-1-glycan interactions.
Galectin-3 is the unique ‘‘chimera-type’’ galectin containing three structurally distinct domains, an atypical N-terminal domain that includes a serine phosphorylation site, important for the regulation of intracellular signaling, a collagen-like sequence sensitive to proteolysis by MMP-2 and MMP-9 matrix metalloproteinases and a C-terminus containing one carbohydrate-recognition domain (CRD) containing an Asp-Trp-Gly-Arg motif. This sequence motif is also present in members of the B-cell lymphoma 2 (Bcl-2) family of apoptosis regulators, and is responsible for the antiapoptotic activity of galectin-3[97]. In solution, galectin-3 largely occurs as a monomer[98]. Although in the absence of its binding partners it can form homodimers by self-association through its CRDs[99], in the presence of carbohydrate ligands, galectin-3 can polymerize up to pentamers through its N-terminal domain[99,100].
Galectin-3 is mainly localized at the cytoplasmic compartment, but it is also present within the nucleus, in the cell surface or in the extracellular space[29,101]. Translocation of this lectin from the cytoplasm to the nucleus is mediated by its N-terminal domain[102], whilst translocation from nucleus to the cytoplasm involves a nuclear export sequence located within its CRD[103] and occurs through nucleoporin NP98[104]. Notably, the N-terminal domain is also required for the secretion of the lectin to the extracellular milieu[105].
Galectin-3 has multiple and complex functions. In the cytoplasm, galectin-3 can bind to Bcl-2 and inhibit cellular apoptosis[97]. Also, it can interact with the activated K-Ras (K-Ras-GTP)[106,107] and affect Ras-mediated Akt signaling[108,109]. On the other hand, nuclear galectin-3 acts as a pre-mRNA splicing factor and is involved in spliceosome assembly[110] by forming protein complexes with Gemin4[111]. In the nucleus, Galectin-3 can also regulate gene transcription by enhancing transcription factor association with Spi1 and CRE elements in gene promoter sequences[29]. In addition, β-catenin, a molecule involved in Wnt signaling pathway, was also identified as a novel binding partner of galectin-3 in the nucleus[112].
On the other hand, extracellular galectin-3 mediates cell adhesion and activation and also acts as a chemoattractant for certain cell types[29]. It often forms multimers and thus, it cross-links cell surface ligands forming lattice-like structures which trigger cell signaling[29]. Galectin-3 has been shown to bind glycosylated components of the extracellular matrix, and cell-surface adhesion molecules like integrins[43]. Pro-apoptotic activity of extracellular galectin-3 was observed in several cell types, such as human T leukemia cell lines, human peripheral blood mononuclear cells, and activated mouse T cells[113].
Galectin-3 is widely expressed in human tissues, including immune cells, epithelial cells and sensory neurons (reviewed by[29]). This lectin regulates immune cell activities and contributes to immunosuppression as it induces monocyte and T-cell apoptosis, suppresses IL-15 production and inhibits B-cell differentiation[114,115]. In general, galectin-3 is a powerful pro-inflammatory signal as demonstrated by both in vitro and in vivo assays[29,116]. Extracellular galectin-3 has been demonstrated to activate and modulate the viability of immune and inflammatory cells, although the effects of Galectin-3 in T-cell survival are dependent on whether the protein is produced endogenously (anti-apoptotic) or is secreted to the extracellular medium (pro-apoptotic)[114,116].
Expression of galectin-3 and its intracellular distribution are frequently altered in cancer and pre-cancerous conditions[26], and it is evident that this lectin plays multiple roles in cancer pathogenesis, proliferation and spreading of metastasis[29,62,117]. Pre-clinical and clinical data indicate that expression of galectin-3 is associated with the carcinogenesis and malignant potential in melanoma, head and neck, thyroid, gastric, colon, uterine, and renal cancers[118]. In fact, galectin-3 contributes to tumorigenesis and tumor progression through several different mechanisms, including promotion of oncogenesis, angiogenesis, adhesion, invasion and metastasis[101,115].
The mechanisms of regulation of galectin-3 expression are still poorly understood. The promoter region of the human galectin-3 gene (LGALS3) contains several regulatory elements for activation by the SP1, AP-1, CREB, and NF-κB transcription factors[119]. In this regard, c-Jun, CREB, and NF-κB have been implicated in activation of the LGALS3 gene[29,49]. Galectin-3 expression is also regulated by methylation of CpG islands in the promoter region. It has been demonstrated that demethylation of LGALS3 promoter induces expression of galectin-3 in thyroid carcinoma[120,121]. Recently, Margadant et al[122] demonstrated that, in cells from epithelial origin, integrin β1 specifically triggers transcriptional activation of galectin-3 through a mechanism that involves demethylation of the LGALS3 promoter. Further, it has been shown that the cell-surface glycoprotein MUC1 controls galectin-3 expression in an epigenetic manner in cancer cells, through a miRNA-dependent mechanism[123].
Hsu and colleagues demonstrated using immunohistochemistry and immunoblot analysis, that normal hepatocytes do not express galectin-3; however this galectin is prominently up-regulated in HCC tissues and in HCC cell lines[124]. Increased expression of galectin-3 in HCC was independent of whether the patients were previously exposed to hepatitis B virus (HBV). However, galectin-3 expression in HCC was positively influenced by HBV infection through a mechanism that included transactivation of the murine LGALS3 gene promoter[124]. Accordingly, using cDNA microarray and gene expression profiling, Chung et al[79] reported the up-regulation of Galectin-3 in HCC human tissues with respect to their normal counterparts. Moreover, by analyzing gene expression patterns, Luo et al[125] also reported the overexpression of galectin-3 gene in HCC tissues respect to normal liver and adjacent non-tumoral tissues.
Interestingly, expression of galectin-3 correlated with histological differentiation and vascular invasion in HCC patients[126]. In particular, higher expression rate of nuclear galectin-3 denoted worse prognosis in this pathology and serum galectin-3 levels were found to be increased in HCC patients compared to those suffering chronic liver disease[126]. These results highlighted a central role for galectin-3 in HCC development and progression (Figure 1 and Table 1).
HCC is a hypervascular tumor in which angiogenesis plays a critical role. Tumor-associated capillary endothelial cells (TECs) in HCC are known to originate from liver sinusoid endothelial cells (SECs), which then undergo a capillarization process to become morphologically and functionally different TECs[127]. Using two-dimensional gel electrophoresis coupled to mass spectrometry, Jia et al[128] observed that galectin-3 is up-regulated in TECs, respect to SECs. This result validated by immunoblot and immunohistochemistry, demonstrated that galectin-3 is generally absent in liver SECs, but is significantly up-regulated in HCC TECs (Table 1)[128]. Further investigation is required to reveal whether galectin-3 produced in HCC TECs could influence HCC angiogenesis.
EGFR family is an important mediator of cancer cell transformation, proliferation, maintenance, and survival[129]. Paradoxically, high concentrations of epidermal growth factor (EGF) initiates different signaling cascades and mainly induces apoptosis of tumor cells expressing high levels of EGF receptor[130]. Recently, the role of galectin-3 in EGF-induced apoptosis on HepG2 cells was investigated[131]. Indeed, high concentrations of EGF inhibited proliferation and induced apoptosis of these cells, concomitantly with a reduced expression of galectin-3 at both mRNA and protein levels[131]. Also, high levels of EGF down-regulated the expression of cytoplasmic galectin-3. Remarkably, the reduced expression of galectin-3 in EGF-treated cells was associated with reduced phosphorylation of Akt and ERK. Moreover, over-expression of galectin-3 in HepG2 cells blocked EGF-induced growth inhibition and apoptosis[131]. Thus, cellular proliferation and/or apoptosis induced by EGF signaling pathway in HCC cells might rely on the expression levels of galectin-3.
Collectively, these results demonstrate that galectin-3 over-expression correlates with HCC progression (Figure 1 and Table 1) and suggest that this lectin could serve as a novel biomarker and therapeutic target in HCC.
Expression of galectin-3 is increased in liver fibrosis regardless of the initiating agent or disease process[94,132]. In vitro experiments and different experimental models of liver injury and fibrosis demonstrated that galectin-3 stimulated the proliferation rate of cultured activated HSCs and is also involved in myofibroblast activation, identifying galectin-3 as a potential therapeutic target in the treatment of liver fibrosis (Table 3)[94,132-134].
Liver fibrosis leads to progressive liver insufficiency, portal hypertension and ultimately to cirrhosis and/or HCC[135]. In patients with liver cirrhosis galectin-3 is not extracted by the liver[136], and also, its expression is induced in hepatocytes of cirrhotic liver[124,136]. Furthermore, galectin-3 was negatively associated with liver function in patients with alcoholic liver cirrhosis, an effect which might be partly explained by the impaired hepatic removal and/or by higher hepatic synthesis of galectin-3 (Table 3)[136].
As mentioned before, cholangiocarcinoma frequently occurs in a context of inflammation and fibrosis of bile ducts. Shimonishi et al[96] examined galectin-3 expression pattern in intrahepatic cholangiocarcinoma (ICC), and found that 93% of the ICC samples analyzed were positive for this lectin. The expression was more intense in well-differentiated ICC, and was significantly decreased in dedifferentiated areas or poorly differentiated ICCs, indicating that galectin-3 expression is rather related to the preneoplastic and early neoplastic stages of ICC, and tends to disappear at later stages of ICC (Table 3)[96,137]. Also, it has been demonstrated that galectin-3 played a role in apoptosis and response to chemotherapy in cholangiocarcinoma cell lines (Table 3)[138]. These results highlight the possibility of targeting galectin-3 as an alternative therapeutic approach in cholangiocarcinoma.
Non-alcoholic fatty liver disease (NAFLD) is increasingly recognized as a condition in which excess fat accumulates in hepatocytes. NASH, a severe form of NAFLD in which inflammation and fibrosis of the liver take place, may eventually progress to end-stage liver disease and ultimately, to HCC[139]. Controversial results have been published on the effect of galectin-3 deficiency in models of hepatic steatosis/inflammation, with studies indicating either protection or increased disease severity in galectin-3 knock-out (KO) mice (Table 2)[140-143]. On one hand, it has been demonstrated that in choline-deficient L-amino-acid (CDAA) diet-induced NAFLD/NASH hepatic injury was more severe in galectin-3 KO mice, as compared to wild type mice[142].
On the other hand, Iacobini et al[143] reported a complete prevention or marked attenuation of NASH induced by an atherogenic diet in galectin-3 KO mice. In these animals, the earlier steps of NASH, e.g., steatosis, hepatocyte injury, and inflammation, were dramatically influenced[143]. Further research is needed to elucidate the protective or promoting roles of galectin-3 in liver steatosis and inflammation.
Excess fatty acid oxidation and generation of reactive carbonyls with formation of advanced lipoxidation and glycation end products (ALEs and AGEs, respectively) are involved in NASH. Several AGE-binding proteins have been identified including galectin-3, which has been widely recognized as an AGE receptor (AGE-R3)[144]. Butscheid et al[145] explored the expression of galectin-3 and RAGE, a member of the immunoglobulin superfamily which also serves as a receptor for AGEs, in specific cell types and histological structures of human liver biopsy specimens from patients with varying degrees of hepatic impairment (steatosis hepatitis, hepatitis, cholestasis and cirrhosis). They observed that when liver function is impaired and AGE levels rise, over-expression of galectin-3 appears to contribute to tissue protection (Table 2)[145].
Acetaminophen (APAP)-induced hepatotoxicity is a major cause of acute liver failure[146]. Evidence suggests that activated macrophages contribute to the pathogenic response to APAP and, two major phenotypically distinct subpopulations have been identified: classically activated (M1-type) macrophages which show pro-inflammatory function and alternatively activated (M2) macrophages which often display anti-inflammatory wound repair activities[147]. It appears that the outcome of tissue injury depends on which macrophage subpopulation predominates. In wild type mice, galectin-3 is markedly up-regulated in macrophages infiltrating the liver 48-72 h after APAP administration[147]. Interestingly, loss of galectin-3 resulted in reduced hepatotoxicity and decreased expression of proinflammatory mediators[148]. Taken together, the data suggest that galectin-3 plays a key role in promoting late pro-inflammatory responses, classical macrophage activation and perpetuating injury in the liver following APAP intoxication (Table 2).
Supporting these findings, Volarevic et al[149] showed that galectin-3 deficiency leads to a marked attenuation of Con A-induced hepatitis. This effect was associated with a decreased number of effector cells in the liver. Moreover, pretreatment of wild type mice with a selective inhibitor of galectin-3 (TD139) attenuated Con A-induced liver injury and reduced the number of CD4+ and CD8+ T cells (Table 2)[149]. Hence, galectin-3 plays an important pro-inflammatory role in Con-A-induced hepatitis and may function as a potential target for therapeutic intervention in acute liver diseases.
Galectin-4 is a “tandem-repeat” galectin, which possesses two CRDs and is primarily expressed in epithelial cells along the gastrointestinal tract[150]. Recently, this lectin has been reported as a major component of lipid rafts in brush border membranes of small intestinal epithelial cells[151]. In a human colon adenocarcinoma cell line, galectin-4 has been proposed to play an important role in the apical delivery of proteins[152].
Galectin-4 expression is altered in human malignancies[46,62,150]. Although controversial data has been published, it is apparent that galectin-4 is significantly down-regulated in colon adenocarcinoma. In fact, it has been recently demonstrated that galectin-4 functions as a tumor suppressor in this type of malignancy[153]. In contrast, galectin-4 expression is higher in HCC[154] and gastric cancer cell[155], as compared to their corresponding normal tissues, suggesting a context-dependent role of galectin-4 in tumor development and progression. Kondoh et al[154] identified several cDNAs that were differentially expressed in surgically resected human HCC as compared to non-tumor liver and normal liver tissues[154]. Non-tumor liver tissues were obtained from patients that suffered cirrhosis associated with HCV infection and, from patients suffering liver cirrhosis but in the absence of HCV or HBV infection. Normal liver tissues that were used as controls were obtained from patients who died of pancreatic carcinoma and subarachnoid bleeding. Interestingly, one of the genes differentially expressed was the galectin-4 gene (LGALS4). Northern blot analysis revealed that galectin-4 mRNA was more abundant in HCCs than in adjacent non-tumor liver tissues or normal liver tissues from non-HCC patients[154]. When HCC cell lines were analyzed (HuH-7 and HepG2 cells), the levels of galectin-4 mRNA were undetectable or low in rapidly growing cells. However, the levels of this lectin increased considerably in HuH-7 cells growing at a higher cell density, although the expression of galectin-4 did not increase in HepG2 cells. Furthermore, the expression of galectin-4 mRNA was also induced in HuH-7 cells cultured with low concentration serum (0.1%)[154]. Thus, although the precise roles of galectin-4 in HCC remained to be elucidated, these results show a possible association between galectin-4 expression and liver malignancy. Functional studies will provide insight to further understand the role of galectin-4 in HCC biology.
Galectin-8 is another member of the “tandem-repeat”-type family of galectins, which possesses two CRDs and thus, behaves as a bivalent molecule. The galectin-8 gene (LGALS8) encodes numerous mRNAs (most likely seven) generated through alternative splicing, mostly in intron VIII[156]. Because the N-terminal domain of galectin-8 intrinsically dimerizes[157], cleavage of the linker region between galectin-8N and galectin-8C may allow the possibility to dissect potential signaling pathways initiated by each separate domain[46].
This lectin has been initially cloned from a rat liver cDNA library[158]. Using Northern analysis it was established that galectin-8 mRNA is highly expressed in lungs and, to a lesser extent in the liver, kidneys, spleen, hind-limb and cardiac muscles in the rat[158]. The role of galectin-8 has been mostly investigated in relation to tumor malignancy[62,156] in a variety of different tumors from different origin[62,159]. Immunohistochemical studies revealed that galectin-8 expression is increased in cancerous versus normal tissues in the lung, bladder, kidney, prostate and stomach. However, in the liver and also in large intestine, pancreas, larynx and skin, immunohistochemical analysis revealed decreased expression of this lectin in cancerous versus normal tissues, suggesting tissue-specific regulation of galectin-8 expression in cancer[159]. In normal and cirrhotic livers, the staining intensities of galectin-8-positive cells appeared to be moderate to strong. On the contrary, in hepatoblastomas and hepatocarcinomas the staining intensity of positive cells was weak to moderate. Collectively, these experiments revealed tissue-specific regulation of galectin-8 expression upon malignant transformation of various tissue types of epithelial origin. Further investigation is necessary to further delineate the functional roles of galectin-8 in liver carcinogenesis and to determine if galectin-8 downregulation is associated with poor prognosis of HCC.
Galectin-9 is a “tandem-repeat” galectin originally isolated from mouse embryonic kidney cells[160]. Galectin-9 consists of two different CRDs joined by a flexible peptide linker, with 39% amino acid sequence homology. The C-terminal CRD and the N-terminal CRD share high affinity for both branched N-glycans and repeated oligo-lactosamines. Further, the N-CRD exhibits striking affinity for the Forssman pentasaccharide and polymerized N-acetyllactosamine[161,162]. Alternative splicing leads to the formation of three splice variants that vary only in the length of the peptide linker. The 35.9 kDa medium-sized isoform (galectin-9M) corresponds to authentic galectin-9 whereas the long and small-sized isoforms (galectin-9L and S) have a 32-amino acid insertion and a 12-amino acid deletion, respectively in the linker peptide[36]. The length of this region influences the rotational flexibility of the two CRDs in the space, impacting on galectin-9 valency[163].
Human galectin-9 was first identified as a tumor antigen in Hodgkin’s lymphoma, a condition characterized by abundant blood and tissue eosinophilia[164] and it is widely distributed within the immune system. This galectin is known to play a variety of cellular roles, including modulation of cell differentiation, adhesion, aggregation, and cell death[165]. Through modulation of cell signaling, this lectin can regulate multiple physiological and pathological processes such as immunity, inflammation, and cancer.
Galectin-9 has been identified as a ligand for the T-cell immunoglobulin mucin domain 3 (TIM-3), a membrane glycoprotein expressed on the surface of Th1, Th17 and citotoxic T cells, as well as in natural killer (NK) cells, monocytes, dendritic cells, macrophages and mast cells (reviewed by[166]). The galectin-9/TIM-3 pathway plays a dual role in immunity. On one hand, it favors a pro-inflammatory response, induces maturation of monocyte-derived dendritic cells, and through this process, enhances Th1-type immune responses[167]. On the other hand, galectin-9 contributes to apoptosis of thymocytes and peripheral T cells, implicating a dual role of the Galectin-9/TIM-3 axis in both T-cell maturation and negative regulation of T-cell-mediated immune reactions[168,169]. Blocking or activation of the Galectin-9/TIM-3 signaling pathway has been found to affect the evolution of many diseases, including autoimmune diseases, allergic disorders, graft rejection and anti-viral immunity (reviewed by[170]). Due to its potent roles in T cell suppression, galectin-9 has been considered as a therapeutic candidate for autoimmune and inflammatory diseases[167,171].
Although most studies indicate that TIM-3 is involved in galectin-9 mediated signaling in T cells, multiple mechanisms and alternative receptors have been also proposed for this lectin[163,172,173]. More recently, a publication by Leitner et al[174] suggested that TIM-3 does not act as a receptor for galectin-9. These controversial results emphasize the involvement of distinct glycosylated receptors in galectin-9 effects.
In spite of considerable evidence indicating the role of galectin-9 in tumor biology and inflammation, the mechanisms governing expression of this protein are poorly understood. So far, IFN-γ has been shown to induce galectin-9 expression in fibroblasts[175], endothelial cells[176] and on Kupffer cell[177]. Additional modulators of galectin-9 include interleukin-1β (IL-1β) and interleukin-5 (IL-5) in astrocytes[178] and eosinophils[179] respectively. Interestingly, decreased galectin-9 expression typically correlates with tumor progression and metastasis formation in various types of cancer[166].
Galectin-9 has been identified as a possible prognostic marker in breast cancer, melanoma, and oral squamous cell carcinoma[180]. Most recently, Zhang et al[181] examined the relationship between galectin-9 expression and HCC, using an in vitro approach and immunohistochemistry on HCC tissues. The authors found that silencing galectin-9 expression in HepG2 HCC cells through siRNA-mediated strategies resulted in a weakened cell aggregation and increased proliferation and adhesion to ECM[181]. Also, galectin-9 suppression increased tumor cell-endothelial cell adhesion and trans-endothelial invasion of HepG2 cells. Additionally, downregulation of galectin-9 in human HCC tissue specimens represented a significant risk factor for patient survival and significantly correlated with the histopathologic grade of the tumor, lymph node metastasis, vascular invasion and intrahepatic metastasis[181]. These results emphasized an anti-metastasic role for galectin-9 in HCC (Figure 1 and Table 1).
T-cell responses are regulated by multiple mechanisms to maintain homeostasis and to prevent exuberant tissue inflammation and autoimmune disease. Whilst these regulatory mechanisms are critical to terminate excessive inflammatory responses, they can excessively constrain antiviral immunity in settings of persistent viral infection[182]. Galectin-9 is present at significantly higher levels in sera from patients infected with HCV or HBV compared to normal healthy controls[177,182,183]. Galectin-9 is expressed mainly in Kupffer cells[177,182], but is also present in inflammatory leucocytes and hepatocytes[183]. Recently, it has been reported that progression to persistent infection of HCV was accompanied by increased plasma levels of galectin-9[184].
In patients chronically infected with HCV or HBV, multiple regulatory mechanisms act in concert to induce failure of the immune response and facilitate viral persistence. Interestingly, it has been demonstrated that galectin-9 plays a key role in limiting T-cell responses in the liver and facilitating the establishment of viral persistence. Galectin-9 induces the secretion of pro-inflammatory cytokines from monocytes and macrophages[177] that can further amplify immunopathology associated with HCV/HBV infection. As a counter-effect, galectin-9 induces TIM-3-mediated apoptosis of effector T cells[177,182] and favors the expansion of Tregs[177,184,185] thereby attenuating adaptive immune responses.
Li et al[186] studied the relevance of galectin-9 in patients with HBV-associated HCC. By flow cytometry analysis, the authors found that tumor cells and T cells expressed low amounts of galectin-9 while dendritic cells expressed moderate levels of this protein and Kupffer cells showed the highest expression in HBV-associated HCC tissues in comparison to non-tumor adjacent tissues[186]. The authors also observed that in HBV-positive patients the percentage of galectin-9+ Kupffer cells was higher in tumor tissues than in normal adjacent tissues. However, in HBV-negative patients the expression of galectin-9 in Kupffer cells was negligible in both HCC and adjacent tissues. Interestingly, IFN-γ derived from tumor-infiltrating T cells contributed to the increased galectin-9 expression in the HCC microenvironment[186]. In addition, high numbers of TIM-3+ T cells were detected in HBV-associated HCC, which expressed senescence markers and exhibited decreased proliferative ability and impaired effector function when compared with TIM-3- T cells. Therefore, the TIM-3/galectin-9 signaling axis mediates T-cell dysfunction and predicts poor prognosis in patients with HBV-associated HCC[186].
Although these data indicates a major role for galectin-9 in regulating liver immune responses, the observation that this galectin predominantly dampens immune function seems hard to reconcile with the poor outcome in patients with low galectin-9 expression. Possibly, galectin-9 expression is lost during the course of tumorigenesis, enabling tumor cells to metastasize more easily while alternatives modes of escape are being developed[180] (e.g., the up-regulation of galectin-1) (Figure 1 and Table 1). A better understanding of the mechanisms underlying galectin-9 functions is required to elucidate its possible role as a promising target in HCC.
The ischemia and reperfusion injury (IRI), an inflammatory event controlled by an exogenous antigen-independent insult that stimulates innate immunity, remains a critical problem in clinical organ transplantation. Liver IRI occurs frequently after major hepatic resection or liver transplantation. It has been demonstrated that CD4+ T cells are the key mediators of IRI-triggered liver inflammation[187]. Kupffer cells release pro-inflammatory mediators such as TNF and IL-6[188], and CD4+ T cells amplify Kupffer cell activity[189]. In this context, blockade of the TIM-3/galectin-9 pathway exacerbated local inflammation and liver damage (Table 2)[190]. These results suggest the importance of TIM-3/galectin-9 signaling in the maintenance of liver homeostasis and controlling dysregulated liver immune response, for example during IRI.
Similar results were observed in the murine model of liver injury, Con A-induced hepatitis, where T cell activation plays a crucial role. Blockade of TIM-3 using an anti-TIM-3 Ab resulted in more severe liver damage. On the contrary, biochemical and histopathological data indicated that a single injection of galectin-9 was sufficient to protect mice against Con A-induced hepatitis (Table 2)[191].
Another progressive inflammatory liver disorder is autoimmune hepatitis (AIH), where a defective control of CD4+ T cells takes place. Liberal et al[192] showed that patients with AIH had reduced levels of TIM-3 and galectin-9 on effector CD4+ T cells and Treg cells, respectively, as compared to healthy individuals[192]. Reduced signaling of the TIM-3/galectin-9 axis contributed to impaired control during AIH by rendering effector cells less prone to Treg cell control and Tregs less capable of suppressing effector responses.
A distinct subset of cells, referred as NKT cells has been characterized by the expression of a semi-invariant T cell receptor (TCR) and surface antigens typical of natural killer (NK) cells. These cells exhibit features of both cell types and act as a bridging system between innate and adaptive immunity[193]. NKT cells are particularly enriched within the liver and regulate immune responses through rapid secretion of large amounts of both Th1 and Th2 cytokines following stimulation[194]. The TIM-3/galectin-9 signaling pathway also plays a critical role in the homeostasis of hepatic NKT cells. It has been demonstrated that galectin-9 limits the inflammatory response in a mouse model of diet-induced nonalcoholic fatty liver disease (NAFLD) (Table 2)[195].
In summary, these observations validated the relevance of the TIM-3/galectin-9 signaling axis in maintaining a balanced local immune microenvironment in the liver. Dysregulation of this axis can lead to a chronic inflammatory liver disorder which can eventually develop into an HCC.
Because of their roles in tumor progression, galectins have evolved as promising targets for cancer therapy. A variety of studies revealed the involvement of this evolutionarily conserved protein family in murine and human cancers[26,52-56]. Modified citrus pectin, peptides, anti-galectin neutralizing antibodies and chemical inhibitors that antagonize galectins CRDs have been demonstrated the ability to reduce tumor volume, metastasis, angiogenesis, potentiate immune responses and increase host survival in various tumor-type models[73,75,196-198].
Current literature shows that the “proto-type” galectin-1, the “chimera” galectin-3 and “tandem-repeat” galectin-4 are increased in HCC cells compared to their normal counterparts. On the other hand, expression of “tandem-repeat” galectin-8 and galectin-9 is decreased in tumor hepatocytes. The aberrant expression (up- or down-regulation) of these galectins correlates with tumor growth, HCC adhesion, migration and invasion, tumor aggressiveness, metastasis, postoperative recurrence and poor prognosis (Figure 1 and Table 1). It is noteworthy that galectins also play key roles in other liver pathologies associated with chronic inflammation and fibrosis (Tables 2 and 3). Although research in this field is just beginning, the role for these galectins in HCC biology is substantiated by a wide range of accumulating evidence from animal models and human samples. Further functional studies are crucial to delineate the precise mechanisms by which galectins promote liver carcinogenesis, HCC progression, aggressiveness, inflammation and metastasis. Hopefully, in a near future, galectin-based therapies can be developed for the treatment of HCC, liver-associated fibrosis and liver chronic inflammatory disorders.
We apologize to the many authors whose papers could not be cited owing to space limitations.
P- Reviewers: López de Heredia M, Kumar S S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11836] [Article Influence: 845.4] [Reference Citation Analysis (4)] |
| 2. | Bosetti C, Levi F, Boffetta P, Lucchini F, Negri E, La Vecchia C. Trends in mortality from hepatocellular carcinoma in Europe, 1980-2004. Hepatology. 2008;48:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 181] [Article Influence: 10.6] [Reference Citation Analysis (1)] |
| 3. | Qiu D, Katanoda K, Marugame T, Sobue T. A Joinpoint regression analysis of long-term trends in cancer mortality in Japan (1958-2004). Int J Cancer. 2009;124:443-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (2)] |
| 4. | El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2881] [Cited by in RCA: 3088] [Article Influence: 220.6] [Reference Citation Analysis (0)] |
| 5. | Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6:674-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1461] [Cited by in RCA: 1568] [Article Influence: 82.5] [Reference Citation Analysis (0)] |
| 6. | But DY, Lai CL, Yuen MF. Natural history of hepatitis-related hepatocellular carcinoma. World J Gastroenterol. 2008;14:1652-1656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 145] [Cited by in RCA: 157] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 7. | El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264-1273.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2183] [Cited by in RCA: 2508] [Article Influence: 192.9] [Reference Citation Analysis (2)] |
| 8. | Zhang DY, Friedman SL. Fibrosis-dependent mechanisms of hepatocarcinogenesis. Hepatology. 2012;56:769-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 322] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 9. | Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J Hepatol. 2012;56:1384-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 654] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 10. | Yu J, Shen J, Sun TT, Zhang X, Wong N. Obesity, insulin resistance, NASH and hepatocellular carcinoma. Semin Cancer Biol. 2013;23:483-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 11. | Villanueva A, Hernandez-Gea V, Llovet JM. Medical therapies for hepatocellular carcinoma: a critical view of the evidence. Nat Rev Gastroenterol Hepatol. 2013;10:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 253] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 12. | de Lope CR, Tremosini S, Forner A, Reig M, Bruix J. Management of HCC. J Hepatol. 2012;56 Suppl 1:S75-S87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 484] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 13. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3596] [Article Influence: 276.6] [Reference Citation Analysis (4)] |
| 14. | Mancuso A. Management of hepatocellular carcinoma: Enlightening the gray zones. World J Hepatol. 2013;5:302-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Yamamoto J, Kosuge T, Takayama T, Shimada K, Yamasaki S, Ozaki H, Yamaguchi N, Makuuchi M. Recurrence of hepatocellular carcinoma after surgery. Br J Surg. 1996;83:1219-1222. [PubMed] |
| 16. | Ercolani G, Grazi GL, Ravaioli M, Del Gaudio M, Gardini A, Cescon M, Varotti G, Cetta F, Cavallari A. Liver resection for hepatocellular carcinoma on cirrhosis: univariate and multivariate analysis of risk factors for intrahepatic recurrence. Ann Surg. 2003;237:536-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 262] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 17. | Vivarelli M, Risaliti A. Liver transplantation for hepatocellular carcinoma on cirrhosis: strategies to avoid tumor recurrence. World J Gastroenterol. 2011;17:4741-4746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Pang RW, Poon RT. From molecular biology to targeted therapies for hepatocellular carcinoma: the future is now. Oncology. 2007;72 Suppl 1:30-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 137] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 19. | Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology. 2008;48:1312-1327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 823] [Cited by in RCA: 832] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 20. | Shen YC, Hsu C, Cheng AL. Molecular targeted therapy for advanced hepatocellular carcinoma: current status and future perspectives. J Gastroenterol. 2010;45:794-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10270] [Article Influence: 604.1] [Reference Citation Analysis (2)] |
| 22. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4652] [Article Influence: 273.6] [Reference Citation Analysis (0)] |
| 23. | Matar P, Alaniz L, Rozados V, Aquino JB, Malvicini M, Atorrasagasti C, Gidekel M, Silva M, Scharovsky OG, Mazzolini G. Immunotherapy for liver tumors: present status and future prospects. J Biomed Sci. 2009;16:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Barondes SH, Castronovo V, Cooper DN, Cummings RD, Drickamer K, Feizi T, Gitt MA, Hirabayashi J, Hughes C, Kasai K. Galectins: a family of animal beta-galactoside-binding lectins. Cell. 1994;76:597-598. [PubMed] |
| 25. | Barondes SH, Cooper DN, Gitt MA, Leffler H. Galectins. Structure and function of a large family of animal lectins. J Biol Chem. 1994;269:20807-20810. [PubMed] |
| 26. | Yang RY, Rabinovich GA, Liu FT. Galectins: structure, function and therapeutic potential. Expert Rev Mol Med. 2008;10:e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 621] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 27. | Chiariotti L, Salvatore P, Frunzio R, Bruni CB. Galectin genes: regulation of expression. Glycoconj J. 2004;19:441-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Camby I, Le Mercier M, Lefranc F, Kiss R. Galectin-1: a small protein with major functions. Glycobiology. 2006;16:137R-157R. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 688] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 29. | Dumic J, Dabelic S, Flögel M. Galectin-3: an open-ended story. Biochim Biophys Acta. 2006;1760:616-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 854] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 30. | Cooper DN, Barondes SH. God must love galectins; he made so many of them. Glycobiology. 1999;9:979-984. [PubMed] |
| 31. | Hughes RC. Secretion of the galectin family of mammalian carbohydrate-binding proteins. Biochim Biophys Acta. 1999;1473:172-185. [PubMed] |
| 32. | Nickel W. Unconventional secretory routes: direct protein export across the plasma membrane of mammalian cells. Traffic. 2005;6:607-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 252] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 33. | Cooper DN, Barondes SH. Evidence for export of a muscle lectin from cytosol to extracellular matrix and for a novel secretory mechanism. J Cell Biol. 1990;110:1681-1691. [PubMed] |
| 34. | Lindstedt R, Apodaca G, Barondes SH, Mostov KE, Leffler H. Apical secretion of a cytosolic protein by Madin-Darby canine kidney cells. Evidence for polarized release of an endogenous lectin by a nonclassical secretory pathway. J Biol Chem. 1993;268:11750-11757. [PubMed] |
| 35. | Sato S, Burdett I, Hughes RC. Secretion of the baby hamster kidney 30-kDa galactose-binding lectin from polarized and nonpolarized cells: a pathway independent of the endoplasmic reticulum-Golgi complex. Exp Cell Res. 1993;207:8-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 144] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 36. | Chabot S, Kashio Y, Seki M, Shirato Y, Nakamura K, Nishi N, Nakamura T, Matsumoto R, Hirashima M. Regulation of galectin-9 expression and release in Jurkat T cell line cells. Glycobiology. 2002;12:111-118. [PubMed] |
| 37. | Oomizu S, Arikawa T, Niki T, Kadowaki T, Ueno M, Nishi N, Yamauchi A, Hattori T, Masaki T, Hirashima M. Cell surface galectin-9 expressing Th cells regulate Th17 and Foxp3+ Treg development by galectin-9 secretion. PLoS One. 2012;7:e48574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 38. | Rabinovich GA, Toscano MA, Jackson SS, Vasta GR. Functions of cell surface galectin-glycoprotein lattices. Curr Opin Struct Biol. 2007;17:513-520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 323] [Cited by in RCA: 304] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 39. | Garner OB, Baum LG. Galectin-glycan lattices regulate cell-surface glycoprotein organization and signalling. Biochem Soc Trans. 2008;36:1472-1477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 166] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 40. | Laderach DJ, Compagno D, Toscano MA, Croci DO, Dergan-Dylon S, Salatino M, Rabinovich GA. Dissecting the signal transduction pathways triggered by galectin-glycan interactions in physiological and pathological settings. IUBMB Life. 2010;62:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Fajka-Boja R, Blaskó A, Kovács-Sólyom F, Szebeni GJ, Tóth GK, Monostori E. Co-localization of galectin-1 with GM1 ganglioside in the course of its clathrin- and raft-dependent endocytosis. Cell Mol Life Sci. 2008;65:2586-2593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 42. | Liu FT, Patterson RJ, Wang JL. Intracellular functions of galectins. Biochim Biophys Acta. 2002;1572:263-273. [PubMed] |
| 43. | Elola MT, Wolfenstein-Todel C, Troncoso MF, Vasta GR, Rabinovich GA. Galectins: matricellular glycan-binding proteins linking cell adhesion, migration, and survival. Cell Mol Life Sci. 2007;64:1679-1700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 295] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 44. | Liu FT, Rabinovich GA. Galectins: regulators of acute and chronic inflammation. Ann N Y Acad Sci. 2010;1183:158-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 303] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 45. | Rabinovich GA, Croci DO. Regulatory circuits mediated by lectin-glycan interactions in autoimmunity and cancer. Immunity. 2012;36:322-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 260] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 46. | Troncoso MF, Elola MT, Croci DO, Rabinovich GA. Integrating structure and function of ‘tandem-repeat’ galectins. Front Biosci (Schol Ed). 2012;4:864-887. [PubMed] |
| 47. | Lahm H, André S, Hoeflich A, Kaltner H, Siebert HC, Sordat B, von der Lieth CW, Wolf E, Gabius HJ. Tumor galectinology: insights into the complex network of a family of endogenous lectins. Glycoconj J. 2004;20:227-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 108] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 48. | van den Brûle F, Califice S, Castronovo V. Expression of galectins in cancer: a critical review. Glycoconj J. 2004;19:537-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 132] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 49. | Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5:29-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1074] [Cited by in RCA: 1114] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 50. | Cedeno-Laurent F, Dimitroff CJ. Galectins and their ligands: negative regulators of anti-tumor immunity. Glycoconj J. 2012;29:619-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 51. | Compagno D, Laderach DJ, Gentilini L, Jaworski FM, Rabinovich GA. Delineating the “galectin signature” of the tumor microenvironment. Oncoimmunology. 2013;2:e23565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 52. | Thijssen VL, Rabinovich GA, Griffioen AW. Vascular galectins: Regulators of tumor progression and targets for cancer therapy. Cytokine Growth Factor Rev. 2013;24:547-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 53. | Ingrassia L, Camby I, Lefranc F, Mathieu V, Nshimyumukiza P, Darro F, Kiss R. Anti-galectin compounds as potential anti-cancer drugs. Curr Med Chem. 2006;13:3513-3527. [PubMed] |
| 54. | Hasan SS, Ashraf GM, Banu N. Galectins - potential targets for cancer therapy. Cancer Lett. 2007;253:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 55. | Ito K, Stannard K, Gabutero E, Clark AM, Neo SY, Onturk S, Blanchard H, Ralph SJ. Galectin-1 as a potent target for cancer therapy: role in the tumor microenvironment. Cancer Metastasis Rev. 2012;31:763-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 56. | Wiersma VR, de Bruyn M, Helfrich W, Bremer E. Therapeutic potential of Galectin-9 in human disease. Med Res Rev. 2013;33 Suppl 1:E102-E126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 57. | Danguy A, Camby I, Kiss R. Galectins and cancer. Biochim Biophys Acta. 2002;1572:285-293. [PubMed] |
| 58. | Rabinovich GA. Galectin-1 as a potential cancer target. Br J Cancer. 2005;92:1188-1192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 184] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 59. | Martinez VG, Pellizzari EH, Díaz ES, Cigorraga SB, Lustig L, Denduchis B, Wolfenstein-Todel C, Iglesias MM. Galectin-1, a cell adhesion modulator, induces apoptosis of rat Leydig cells in vitro. Glycobiology. 2004;14:127-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 60. | Elola MT, Chiesa ME, Alberti AF, Mordoh J, Fink NE. Galectin-1 receptors in different cell types. J Biomed Sci. 2005;12:13-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 61. | Cedeno-Laurent F, Dimitroff CJ. Galectin-1 research in T cell immunity: past, present and future. Clin Immunol. 2012;142:107-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 62. | Balan V, Nangia-Makker P, Raz A. Galectins as Cancer Biomarkers. Cancers (Basel). 2010;2:592-610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 63. | Lefranc F, Mathieu V, Kiss R. Galectin-1-mediated biochemical controls of melanoma and glioma aggressive behavior. World J Biol Chem. 2011;2:193-201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 64. | Chung LY, Tang SJ, Sun GH, Chou TY, Yeh TS, Yu SL, Sun KH. Galectin-1 promotes lung cancer progression and chemoresistance by upregulating p38 MAPK, ERK, and cyclooxygenase-2. Clin Cancer Res. 2012;18:4037-4047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 65. | Laderach DJ, Gentilini LD, Giribaldi L, Delgado VC, Nugnes L, Croci DO, Al Nakouzi N, Sacca P, Casas G, Mazza O. A unique galectin signature in human prostate cancer progression suggests galectin-1 as a key target for treatment of advanced disease. Cancer Res. 2013;73:86-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 66. | Verschuere T, Van Woensel M, Fieuws S, Lefranc F, Mathieu V, Kiss R, Van Gool SW, De Vleeschouwer S. Altered galectin-1 serum levels in patients diagnosed with high-grade glioma. J Neurooncol. 2013;115:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 67. | Chen J, Zhou SJ, Zhang Y, Zhang GQ, Zha TZ, Feng YZ, Zhang K. Clinicopathological and prognostic significance of galectin-1 and vascular endothelial growth factor expression in gastric cancer. World J Gastroenterol. 2013;19:2073-2079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 68. | Jung EJ, Moon HG, Cho BI, Jeong CY, Joo YT, Lee YJ, Hong SC, Choi SK, Ha WS, Kim JW. Galectin-1 expression in cancer-associated stromal cells correlates tumor invasiveness and tumor progression in breast cancer. Int J Cancer. 2007;120:2331-2338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 130] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 69. | Dalotto-Moreno T, Croci DO, Cerliani JP, Martinez-Allo VC, Dergan-Dylon S, Méndez-Huergo SP, Stupirski JC, Mazal D, Osinaga E, Toscano MA. Targeting galectin-1 overcomes breast cancer-associated immunosuppression and prevents metastatic disease. Cancer Res. 2013;73:1107-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 210] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 70. | Kim HJ, Do IG, Jeon HK, Cho YJ, Park YA, Choi JJ, Sung CO, Lee YY, Choi CH, Kim TJ. Galectin 1 expression is associated with tumor invasion and metastasis in stage IB to IIA cervical cancer. Hum Pathol. 2013;44:62-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 71. | Hsu YL, Wu CY, Hung JY, Lin YS, Huang MS, Kuo PL. Galectin-1 promotes lung cancer tumor metastasis by potentiating integrin α6β4 and Notch1/Jagged2 signaling pathway. Carcinogenesis. 2013;34:1370-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 72. | Thijssen VL, Barkan B, Shoji H, Aries IM, Mathieu V, Deltour L, Hackeng TM, Kiss R, Kloog Y, Poirier F. Tumor cells secrete galectin-1 to enhance endothelial cell activity. Cancer Res. 2010;70:6216-6224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 195] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 73. | Thijssen VL, Postel R, Brandwijk RJ, Dings RP, Nesmelova I, Satijn S, Verhofstad N, Nakabeppu Y, Baum LG, Bakkers J. Galectin-1 is essential in tumor angiogenesis and is a target for antiangiogenesis therapy. Proc Natl Acad Sci USA. 2006;103:15975-15980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 381] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 74. | Le QT, Shi G, Cao H, Nelson DW, Wang Y, Chen EY, Zhao S, Kong C, Richardson D, O’Byrne KJ. Galectin-1: a link between tumor hypoxia and tumor immune privilege. J Clin Oncol. 2005;23:8932-8941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 192] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 75. | Croci DO, Salatino M, Rubinstein N, Cerliani JP, Cavallin LE, Leung HJ, Ouyang J, Ilarregui JM, Toscano MA, Domaica CI. Disrupting galectin-1 interactions with N-glycans suppresses hypoxia-driven angiogenesis and tumorigenesis in Kaposi’s sarcoma. J Exp Med. 2012;209:1985-2000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 167] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 76. | Rubinstein N, Alvarez M, Zwirner NW, Toscano MA, Ilarregui JM, Bravo A, Mordoh J, Fainboim L, Podhajcer OL, Rabinovich GA. Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection; A potential mechanism of tumor-immune privilege. Cancer Cell. 2004;5:241-251. [PubMed] |
| 77. | Juszczynski P, Ouyang J, Monti S, Rodig SJ, Takeyama K, Abramson J, Chen W, Kutok JL, Rabinovich GA, Shipp MA. The AP1-dependent secretion of galectin-1 by Reed Sternberg cells fosters immune privilege in classical Hodgkin lymphoma. Proc Natl Acad Sci USA. 2007;104:13134-13139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 255] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 78. | Banh A, Zhang J, Cao H, Bouley DM, Kwok S, Kong C, Giaccia AJ, Koong AC, Le QT. Tumor galectin-1 mediates tumor growth and metastasis through regulation of T-cell apoptosis. Cancer Res. 2011;71:4423-4431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 79. | Chung EJ, Sung YK, Farooq M, Kim Y, Im S, Tak WY, Hwang YJ, Kim YI, Han HS, Kim JC. Gene expression profile analysis in human hepatocellular carcinoma by cDNA microarray. Mol Cells. 2002;14:382-387. [PubMed] |
| 80. | Kondoh N, Hada A, Ryo A, Shuda M, Arai M, Matsubara O, Kimura F, Wakatsuki T, Yamamoto M. Activation of Galectin-1 gene in human hepatocellular carcinoma involves methylation-sensitive complex formations at the transcriptional upstream and downstream elements. Int J Oncol. 2003;23:1575-1583. [PubMed] |
| 81. | Spano D, Russo R, Di Maso V, Rosso N, Terracciano LM, Roncalli M, Tornillo L, Capasso M, Tiribelli C, Iolascon A. Galectin-1 and its involvement in hepatocellular carcinoma aggressiveness. Mol Med. 2010;16:102-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 82. | Wu H, Chen P, Liao R, Li YW, Yi Y, Wang JX, Sun TW, Zhou J, Shi YH, Yang XR. Overexpression of galectin-1 is associated with poor prognosis in human hepatocellular carcinoma following resection. J Gastroenterol Hepatol. 2012;27:1312-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 83. | Cimmino F, Schulte JH, Zollo M, Koster J, Versteeg R, Iolascon A, Eggert A, Schramm A. Galectin-1 is a major effector of TrkB-mediated neuroblastoma aggressiveness. Oncogene. 2009;28:2015-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 84. | Wu MH, Hong TM, Cheng HW, Pan SH, Liang YR, Hong HC, Chiang WF, Wong TY, Shieh DB, Shiau AL. Galectin-1-mediated tumor invasion and metastasis, up-regulated matrix metalloproteinase expression, and reorganized actin cytoskeletons. Mol Cancer Res. 2009;7:311-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 119] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 85. | Espelt MV, Croci DO, Bacigalupo ML, Carabias P, Manzi M, Elola MT, Muñoz MC, Dominici FP, Wolfenstein-Todel C, Rabinovich GA. Novel roles of galectin-1 in hepatocellular carcinoma cell adhesion, polarization, and in vivo tumor growth. Hepatology. 2011;53:2097-2106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 86. | Garín MI, Chu CC, Golshayan D, Cernuda-Morollón E, Wait R, Lechler RI. Galectin-1: a key effector of regulation mediated by CD4+CD25+ T cells. Blood. 2007;109:2058-2065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 379] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 87. | Orentas RJ, Kohler ME, Johnson BD. Suppression of anti-cancer immunity by regulatory T cells: back to the future. Semin Cancer Biol. 2006;16:137-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 88. | Tiegs G, Hentschel J, Wendel A. A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J Clin Invest. 1992;90:196-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 812] [Cited by in RCA: 888] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 89. | Santucci L, Fiorucci S, Cammilleri F, Servillo G, Federici B, Morelli A. Galectin-1 exerts immunomodulatory and protective effects on concanavalin A-induced hepatitis in mice. Hepatology. 2000;31:399-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 121] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 90. | Mauad TH, van Nieuwkerk CM, Dingemans KP, Smit JJ, Schinkel AH, Notenboom RG, van den Bergh Weerman MA, Verkruisen RP, Groen AK, Oude Elferink RP. Mice with homozygous disruption of the mdr2 P-glycoprotein gene. A novel animal model for studies of nonsuppurative inflammatory cholangitis and hepatocarcinogenesis. Am J Pathol. 1994;145:1237-1245. [PubMed] |
| 91. | Potikha T, Stoyanov E, Pappo O, Frolov A, Mizrahi L, Olam D, Shnitzer-Perlman T, Weiss I, Barashi N, Peled A. Interstrain differences in chronic hepatitis and tumor development in a murine model of inflammation-mediated hepatocarcinogenesis. Hepatology. 2013;58:192-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 92. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3381] [Cited by in RCA: 4120] [Article Influence: 206.0] [Reference Citation Analysis (3)] |
| 93. | Kristensen DB, Kawada N, Imamura K, Miyamoto Y, Tateno C, Seki S, Kuroki T, Yoshizato K. Proteome analysis of rat hepatic stellate cells. Hepatology. 2000;32:268-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 156] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 94. | Maeda N, Kawada N, Seki S, Arakawa T, Ikeda K, Iwao H, Okuyama H, Hirabayashi J, Kasai K, Yoshizato K. Stimulation of proliferation of rat hepatic stellate cells by galectin-1 and galectin-3 through different intracellular signaling pathways. J Biol Chem. 2003;278:18938-18944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 119] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 95. | Glaser S, Francis H, Demorrow S, Lesage G, Fava G, Marzioni M, Venter J, Alpini G. Heterogeneity of the intrahepatic biliary epithelium. World J Gastroenterol. 2006;12:3523-3536. [PubMed] |
| 96. | Shimonishi T, Miyazaki K, Kono N, Sabit H, Tuneyama K, Harada K, Hirabayashi J, Kasai K, Nakanuma Y. Expression of endogenous galectin-1 and galectin-3 in intrahepatic cholangiocarcinoma. Hum Pathol. 2001;32:302-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 97. | Yang RY, Hsu DK, Liu FT. Expression of galectin-3 modulates T-cell growth and apoptosis. Proc Natl Acad Sci USA. 1996;93:6737-6742. [PubMed] |
| 98. | Morris S, Ahmad N, André S, Kaltner H, Gabius HJ, Brenowitz M, Brewer F. Quaternary solution structures of galectins-1, -3, and -7. Glycobiology. 2004;14:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 88] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 99. | Yang RY, Hill PN, Hsu DK, Liu FT. Role of the carboxyl-terminal lectin domain in self-association of galectin-3. Biochemistry. 1998;37:4086-4092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 97] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 100. | Ahmad N, Gabius HJ, André S, Kaltner H, Sabesan S, Roy R, Liu B, Macaluso F, Brewer CF. Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J Biol Chem. 2004;279:10841-10847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 404] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 101. | Newlaczyl AU, Yu LG. Galectin-3--a jack-of-all-trades in cancer. Cancer Lett. 2011;313:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 279] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 102. | Gong HC, Honjo Y, Nangia-Makker P, Hogan V, Mazurak N, Bresalier RS, Raz A. The NH2 terminus of galectin-3 governs cellular compartmentalization and functions in cancer cells. Cancer Res. 1999;59:6239-6245. [PubMed] |
| 103. | Tsay YG, Lin NY, Voss PG, Patterson RJ, Wang JL. Export of galectin-3 from nuclei of digitonin-permeabilized mouse 3T3 fibroblasts. Exp Cell Res. 1999;252:250-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 104. | Funasaka T, Balan V, Raz A, Wong RW. Nucleoporin Nup98 mediates galectin-3 nuclear-cytoplasmic trafficking. Biochem Biophys Res Commun. 2013;434:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 105. | Menon RP, Hughes RC. Determinants in the N-terminal domains of galectin-3 for secretion by a novel pathway circumventing the endoplasmic reticulum-Golgi complex. Eur J Biochem. 1999;264:569-576. [PubMed] |
| 106. | Elad-Sfadia G, Haklai R, Balan E, Kloog Y. Galectin-3 augments K-Ras activation and triggers a Ras signal that attenuates ERK but not phosphoinositide 3-kinase activity. J Biol Chem. 2004;279:34922-34930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 240] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 107. | Shalom-Feuerstein R, Cooks T, Raz A, Kloog Y. Galectin-3 regulates a molecular switch from N-Ras to K-Ras usage in human breast carcinoma cells. Cancer Res. 2005;65:7292-7300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 108. | Lee YJ, Song YK, Song JJ, Siervo-Sassi RR, Kim HR, Li L, Spitz DR, Lokshin A, Kim JH. Reconstitution of galectin-3 alters glutathione content and potentiates TRAIL-induced cytotoxicity by dephosphorylation of Akt. Exp Cell Res. 2003;288:21-34. [PubMed] |
| 109. | Oka N, Nakahara S, Takenaka Y, Fukumori T, Hogan V, Kanayama HO, Yanagawa T, Raz A. Galectin-3 inhibits tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by activating Akt in human bladder carcinoma cells. Cancer Res. 2005;65:7546-7553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 106] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 110. | Dagher SF, Wang JL, Patterson RJ. Identification of galectin-3 as a factor in pre-mRNA splicing. Proc Natl Acad Sci USA. 1995;92:1213-1217. [PubMed] |
| 111. | Park JW, Voss PG, Grabski S, Wang JL, Patterson RJ. Association of galectin-1 and galectin-3 with Gemin4 in complexes containing the SMN protein. Nucleic Acids Res. 2001;29:3595-3602. [PubMed] |
| 112. | Shimura T, Takenaka Y, Fukumori T, Tsutsumi S, Okada K, Hogan V, Kikuchi A, Kuwano H, Raz A. Implication of galectin-3 in Wnt signaling. Cancer Res. 2005;65:3535-3537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 113. | Fukumori T, Takenaka Y, Yoshii T, Kim HR, Hogan V, Inohara H, Kagawa S, Raz A. CD29 and CD7 mediate galectin-3-induced type II T-cell apoptosis. Cancer Res. 2003;63:8302-8311. [PubMed] |
| 114. | Hsu DK, Chen HY, Liu FT. Galectin-3 regulates T-cell functions. Immunol Rev. 2009;230:114-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 115. | Radosavljevic G, Volarevic V, Jovanovic I, Milovanovic M, Pejnovic N, Arsenijevic N, Hsu DK, Lukic ML. The roles of Galectin-3 in autoimmunity and tumor progression. Immunol Res. 2012;52:100-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 116. | Henderson NC, Sethi T. The regulation of inflammation by galectin-3. Immunol Rev. 2009;230:160-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 408] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 117. | Califice S, Castronovo V, Van Den Brûle F. Galectin-3 and cancer (Review). Int J Oncol. 2004;25:983-992. [PubMed] |
| 118. | Fukumori T, Kanayama HO, Raz A. The role of galectin-3 in cancer drug resistance. Drug Resist Updat. 2007;10:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 115] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 119. | Kadrofske MM, Openo KP, Wang JL. The human LGALS3 (galectin-3) gene: determination of the gene structure and functional characterization of the promoter. Arch Biochem Biophys. 1998;349:7-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 83] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 120. | Keller S, Angrisano T, Florio E, Pero R, Decaussin-Petrucci M, Troncone G, Capasso M, Lembo F, Fusco A, Chiariotti L. DNA methylation state of the galectin-3 gene represents a potential new marker of thyroid malignancy. Oncol Lett. 2013;6:86-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 121. | Ruebel KH, Jin L, Qian X, Scheithauer BW, Kovacs K, Nakamura N, Zhang H, Raz A, Lloyd RV. Effects of DNA methylation on galectin-3 expression in pituitary tumors. Cancer Res. 2005;65:1136-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 122. | Margadant C, van den Bout I, van Boxtel AL, Thijssen VL, Sonnenberg A. Epigenetic regulation of galectin-3 expression by β1 integrins promotes cell adhesion and migration. J Biol Chem. 2012;287:44684-44693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 123. | Ramasamy S, Duraisamy S, Barbashov S, Kawano T, Kharbanda S, Kufe D. The MUC1 and galectin-3 oncoproteins function in a microRNA-dependent regulatory loop. Mol Cell. 2007;27:992-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 154] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 124. | Hsu DK, Dowling CA, Jeng KC, Chen JT, Yang RY, Liu FT. Galectin-3 expression is induced in cirrhotic liver and hepatocellular carcinoma. Int J Cancer. 1999;81:519-526. [PubMed] |
| 125. | Luo JH, Ren B, Keryanov S, Tseng GC, Rao UN, Monga SP, Strom S, Demetris AJ, Nalesnik M, Yu YP. Transcriptomic and genomic analysis of human hepatocellular carcinomas and hepatoblastomas. Hepatology. 2006;44:1012-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 271] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 126. | Matsuda Y, Yamagiwa Y, Fukushima K, Ueno Y, Shimosegawa T. Expression of galectin-3 involved in prognosis of patients with hepatocellular carcinoma. Hepatol Res. 2008;38:1098-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 127. | Frachon S, Gouysse G, Dumortier J, Couvelard A, Nejjari M, Mion F, Berger F, Paliard P, Boillot O, Scoazec JY. Endothelial cell marker expression in dysplastic lesions of the liver: an immunohistochemical study. J Hepatol. 2001;34:850-857. [PubMed] |
| 128. | Jia J, Wang J, Teh M, Sun W, Zhang J, Kee I, Chow PK, Liang RC, Chung MC, Ge R. Identification of proteins differentially expressed between capillary endothelial cells of hepatocellular carcinoma and normal liver in an orthotopic rat tumor model using 2-D DIGE. Proteomics. 2010;10:224-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 129. | Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117-1134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3896] [Cited by in RCA: 3518] [Article Influence: 234.5] [Reference Citation Analysis (0)] |
| 130. | Krall JA, Beyer EM, MacBeath G. High- and low-affinity epidermal growth factor receptor-ligand interactions activate distinct signaling pathways. PLoS One. 2011;6:e15945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 131. | Hu Z, Jiang X, Xu Y, Lu N, Wang W, Luo J, Zou H, Zheng D, Feng X. Downregulation of galectin-3 by EGF mediates the apoptosis of HepG2 cells. Mol Cell Biochem. 2012;369:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 132. | Henderson NC, Mackinnon AC, Farnworth SL, Poirier F, Russo FP, Iredale JP, Haslett C, Simpson KJ, Sethi T. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc Natl Acad Sci USA. 2006;103:5060-5065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 515] [Cited by in RCA: 485] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 133. | Yamazaki K, Kawai A, Kawaguchi M, Hibino Y, Li F, Sasahara M, Tsukada K, Hiraga K. Simultaneous induction of galectin-3 phosphorylated on tyrosine residue, p21(WAF1/Cip1/Sdi1), and the proliferating cell nuclear antigen at a distinctive period of repair of hepatocytes injured by CCl4. Biochem Biophys Res Commun. 2001;280:1077-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 134. | Jiang JX, Chen X, Hsu DK, Baghy K, Serizawa N, Scott F, Takada Y, Takada Y, Fukada H, Chen J. Galectin-3 modulates phagocytosis-induced stellate cell activation and liver fibrosis in vivo. Am J Physiol Gastrointest Liver Physiol. 2012;302:G439-G446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 135. | Jiao J, Friedman SL, Aloman C. Hepatic fibrosis. Curr Opin Gastroenterol. 2009;25:223-229. [PubMed] |
| 136. | Wanninger J, Weigert J, Wiest R, Bauer S, Karrasch T, Farkas S, Scherer MN, Walter R, Weiss TS, Hellerbrand C. Systemic and hepatic vein galectin-3 are increased in patients with alcoholic liver cirrhosis and negatively correlate with liver function. Cytokine. 2011;55:435-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 137. | Junking M, Wongkham C, Sripa B, Sawanyawisuth K, Araki N, Wongkham S. Decreased expression of galectin-3 is associated with metastatic potential of liver fluke-associated cholangiocarcinoma. Eur J Cancer. 2008;44:619-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 138. | Wongkham S, Junking M, Wongkham C, Sripa B, Chur-In S, Araki N. Suppression of galectin-3 expression enhances apoptosis and chemosensitivity in liver fluke-associated cholangiocarcinoma. Cancer Sci. 2009;100:2077-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 139. | Erickson SK. Nonalcoholic fatty liver disease. J Lipid Res. 2009;50 Suppl:S412-S416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 156] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 140. | Nomoto K, Tsuneyama K, Abdel Aziz HO, Takahashi H, Murai Y, Cui ZG, Fujimoto M, Kato I, Hiraga K, Hsu DK. Disrupted galectin-3 causes non-alcoholic fatty liver disease in male mice. J Pathol. 2006;210:469-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 141. | Nakanishi Y, Tsuneyama K, Nomoto K, Fujimoto M, Salunga TL, Nakajima T, Miwa S, Murai Y, Hayashi S, Kato I. Nonalcoholic steatohepatitis and hepatocellular carcinoma in galectin-3 knockout mice. Hepatol Res. 2008;38:1241-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 142. | Nomoto K, Nishida T, Nakanishi Y, Fujimoto M, Takasaki I, Tabuchi Y, Tsuneyama K. Deficiency in galectin-3 promotes hepatic injury in CDAA diet-induced nonalcoholic fatty liver disease. ScientificWorldJournal. 2012;2012:959824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 143. | Iacobini C, Menini S, Ricci C, Blasetti Fantauzzi C, Scipioni A, Salvi L, Cordone S, Delucchi F, Serino M, Federici M. Galectin-3 ablation protects mice from diet-induced NASH: a major scavenging role for galectin-3 in liver. J Hepatol. 2011;54:975-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 144. | Vlassara H, Li YM, Imani F, Wojciechowicz D, Yang Z, Liu FT, Cerami A. Identification of galectin-3 as a high-affinity binding protein for advanced glycation end products (AGE): a new member of the AGE-receptor complex. Mol Med. 1995;1:634-646. [PubMed] |
| 145. | Butscheid M, Hauptvogel P, Fritz P, Klotz U, Alscher DM. Hepatic expression of galectin-3 and receptor for advanced glycation end products in patients with liver disease. J Clin Pathol. 2007;60:415-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 146. | Lee WM. Acetaminophen-related acute liver failure in the United States. Hepatol Res. 2008;38 Suppl 1:S3-S8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 147. | Dragomir AC, Sun R, Mishin V, Hall LB, Laskin JD, Laskin DL. Role of galectin-3 in acetaminophen-induced hepatotoxicity and inflammatory mediator production. Toxicol Sci. 2012;127:609-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 148. | Dragomir AC, Sun R, Choi H, Laskin JD, Laskin DL. Role of galectin-3 in classical and alternative macrophage activation in the liver following acetaminophen intoxication. J Immunol. 2012;189:5934-5941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 149. | Volarevic V, Milovanovic M, Ljujic B, Pejnovic N, Arsenijevic N, Nilsson U, Leffler H, Lukic ML. Galectin-3 deficiency prevents concanavalin A-induced hepatitis in mice. Hepatology. 2012;55:1954-1964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 150. | Huflejt ME, Leffler H. Galectin-4 in normal tissues and cancer. Glycoconj J. 2004;20:247-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 132] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 151. | Danielsen EM, Hansen GH. Lipid raft organization and function in the small intestinal brush border. J Physiol Biochem. 2008;64:377-382. [PubMed] |
| 152. | Delacour D, Gouyer V, Zanetta JP, Drobecq H, Leteurtre E, Grard G, Moreau-Hannedouche O, Maes E, Pons A, André S. Galectin-4 and sulfatides in apical membrane trafficking in enterocyte-like cells. J Cell Biol. 2005;169:491-501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 205] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 153. | Satelli A, Rao PS, Thirumala S, Rao US. Galectin-4 functions as a tumor suppressor of human colorectal cancer. Int J Cancer. 2011;129:799-809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 154. | Kondoh N, Wakatsuki T, Ryo A, Hada A, Aihara T, Horiuchi S, Goseki N, Matsubara O, Takenaka K, Shichita M. Identification and characterization of genes associated with human hepatocellular carcinogenesis. Cancer Res. 1999;59:4990-4996. [PubMed] |
| 155. | Hippo Y, Yashiro M, Ishii M, Taniguchi H, Tsutsumi S, Hirakawa K, Kodama T, Aburatani H. Differential gene expression profiles of scirrhous gastric cancer cells with high metastatic potential to peritoneum or lymph nodes. Cancer Res. 2001;61:889-895. [PubMed] |
| 156. | Bidon-Wagner N, Le Pennec JP. Human galectin-8 isoforms and cancer. Glycoconj J. 2004;19:557-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 157. | Stowell SR, Arthur CM, Slanina KA, Horton JR, Smith DF, Cummings RD. Dimeric Galectin-8 induces phosphatidylserine exposure in leukocytes through polylactosamine recognition by the C-terminal domain. J Biol Chem. 2008;283:20547-20559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 158. | Hadari YR, Paz K, Dekel R, Mestrovic T, Accili D, Zick Y. Galectin-8. A new rat lectin, related to galectin-4. J Biol Chem. 1995;270:3447-3453. [PubMed] |
| 159. | Danguy A, Rorive S, Decaestecker C, Bronckart Y, Kaltner H, Hadari YR, Goren R, Zich Y, Petein M, Salmon I. Immunohistochemical profile of galectin-8 expression in benign and malignant tumors of epithelial, mesenchymatous and adipous origins, and of the nervous system. Histol Histopathol. 2001;16:861-868. [PubMed] |
| 160. | Wada J, Kanwar YS. Identification and characterization of galectin-9, a novel beta-galactoside-binding mammalian lectin. J Biol Chem. 1997;272:6078-6086. [PubMed] |
| 161. | Hirabayashi J, Hashidate T, Arata Y, Nishi N, Nakamura T, Hirashima M, Urashima T, Oka T, Futai M, Muller WE. Oligosaccharide specificity of galectins: a search by frontal affinity chromatography. Biochim Biophys Acta. 2002;1572:232-254. [PubMed] |
| 162. | Sato M, Nishi N, Shoji H, Seki M, Hashidate T, Hirabayashi J, Kasai Ki K, Hata Y, Suzuki S, Hirashima M. Functional analysis of the carbohydrate recognition domains and a linker peptide of galectin-9 as to eosinophil chemoattractant activity. Glycobiology. 2002;12:191-197. [PubMed] |
| 163. | Bi S, Earl LA, Jacobs L, Baum LG. Structural features of galectin-9 and galectin-1 that determine distinct T cell death pathways. J Biol Chem. 2008;283:12248-12258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 164. | Türeci O, Schmitt H, Fadle N, Pfreundschuh M, Sahin U. Molecular definition of a novel human galectin which is immunogenic in patients with Hodgkin’s disease. J Biol Chem. 1997;272:6416-6422. [PubMed] |
| 165. | Hirashima M, Kashio Y, Nishi N, Yamauchi A, Imaizumi TA, Kageshita T, Saita N, Nakamura T. Galectin-9 in physiological and pathological conditions. Glycoconj J. 2004;19:593-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 147] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 166. | Fujihara S, Mori H, Kobara H, Rafiq K, Niki T, Hirashima M, Masaki T. Galectin-9 in cancer therapy. Recent Pat Endocr Metab Immune Drug Discov. 2013;7:130-137. [PubMed] |
| 167. | Anderson AC, Anderson DE, Bregoli L, Hastings WD, Kassam N, Lei C, Chandwaskar R, Karman J, Su EW, Hirashima M. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science. 2007;318:1141-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 571] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 168. | Sánchez-Fueyo A, Tian J, Picarella D, Domenig C, Zheng XX, Sabatos CA, Manlongat N, Bender O, Kamradt T, Kuchroo VK. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat Immunol. 2003;4:1093-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 578] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 169. | Zhu C, Anderson AC, Kuchroo VK. TIM-3 and its regulatory role in immune responses. Curr Top Microbiol Immunol. 2011;350:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 170. | Li Y, Feng J, Geng S, Geng S, Wei H, Chen G, Li X, Wang L, Wang R, Peng H. The N- and C-terminal carbohydrate recognition domains of galectin-9 contribute differently to its multiple functions in innate immunity and adaptive immunity. Mol Immunol. 2011;48:670-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 171. | Chou FC, Shieh SJ, Sytwu HK. Attenuation of Th1 response through galectin-9 and T-cell Ig mucin 3 interaction inhibits autoimmune diabetes in NOD mice. Eur J Immunol. 2009;39:2403-2411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 172. | Su EW, Bi S, Kane LP. Galectin-9 regulates T helper cell function independently of Tim-3. Glycobiology. 2011;21:1258-1265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 173. | Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1334] [Cited by in RCA: 1590] [Article Influence: 79.5] [Reference Citation Analysis (0)] |
| 174. | Leitner J, Rieger A, Pickl WF, Zlabinger G, Grabmeier-Pfistershammer K, Steinberger P. TIM-3 does not act as a receptor for galectin-9. PLoS Pathog. 2013;9:e1003253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 175. | Asakura H, Kashio Y, Nakamura K, Seki M, Dai S, Shirato Y, Abedin MJ, Yoshida N, Nishi N, Imaizumi T. Selective eosinophil adhesion to fibroblast via IFN-gamma-induced galectin-9. J Immunol. 2002;169:5912-5918. [PubMed] |
| 176. | Imaizumi T, Kumagai M, Sasaki N, Kurotaki H, Mori F, Seki M, Nishi N, Fujimoto K, Tanji K, Shibata T. Interferon-gamma stimulates the expression of galectin-9 in cultured human endothelial cells. J Leukoc Biol. 2002;72:486-491. [PubMed] |
| 177. | Mengshol JA, Golden-Mason L, Arikawa T, Smith M, Niki T, McWilliams R, Randall JA, McMahan R, Zimmerman MA, Rangachari M. A crucial role for Kupffer cell-derived galectin-9 in regulation of T cell immunity in hepatitis C infection. PLoS One. 2010;5:e9504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 160] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 178. | Yoshida H, Imaizumi T, Kumagai M, Kimura K, Satoh C, Hanada N, Fujimoto K, Nishi N, Tanji K, Matsumiya T. Interleukin-1beta stimulates galectin-9 expression in human astrocytes. Neuroreport. 2001;12:3755-3758. [PubMed] |
| 179. | Saita N, Goto E, Yamamoto T, Cho I, Tsumori K, Kohrogi H, Maruo K, Ono T, Takeya M, Kashio Y. Association of galectin-9 with eosinophil apoptosis. Int Arch Allergy Immunol. 2002;128:42-50. [PubMed] |
| 180. | Heusschen R, Griffioen AW, Thijssen VL. Galectin-9 in tumor biology: a jack of multiple trades. Biochim Biophys Acta. 2013;1836:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 181. | Zhang ZY, Dong JH, Chen YW, Wang XQ, Li CH, Wang J, Wang GQ, Li HL, Wang XD. Galectin-9 acts as a prognostic factor with antimetastatic potential in hepatocellular carcinoma. Asian Pac J Cancer Prev. 2012;13:2503-2509. [PubMed] |
| 182. | Nebbia G, Peppa D, Schurich A, Khanna P, Singh HD, Cheng Y, Rosenberg W, Dusheiko G, Gilson R, ChinAleong J. Upregulation of the Tim-3/galectin-9 pathway of T cell exhaustion in chronic hepatitis B virus infection. PLoS One. 2012;7:e47648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 231] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 183. | Barjon C, Niki T, Vérillaud B, Opolon P, Bedossa P, Hirashima M, Blanchin S, Wassef M, Rosen HR, Jimenez AS. A novel monoclonal antibody for detection of galectin-9 in tissue sections: application to human tissues infected by oncogenic viruses. Infect Agent Cancer. 2012;7:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 184. | Kared H, Fabre T, Bédard N, Bruneau J, Shoukry NH. Galectin-9 and IL-21 mediate cross-regulation between Th17 and Treg cells during acute hepatitis C. PLoS Pathog. 2013;9:e1003422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 185. | Ji XJ, Ma CJ, Wang JM, Wu XY, Niki T, Hirashima M, Moorman JP, Yao ZQ. HCV-infected hepatocytes drive CD4+ CD25+ Foxp3+ regulatory T-cell development through the Tim-3/Gal-9 pathway. Eur J Immunol. 2013;43:458-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 186. | Li H, Wu K, Tao K, Chen L, Zheng Q, Lu X, Liu J, Shi L, Liu C, Wang G. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology. 2012;56:1342-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 394] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 187. | Zwacka RM, Zhang Y, Halldorson J, Schlossberg H, Dudus L, Engelhardt JF. CD4(+) T-lymphocytes mediate ischemia/reperfusion-induced inflammatory responses in mouse liver. J Clin Invest. 1997;100:279-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 291] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 188. | Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol. 1991;260:G355-G362. [PubMed] |
| 189. | Hanschen M, Zahler S, Krombach F, Khandoga A. Reciprocal activation between CD4+ T cells and Kupffer cells during hepatic ischemia-reperfusion. Transplantation. 2008;86:710-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 190. | Uchida Y, Ke B, Freitas MC, Yagita H, Akiba H, Busuttil RW, Najafian N, Kupiec-Weglinski JW. T-cell immunoglobulin mucin-3 determines severity of liver ischemia/reperfusion injury in mice in a TLR4-dependent manner. Gastroenterology. 2010;139:2195-2206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 191. | Lv K, Zhang Y, Zhang M, Zhong M, Suo Q. Galectin-9 ameliorates Con A-induced hepatitis by inducing CD4(+)CD25(low/int) effector T-Cell apoptosis and increasing regulatory T cell number. PLoS One. 2012;7:e48379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 192. | Liberal R, Grant CR, Holder BS, Ma Y, Mieli-Vergani G, Vergani D, Longhi MS. The impaired immune regulation of autoimmune hepatitis is linked to a defective galectin-9/tim-3 pathway. Hepatology. 2012;56:677-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 193. | Ronchi F, Falcone M. Immune regulation by invariant NKT cells in autoimmunity. Front Biosci. 2008;13:4827-4837. [PubMed] |
| 194. | Godfrey DI, Hammond KJ, Poulton LD, Smyth MJ, Baxter AG. NKT cells: facts, functions and fallacies. Immunol Today. 2000;21:573-583. [PubMed] |
| 195. | Tang ZH, Liang S, Potter J, Jiang X, Mao HQ, Li Z. Tim-3/galectin-9 regulate the homeostasis of hepatic NKT cells in a murine model of nonalcoholic fatty liver disease. J Immunol. 2013;190:1788-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 196. | Glinskii OV, Sud S, Mossine VV, Mawhinney TP, Anthony DC, Glinsky GV, Pienta KJ, Glinsky VV. Inhibition of prostate cancer bone metastasis by synthetic TF antigen mimic/galectin-3 inhibitor lactulose-L-leucine. Neoplasia. 2012;14:65-73. [PubMed] |
| 197. | Liu HY, Huang ZL, Yang GH, Lu WQ, Yu NR. Inhibitory effect of modified citrus pectin on liver metastases in a mouse colon cancer model. World J Gastroenterol. 2008;14:7386-7391. [PubMed] |
| 198. | Nangia-Makker P, Hogan V, Honjo Y, Baccarini S, Tait L, Bresalier R, Raz A. Inhibition of human cancer cell growth and metastasis in nude mice by oral intake of modified citrus pectin. J Natl Cancer Inst. 2002;94:1854-1862. [PubMed] |