Published online Dec 14, 2013. doi: 10.3748/wjg.v19.i46.8552
Revised: September 28, 2013
Accepted: October 13, 2013
Published online: December 14, 2013
Processing time: 114 Days and 0.4 Hours
Cystic fibrosis (CF) causes chronic infections in the respiratory tract and alters the digestive tract. This paper reviews the most important aspects of drug treatment and changes in the digestive tract of patients with CF. This is a review of the literature, emphasizing the discoveries made within the last 15 years by analyzing scientific papers published in journals indexed in the Scientific Electronic Library Online, Sciences Information, United States National Library of Medicine and Medical Literature Analysis and Retrieval System Online databases, both in English and Portuguese, using the key words: cystic fibrosis, medication, therapeutic, absorption, digestion. Randomized, observational, experimental, and epidemiological clinical studies were selected, among others, with statistical significance of 5%. This review evaluates the changes found in the digestive tract of CF patients including pancreatic insufficiency, constipation and liver diseases. Changes in nutritional status are also described. Clinical treatment, nutritional supplementation and drug management were classified in this review as essential to the quality of life of CF patients, and became available through public policies for monitoring and treating CF. The information gathered on CF and a multi professional approach to the disease is essential in the treatment of these patients.
Core tip: Cystic fibrosis (CF) has been studied in Brazil and in many other countries. Digestive manifestations may significantly compromise the nutritional status of CF patients, leading to numerous symptoms. Supplementation with enzymes, vitamins and nutrients is usually necessary. When infections are present, antibiotics are necessary, and these infections are often multisystemic, involving the digestive tract. The pharmaceutical assistance included in public policies, especially those which are financed, and the constant incentive to study the digestive manifestations in CF patients are essential, as without them, there would be infinite clinical changes which would compromise patient survival.
- Citation: Haack A, Aragão GG, Novaes MRCG. Pathophysiology of cystic fibrosis and drugs used in associated digestive tract diseases. World J Gastroenterol 2013; 19(46): 8552-8561
- URL: https://www.wjgnet.com/1007-9327/full/v19/i46/8552.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i46.8552
Cystic fibrosis (CF) is a chronic progressive disease, it exists in every ethnic group and it is equally common in both sexes. The CF gene has been isolated, cloned and sequenced, enabling the study of biochemical mechanisms responsible for the physiopathogenesis of the disease. It also enables easier treatment of the patient’s complications, such as the thick and viscous fluids which obstruct the lungs, the pancreas and the biliary duct[1,2].
The prevalence of CF varies according to ethnicity, from 1/1800 to 1/5000 in Caucasians born alive in Europe, in the United States and in Canada, 1/14000 in Afro-Americans, and 1/40000 in Finland. It is considered a rare disease among Asians and Africans. In Brazil, local studies show variable statistical data which suggest an approximate incidence of 1/7000. The average lifetime of CF patients has increased in the last few years, which is the result of early diagnosis and specialized treatment in the early stages of the disease[1,3,4].
The treatment of CF aims to clear the lungs using aerosols and respiratory physiotherapy, and to maintain nutritional status with nutrient supplementation and pancreatic enzymes. Recent medical advances have improved survival, but with increased costs, especially when the disease has progressed and when hospitalization is required. When infections are present, antibiotics are necessary, usually due to clinical complications which are often multisystemic, and involve the digestive tract[5,6]. Due to many involved systems and the variety and chronicity of the disease, a multitask approach is essential to help the patients and their families to comprehend the disease and undergo medical treatment[7].
The current therapy for CF includes the maintenance of nutritional status, clearance of the pulmonary tract, utilization of antibiotics and other medication, treatment and monitoring of gastric, pancreatic and hepatobiliary changes, in addition to dietary supplementation with hypercaloric and hyperproteic foods, and the utilization of enzymes, minerals and vitamins[1,8,9].
When chronic CF is diagnosed, with many clinical manifestations, the continuous use of medication (antibiotics, bronchodilators, mucolytics) and related procedures (respiratory physiotherapy, oxygen therapy, lung transplantation, digestive enzyme replacement and nutritional support) are required[8,10]. Due to the chronicity and the need for precautions in CF, the development of a Reference Center and the establishment of an organization that involves family members is crucial, together with an increase in cooperation between groups of CF patients and other organizations[4,11,12].
CF requires the continuous use of medication which increases the average cost of treatment, and is too expensive for families. For that reason, CF patients and their families have the right to receive government help under the Unique Health System. The clinical record of the Health Ministry guarantees access to alpha dornase for pulmonary complications and pancreatic enzymes in patients with pancreatic insufficiency[3]. There are many deeds in every unit of the federation, including the Distrito Federal, to promote early diagnosis and even provide special formulas such as the alimentary supplements provided by Ordinance number 94/1809, published at the Distrito Federal in 2009[13].
In Brazil, the dedication to diagnosing CF during infancy is significant, with the use of programs for newborn screening or sweat testing. It is known that early treatment, including drug treatment, contributes to the prognosis and survival of CF patients[14-17].
The objective of this study was to review the most important aspects of drug treatment and changes in the digestive tract of patients with CF. We also aimed to assess the pharmaceutical monitoring offered to CF patients undergoing treatment by public agents from the public health care system.
This review focused on CF literature over the last 15 years, and included scientific papers indexed in the databases of Scientific Electronic Library Online, Sciences Information, United States National Library of Medicine and Medical Literature Analysis and Retrieval System Online, using the key words: cystic fibrosis, medication, therapeutic, absorption, digestion. Studies in English and Portuguese were selected.
The survey focused on the major advances in the understanding of CF during this period, both in understanding the disease and its treatment.
Articles that included at least one of the mentioned key words were selected. Controlled clinical studies were included, as well as observational epidemiological studies and meta-analyses, among others. Papers which did not include information on the diagnosis of CF or adherence to treatment were excluded as were experimental animal studies and gene therapy studies and those published in languages other than English and Portuguese.
The manifestation of CF is very changeable and may appear in the neonatal period or later in life. Some patients are completely asymptomatic for several years. The most common clinical signs of CF include a chronic cough, chronic diarrhea and malnutrition; however, the disease can appear in other ways, and can affect multiple systems and organs[18].
Mutation of the CF gene causes absence or dysfunction of the cystic fibrosis transmembrane conductance regulator (CFTR) protein, which works as a chloride canal in the apical membranes of epithelial cells. The CFTR also affects the production of mucus, secretory granules and intracellular organelles. This defect affects cells in many organs, not all organs have similar clinical responses, and different organs may be affected. Involvement of the respiratory tract is associated with a higher death rate and leads to death in 90% of patients[18-20].
The most common and important symptom which affects the digestive tract is exocrine pancreatic insufficiency, characterized by chronic diarrhea with undigested food present. A decrease in the secretion of sodium bicarbonate reduces the efficacy of pancreatic enzymes and the precipitation of bile salts, which results in a more acidic pH in the duodenum, contributing to malabsorption[18].
The obstruction of pancreatic canaliculi by mucous plugs prevents the release of enzymes into the duodenum, which causes poor digestion of fat, proteins and carbohydrates. Malabsorption is caused by pre-epithelial dysfunction, which occurs after the rejection of non-hydrolysable nutrients in the lumen. Therefore, malnutrition occurs due to inadequate food digestion and increased energy needs (dietary recommendations) that are rarely achieved by CF patients due to anorexia and recurrent respiratory disease among other diseases[18,21-23].
The endocrine pancreas also undergoes changes and the prevalence of CF related to glucose intolerance has increased proportionally with the rate of survival. The main cause of diabetes is damage caused to the pancreas, leading to a decrease in insulin secretion. Diabetes in CF patients results from microvascular and macrovascular complications associated with accelerated lung deterioration, consequently increasing the death rate. Since nutrition is critical in CF patients, blood glucose should be monitored and the insulin dose should be adapted, with a focus on adequate intake of nutrients[24].
Symptomatic vitamin A and vitamin E deficiency has been reported in patients with CF presenting with deficit nutrient consumption and absorption[25,26].
Many newly diagnosed infants have low levels of one or more fat-soluble vitamins[27,28] and due to the prevalence of fat-soluble vitamin deficiency, all infants with CF should receive standard, age-appropriate non-fat-soluble vitamins and vitamins A, D, E, and K as recommended in the CF Foundation Consensus Report on Nutrition for Pediatrics[29].
Most patients who are vitamin deficient can be treated adequately with the doses of fat-soluble vitamins recommended in the CF Foundation Consensus Report on Nutrition for Pediatric Patients[30].
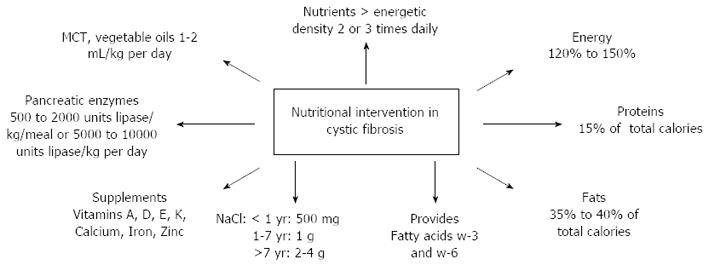
Figure 1 shows relevant information on the nutritional care of CF patients[7].
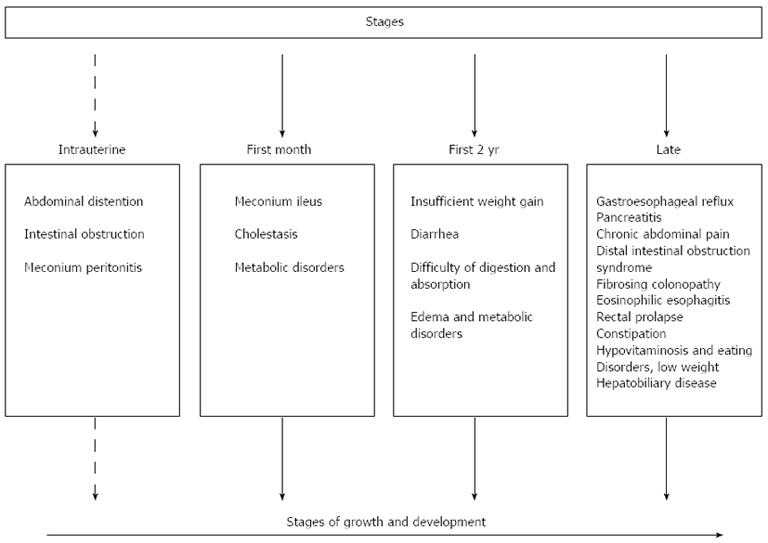
Among other events related to CF, meconium ileus, obstruction of the terminal ileum by thick meconium, is the first signal of pancreatic insufficiency, which affects 15% of babies. Therefore, treating patients with meconium ileus is very important until proved otherwise[31].
Early diagnosis and the treatment of complications of the respiratory and gastrointestinal tract in CF can lead to an improvement in the survival rate of CF patients. Those who live beyond the fourth decade have a higher risk of developing additional diseases associated with chronic manifestations; hence, patients with a higher risk of chronic diseases should be monitored closely to improve the chances of early diagnosis[32].
Figure 2 summarizes the majority of abnormalities observed in the digestive tract of patients diagnosed with CF from intrauterine life to adulthood.
In CF patients gastrointestinal symptoms, such as nausea, vomiting, malnutrition and indigestion are frequent. In addition, gastroesophageal reflux disease, esophageal adenocarcinoma, distal intestinal syndrome and cholelithiasis are often seen in CF patients[33-35].
There is increasing evidence to suggest that chronic inflammation is present in the gastrointestinal tract of CF patients. Some CF patients continue to have many severe gastrointestinal symptoms despite conventional CF treatment[36].
A recent publication indicated the presence of eosinophilic esophagitis (EoE) in CF patients aged from 4, 12 and 15 years. Patients with CF may have clinically persistent emesis, food aversion and failure to thrive. It is possible that EoE has been underappreciated in CF due to symptom overlap with other common gastrointestinal disorders, including gastroesophageal reflux disease, infections, medication side effects or others conditions[37].
Because the symptoms in EoE are non-specific and are also common in CF, when a patient with CF presents with food avoidance, regurgitation, heartburn or dysphagia, EoE should be considered, particularly if symptoms do not respond to empiric treatment and if endoscopic evaluation is contemplated[38-40].
Secretory cells of CF patients show modification in their absorptive-digestive function in the gastrointestinal tract and the entire digestive process is altered, which results in malabsorption of nutrients, malnutrition and several gastrointestinal tract-related symptoms[34,41].
Abdominal pain is a common complaint in CF patients, and distal bowel obstruction syndrome and fibrosing colonopathy are characteristics of gastrointestinal complications in CF patients. The main causes of epigastric pain in patients with CF are gastroesophageal reflux disease, biliary tract disease, pancreatitis and gastritis[42,43].
Among the frequently observed gastrointestinal manifestations, gastroparesis has been diagnosed by a variety of methods and has been described by CF patients. Gastroparesis is a frequent complication of lung or heart-lung transplantation. It is predominantly found in children and individuals with severe deterioration of the pulmonary tract[43,44].
After meconium ileus, the main area affected by distal bowel obstruction syndrome (DIOS) is the right colon. DIOS is more common in patients with pancreatic insufficiency. Several factors can trigger the syndrome, such as dehydration, the use of medicines which interfere with intestinal motility and pancreatic enzyme replacement. The most common signs and symptoms of DIOS are decreased defecation and colic pain in the right lower quadrant. During clinical examination, a reduction in intestinal peristalsis can be observed, with the possibility of cessation at some point. In some cases, a mass in the lower right quadrant can be palpated, which is related to distention of the cecum and right colon[45].
Intestinal obstruction syndrome is similar to meconium ileus; however, one of the differences between these conditions is patient age. Intestinal obstruction syndrome is characterized by the impaction of fecal residues in the terminal ileum and one of the precipitant factors for obstruction is dehydration. This obstruction can be total or partial, and may cause symptoms such as abdominal distention, constipation, anorexia, vomiting, and early satiety, which result in weight loss[45].
Fibrosing colonopathy is another characteristic of CF, and includes a change in the colon submucosa, inflammation, and progressive fibrosis associated with managing the high doses of pancreatic enzymes. The clinical symptoms are pain and abdominal distention after ingesting food, anorexia, difficulty in gaining weight and digestive bleeding[34,46].
The pancreas is one of the main organs affected by dysfunction of the CFTR. The exocrine pancreas is responsible for producing enzymes for food digestion in the intestinal lumen and exocrine pancreatic insufficiency is a well-known complication of CF and leads to fat loss in feces. Loss of function of the pancreas is associated with every genotype of CFTR mutation, leading to pancreatic insufficiency[47-49].
Pancreatic exocrine insufficiency (PEI) is considered the main cause of intestinal malabsorption in CF, affecting 85% to 90% of patients[50], and if inadequately treated high stool energy losses will occur, which is an important determinant of energy imbalance and malnutrition[51].
Intestinal malabsorption is usually of early onset: signs and symptoms of maldigestion are often present at birth, and in the majority of patients, during the first years of life. At the time of diagnosis, at least 50% of infants identified by neonatal screening have PEI, and most of those carrying severe CFTR mutations on both alleles develop PEI during the first years of life[52-55].
PEI is clinically characterized by weight loss or difficulty in gaining weight, diarrhea with a greasy appearance and malabsorption of fat-soluble vitamins A, D, E and K. Thus, the supplementation of these vitamins is routinely recommended, followed by blood examinations to manage the dose and the correct nutrients according to the patient’s needs[27,56-58].
Vitamin D is of great interest in CF due to its role in bone mineralization and its deficiency has been hypothesized to play a role in the development of depression. Hypovitaminosis is almost universal in patients with CF. Insufficient levels are widely reported and is associated with increasing age and obesity. Vitamin D screening and supplementation should be considered in all children with chronic illness, particularly those who are overweight[59-62].
Table 1 shows treatments with fat-soluble vitamin supplementation in CF patients[1].
The primary hepatic changes in CF involve a genetic defect in the CFTR protein, leading to the production of a thick biliary secretion, followed by biliary fibrosis[34]. Cirrhosis, ascites, portal hypertension, esophageal varices and bleeding are complications of hepatobiliary disease associated with CF, and frequently affect teenagers and adults[33].
This dysfunction is predicted to result in defective (sluggish) bile flow, and is associated with a cholangiocyte-induced inflammatory response with activation and proliferation of hepatic stellate cells, which results in cholangitis and fibrosis in focal portal tracts[63-66].
Approximately 5%-10% of CF patients develop multilobular cirrhosis during their first decade of life. Subsequently, most tend to develop signs of hypertension with complications, especially variceal bleeding. Annual examinations are recommended to detect hepatic disease, and when presymptomatic signs are present therapy with ursodeoxycholic acid is recommended, which can prevent disease progression[67,68].
Cystic fibrosis-related liver disease (CFLD) is defined if at least 2 of the following conditions are present on at least 2 consecutive examinations spanning a 1-year period: (1) Ultrasound confirmed hepatomegaly; (2) Elevated serum levels of alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, gamma-glutamyltransferase; and (3) Ultrasound abnormalities other than hepatomegaly (i.e., increased, heterogeneous echogenicity, nodularity, irregular margins, splenomegaly). An ultrasonographic pattern of simple liver steatosis does not represent a diagnostic criterion. In the case of distinct ultrasonographic signs of liver cirrhosis (i.e., coarse nodularity, presence of portal hypertension and rarefaction of peripheral portal veins) and clinical signs (e.g., esophageal varices, splenomegaly) of liver cirrhosis, CFLD patients are classified as cirrhotics[63,69].
Liver disease can only be taken into consideration if the physical examination is abnormal and abnormal hepatic function persists, and the latter has to be proved using ultrasound. If there are any doubts, a liver biopsy is suggested. All patients with liver disease require to be monitored annually to evaluate the progress of hypertension, portal cirrhosis or liver failure. Prophylactic measures for liver disease are nutrition monitoring, bleeding prevention and variceal decompression. In liver transplantation, deterioration of the organ has to be taken into consideration, especially in children with hepatic dysfunction or advanced hypertension[68].
Treatment with pancreatic enzymes in patients with pancreatic insufficiency is associated with an increase in the coefficient of fat absorption, a decrease in bowel movement frequency, an improvement in the consistency of feces and weight gain. One of the aims of pancreatic enzyme replacement therapy is to abolish unpleasant gastrointestinal symptoms[45].
The response to treatment is individually evaluated, and doses are adjusted according to nutritional status. The use of antacids is recommended in patients taking enzymes to increase bioavailability, although, there is insufficient evidence to indicate whether there is an improvement in quality of life or survival[1,70].
In young children whose fat intake is known to vary with age, particular attention needs to be paid to fat malabsorption during pancreatic enzyme supplementation. More importantly, young children often have difficulty swallowing the available enzyme formulations, which may lead to suboptimal compliance and treatment effects[71].
The initial dose of pancreatic enzymes can be calculated based on the weight of the patient taking into consideration the dietary fat intake. 500 to 1000 U of lipase/kg is administered per main meal, the dosage can be increased according to clinical signs, and the maximum daily dose should not exceed 2500 U/kg per meal or 10000 U/kg per day of lipase[3].
Figure 1 summarizes pancreatic enzyme dosage[7].
The guidelines recommend that if dose increases are required, they should be increased with careful monitoring of body weight and stool fat content. When controlled clinical trials are designed to assess the safety and efficacy of pancreatic enzyme replacement therapy, the dose in terms of lipase units is usually limited to a level within the recommended range. However, in everyday clinical practice it is possible that maldigestion is not adequately controlled by the recommended doses in a proportion of CF patients: these patients may, therefore, require higher lipase doses[72-74].
The United Kingdom Cystic Fibrosis database indicates that lipase dose often exceeds 10000 U/kg per day for extended periods in clinical practice, both with standard-dose and high-dose pancreatic enzyme preparations. These high-dose regimens appear to have good safety and tolerability profiles, and fibrosing colonopathy has not been reported in recent years. However, it is essential that the safety and efficacy of higher doses of pancreatic enzyme replacement therapy are fully explored, particularly in the long-term, clinical practice setting[74,75].
There are several options for the treatment of EoE, including pharmaceutical agents and dietary elimination. Consensus recommendations advocate first-line treatment with oral corticosteroids (e.g., fluticasone, budesonide) or dietary therapy depending on patient preference and illness severity[38].
Dietary therapy can be very effective in children if culprit food allergens are identified, and recent data show this to be effective including the elimination of offending agents (targeted elimination diet), or an allergen-free diet consisting only of an elemental formula (elemental diet)[76-78].
The correction of steatorrhea is essential in CF. In the past, diets low in fat were recommended to try to reduce steatorrhea. Currently, restrictive diets have been replaced by hypercaloric diets rich in fat, which is a source of energy, are more economical and their intake should be encouraged[79]. The dose and timing should be followed very strictly, and patients should adhere to treatment. For infants, apple juice or small quantities of milk are consumed, and meals should be carried out in block in order to benefit from the bioavailability of the entire quantity of administered enzyme[49].
Medium chain triglyceride fats should be included in the standard dietary regimen used in the management of any child with CF and failure to thrive. Their use is fully justified due to clinical improvement and alleviation of steatorrhoea[80].
In clinical practice, probiotics have been frequently prescribed for patients suffering from diarrhea to protect the body against pathogens[81].
A probiotic is a “live microbial food ingredient that, when ingested in sufficient quantities, exerts health benefits on the consumer”. Probiotics exert their benefits through several mechanisms; they prevent colonization, cellular adhesion and invasion by pathogenic organisms. The strongest evidence for their clinical effectiveness has been in their use for the prevention of symptoms of lactose intolerance, treatment of diarrhea, and attenuation of antibiotic-associated gastrointestinal side effects[81].
Probiotics reduce the rate of pulmonary exacerbations in patients and may have preventive potential for pulmonary deterioration in CF patients[82-84].
To ensure a continuous effect, probiotics and prebiotics need to be ingested daily. Favorable changes in the composition of intestinal microbiota were observed at doses of 100 g of food product containing 109 colony forming units (cfu) of probiotic microorganisms and doses of 5 to 20 g inulin and/or oligofructose, usually during the administration period of 15 d. Thus, to be of physiological importance to the consumer, probiotics must reach populations greater than 106 to 107 cfu/g or mL bioproduct[85].
The goal of nutritional therapy is to maintain the ideal weight, reduce malabsorption and digestion and control the intake of vitamins and minerals[1]. CF patients require diets with a high energetic rate (120% to 150% of the regular daily need for weight, height and age), hypercaloric, high-fat and high protein, divided into 5-6 meals a day and supplemented with vegetable oils such as medium chain triglycerides. In cases where dietary treatment does not result in weight gain, the diet can be offered in small volumes, several times a day or administered in the evening of through a nasogastric tube or gastrostomy. Enteral tube feeding has been evaluated in pediatric and mixed child and adult populations with CF, demonstrating positive outcomes post-insertion. The diet may be administered through an infusion pump or gravitational and it is recommended that the night diet reaches 40%-50% of the daily energy requirements so that there will be recovery or maintenance of the nutritional state[7,86,87].
CF may include intestinal inflammation and CF patients have altered fatty acid metabolism characterized by an imbalance in the arachidonic/docosahexaenoic acid ratio in favor of the former, which can contribute to an increase in inflammation. Recent studies indicate that changes in fatty acid metabolism are responsible for abnormalities, and dietary supplementation with fish oils high in the omega-3 fatty acids, eicosapentaenoic acid and docosahexaenoic acid may have an anti-inflammatory effect[88-91].
Various anti-inflammatory therapies, including dietary omega-3 polyunsaturated fatty acids supplementation, have been investigated in CF patients. The composition of dietary omega-3 and omega-6 influenced the inflammatory markers in CF and dietetic integration seems to improve clinical condition and the inflammatory pulmonary and intestinal state in patients suffering from CF[92,93].
With a partial bowel obstruction, intestinal disimpaction is stimulated by hypertonic solutions, such as N-acetylcysteine, polyethylene glycol or hypertonic contrast, orally or by using probes. In cases of total obstruction the disimpaction is performed through enemas, while keeping the patient hydrated. After the disimpaction, pancreatic enzyme treatment should be included in the preventive treatment in obstructive conditions, administering lactulose, mineral oil, polyethylene glycol or N-acetylcysteine to the patient. Prokinetic drugs may also be helpful[34].
It is recommended that, in the case of fibrosing colonopathy, there is a reduction in the enzyme dose associated with nutritional support with either semi-elemental or elemental formulas according to the evaluation by the nutritionist for nutritional enteral therapy, and if necessary, associated with parenteral nutrition in the most severe cases. In the case of digestive bleeding, a surgical procedure is prescribed[34,46].
The treatment of liver diseases focuses mainly on preventing disease progression which follows the sequence of cholestasis, fibrosis and cirrhosis. The maintenance of nutritional status is a part of this treatment, and aims to achieve and maintain the ideal weight of the patient, reduce malabsorption and maldigestion and control the intake of vitamins and minerals. However, nutritional treatment consists of enzyme replacement therapy, hypercaloric, high fat and micronutrient supplementation diets[1].
Supplementation with taurine has also been suggested to improve the solubilization of lipid micelles by bile acids. Taurine is a conditionally essential amino acid that possibly improves the micellar phase of fat digestion. Patients with CF and severe steatorrhea, despite appropriate enzyme therapy, showed a significant improvement in the absorption of triglycerides, total fatty acids, and linoleic acid while receiving taurine supplements. Taurine supplementation could be a useful adjunct in the management of patients with CF with ongoing fat malabsorption and essential fatty acid deficiency[94,95].
If CF patients also have taurine deficiency, this will result in malabsorption of bile acid and will require treatment with ursodeoxycholic acid (UDCA). The use of UDCA can increase the need for taurine administration for conjugation of bile acid[33].
UDCA is the drug currently used in CF patients and aims to slow the progression of liver disease. UDCA is a hydrophilic drug and is not significantly concentrated in bile. It has a hepatoprotective effect with rare collateral effects reported[33] and is frequently used in CF. UDCA inhibits the hepatic synthesis of cholesterol and promotes the synthesis of bile acids, thereby restoring the necessary balance between cholesterol and bile salts. The suggested dose is 14-18 mg/kg per day, 2 to 3 times a day up to 30 mg/kg per day[3,96].
Although it is one of the therapeutic options currently used for early changes in the liver, the use of UDCA as a preventive method requires further investigation as there are insufficient data on its long-term use, although adverse effects are rarely reported[97].
Liver transplantation may be necessary in patients with progressive liver failure and/or evidence of major portal hypertension in the absence of significant pulmonary involvement[98,99].
Careful monitoring and treatment should be offered to patients with CF associated liver disease (CFALD) and portal hypertension as they may require supplemental feeding by gastrostomy. However, this could lead to the development of stomal varices, which is an unwanted complication. A recent study evaluated the risk of gastrostomy in a series of seven children with CFALD and portal hypertension. The research concluded that gastrostomy placement for poor nutrition in children with CFALD and portal hypertension is safe and contributes to improved nutritional and pulmonary outcome[100].
CF is a multisystem disease and therefore requires different input from different professional reference centers for the treatment and monitoring of CF, supported by public health policies.
CF has been extensively studied in Brazil and many other countries. Digestive manifestations significantly compromise the nutritional status of the patient and lead to numerous symptoms, organ deterioration, the need for transplantation and resections which can worsen the multisystem disease.
Reference Centers with up-to-date medical teams to monitor and treat CF patients and initiatives such as the Brazilian Cystic Fibrosis Research Group can contribute to the dissemination and standardization of information, in addition to improving the quality of treatment.
The scientific literature contains an important variety of drugs, including many that are available without charges through programs from the Unique Health System, Brazil.
The pharmaceutical assistance and the constant incentive to study digestive manifestations in CF patients are essential, as without them, there would be infinite clinical changes that would compromise patient survival.
P- Reviewer: Wong GLH S- Editor: Ma YJ L- Editor: Webster JR E- Editor: Zhang DN
| 1. | Rosa FR, Dias FG, Nobre LN, Morais HA. Fibrose cística: uma abordagem clínica e nutricional. Rev Nutr. 2008;21:725-737. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Salvatore D, Buzzetti R, Baldo E, Furnari ML, Lucidi V, Manunza D, Marinelli I, Messore B, Neri AS, Raia V. An overview of international literature from cystic fibrosis registries. Part 4: update 2011. J Cyst Fibros. 2012;11:480-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Brasil, Ministério da Saúde. Protocolo Clínico e Diretrizes Terapêuticas. Fibrose Cística-Manifestações Pulmonares. Portaria SAS/MS n 224 de 10 de maio de 2010. Available from: http://portal.saude.gov.br/portal/arquivos/pdf/pcdt_fibrose_cistica_manif_pulm_livro_2010.pdf. |
| 4. | Silva Filho LVRF, Reis FJC, Damaceno N, Hira AY. Registro Brasileiro de Fibrose Cística. Grupo brasileiro de estudos de fibrose cística. Ano 2010. Available from: http://www.gbefc.org.br/gbefc/estudo_gbefc_2010.pdf. |
| 5. | Pizzignacco TP, Mello DF, Lima RG. [The experience of disease in cystic fibrosis: the paths to comprehensive care]. Rev Esc Enferm USP. 2011;45:638-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | van Gool K, Norman R, Delatycki MB, Hall J, Massie J. Understanding the costs of care for cystic fibrosis: an analysis by age and health state. Value Health. 2013;16:345-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Haack A, Carvalho Garbi Novaes MR. Multidisciplinary care in cystic fibrosis: a clinical-nutrition review. Nutr Hosp. 2012;27:362-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 8. | Flume PA, Van Devanter DR. State of progress in treating cystic fibrosis respiratory disease. BMC Med. 2012;10:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Munck A. Nutritional considerations in patients with cystic fibrosis. Expert Rev Respir Med. 2010;4:47-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | George PM, Banya W, Pareek N, Bilton D, Cullinan P, Hodson ME, Simmonds NJ. Improved survival at low lung function in cystic fibrosis: cohort study from 1990 to 2007. BMJ. 2011;342:d1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Alvarez AE, Ribeiro AF, Hessel G, Bertuzzo CS, Ribeiro JD. [Cystic fibrosis at a Brazilian center of excellence: clinical and laboratory characteristics of 104 patients and their association with genotype and disease severity]. J Pediatr (Rio J). 2004;80:371-379. [PubMed] |
| 12. | Ranganathan SC, Parsons F, Gangell C, Brennan S, Stick SM, Sly PD. Evolution of pulmonary inflammation and nutritional status in infants and young children with cystic fibrosis. Thorax. 2011;66:408-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 13. | Distrito Federal. Secretaria de Estado de Saúde. Portaria nº 94, de 20 de maio de 2009. Regulamento técnico para fornecimento de fórmulas para fins especiais para atendimento domiciliar no âmbito do Distrito Federal. Available from: http://www.saude.df.gov.br/sobre-a-secretaria/subsecretarias/502-alimentacao-e-nutricao.html. |
| 14. | Distrito Federal. Secretaria de Estado de Saúde. Lei nº 4.190 de 06 de agosto de 2008. Assegura a todas as crianças nascidas nos hospitais e demais estabelecimentos de atenção à saúde do Distrito Federal o direito ao teste de triagem neonatal, na sua modalidade ampliada. Available from: http://www.buriti.df.gov.br/ftp/diariooficial/2008/08_Agosto/DODF%20155%2011-08-08/Se%C55.pdf. |
| 15. | Wagener JS, Sontag MK, Accurso FJ. Newborn screening for cystic fibrosis. Curr Opin Pediatr. 2003;15:309-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Heidendael JF, Tabbers MM, De Vreede I. False negative newborn screen and neonatal cholestasis in a premature child with cystic fibrosis. Eur J Pediatr. 2013;Epub ahead of print. [PubMed] |
| 17. | Munck A, Houssin E, Roussey M. The importance of sweat testing for older siblings of patients with cystic fibrosis identified by newborn screening. J Pediatr. 2009;155:928-930.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Reis FJ, Damaceno N. [Cystic fibrosis]. J Pediatr (Rio J). 1998;74 Suppl 1:S76-S94. [PubMed] |
| 19. | Kerem E, Viviani L, Zolin A, Macneill S, Hatziagorou E, Ellemunter H, Drevinek P, Gulmans V, Krivec U, Olesen H; on behalf of the ECFS Patient Registry Steering Group. Factors associated with FEV1 decline in cystic fibrosis: analysis of the data of the ECFS Patient Registry. Eur Respir J. 2013;Epub ahead of print. [PubMed] |
| 20. | Abrams SA. Chronic pulmonary insufficiency in children and its effects on growth and development. J Nutr. 2001;131:938S-941S. [PubMed] |
| 21. | Panagopoulou P, Maria F, Nikolaou A, Nousia-Arvanitakis S. Prevalence of Malnutrition and Obesity Among Cystic Fibrosis Patients. Pediatr Int. 2013;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Haack A, Novaes MRG. Clinical and nutritional aspects of cystic fibrosis patients assisted by a home enteral nutrition program in Brazil. Rev Chil Nutr. 2013;40:112. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Wat D, Gelder C, Hibbitts S, Cafferty F, Bowler I, Pierrepoint M, Evans R, Doull I. The role of respiratory viruses in cystic fibrosis. J Cyst Fibros. 2008;7:320-328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 182] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 24. | Brennan AL, Geddes DM, Gyi KM, Baker EH. Clinical importance of cystic fibrosis-related diabetes. J Cyst Fibros. 2004;3:209-222. [PubMed] |
| 25. | Rayner RJ, Tyrrell JC, Hiller EJ, Marenah C, Neugebauer MA, Vernon SA, Brimlow G. Night blindness and conjunctival xerosis caused by vitamin A deficiency in patients with cystic fibrosis. Arch Dis Child. 1989;64:1151-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Sitrin MD, Lieberman F, Jensen WE, Noronha A, Milburn C, Addington W. Vitamin E deficiency and neurologic disease in adults with cystic fibrosis. Ann Intern Med. 1987;107:51-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Feranchak AP, Sontag MK, Wagener JS, Hammond KB, Accurso FJ, Sokol RJ. Prospective, long-term study of fat-soluble vitamin status in children with cystic fibrosis identified by newborn screen. J Pediatr. 1999;135:601-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 99] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Marcus MS, Sondel SA, Farrell PM, Laxova A, Carey PM, Langhough R, Mischler EH. Nutritional status of infants with cystic fibrosis associated with early diagnosis and intervention. Am J Clin Nutr. 1991;54:578-585. [PubMed] |
| 29. | Borowitz D, Robinson KA, Rosenfeld M, Davis SD, Sabadosa KA, Spear SL, Michel SH, Parad RB, White TB, Farrell PM. Cystic Fibrosis Foundation evidence-based guidelines for management of infants with cystic fibrosis. J Pediatr. 2009;155:S73-S93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 284] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 30. | Koscik RL, Farrell PM, Kosorok MR, Zaremba KM, Laxova A, Lai HC, Douglas JA, Rock MJ, Splaingard ML. Cognitive function of children with cystic fibrosis: deleterious effect of early malnutrition. Pediatrics. 2004;113:1549-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 31. | Blackman SM, Deering-Brose R, McWilliams R, Naughton K, Coleman B, Lai T, Algire M, Beck S, Hoover-Fong J, Hamosh A. Relative contribution of genetic and nongenetic modifiers to intestinal obstruction in cystic fibrosis. Gastroenterology. 2006;131:1030-1039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 32. | O’Donnell DH, Ryan R, Hayes B, Fennelly D, Gibney RG. Hepatocellular carcinoma complicating cystic fibrosis related liver disease. J Cyst Fibros. 2009;8:288-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Schoeller CD, Ferreira JEP, Gastaldi LA, Wayhs MLC. Doença gastrintestinal. Fibrose Cística enfoque multidisciplinar. Florianópolis: Hospital Infantil Joana de Gusmão 2009; 213-237. |
| 34. | Monteiro FM, Cunha RCO. Vias aéreas superiores. Fibrose Cística enfoque multidisciplinar. Florianópolis: Hospital Joana de Gusmão 2009; 171-189. |
| 35. | Holt EW, Yimam KK, Liberman MS. Esophageal adenocarcinoma in a 40-year-old man with cystic fibrosis: coincidence or not? Ochsner J. 2013;13:252-255. [PubMed] |
| 36. | Shah N, Tan HL, Sebire N, Suri R, Leuven K. The role of endoscopy and biopsy in the management of severe gastrointestinal disease in cystic fibrosis patients. Pediatr Pulmonol. 2013;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Goralski JL, Lercher DM, Davis SD, Dellon ES. Eosinophilic esophagitis in cystic fibrosis: a case series and review of the literature. J Cyst Fibros. 2013;12:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, Burks AW, Chehade M, Collins MH, Dellon ES. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3-20.e6; quiz 21-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1605] [Cited by in RCA: 1481] [Article Influence: 105.8] [Reference Citation Analysis (1)] |
| 39. | Spergel JM, Brown-Whitehorn TF, Beausoleil JL, Franciosi J, Shuker M, Verma R, Liacouras CA. 14 years of eosinophilic esophagitis: clinical features and prognosis. J Pediatr Gastroenterol Nutr. 2009;48:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 316] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 40. | Dellon ES, Gibbs WB, Fritchie KJ, Rubinas TC, Wilson LA, Woosley JT, Shaheen NJ. Clinical, endoscopic, and histologic findings distinguish eosinophilic esophagitis from gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2009;7:1305-1313; quiz 1261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 282] [Cited by in RCA: 275] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 41. | Yankaskas JR, Marshall BC, Sufian B, Simon RH, Rodman D. Cystic fibrosis adult care: consensus conference report. Chest. 2004;125:1S-39S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 371] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 42. | Tonelli AR, Drane WE, Collins DP, Nichols W, Antony VB, Olson EL. Erythromycin improves gastric emptying half-time in adult cystic fibrosis patients with gastroparesis. J Cyst Fibros. 2009;8:193-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 43. | Franche GLS, Silva FA, Saleb CS. Bacteriologia do aspirado do meato médio em pacientes com fibrose cística. Rev Bras Otorrinolaringol. 2007;73:494-499. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 44. | Berkowitz N, Schulman LL, McGregor C, Markowitz D. Gastroparesis after lung transplantation. Potential role in postoperative respiratory complications. Chest. 1995;108:1602-1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 102] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 45. | Littlewood JM, Wolfe SP, Conway SP. Diagnosis and treatment of intestinal malabsorption in cystic fibrosis. Pediatr Pulmonol. 2006;41:35-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 46. | Witt H. Chronic pancreatitis and cystic fibrosis. Gut. 2003;52 Suppl 2:ii31-ii41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 47. | Ooi CY, Durie PR. Cystic fibrosis transmembrane conductance regulator (CFTR) gene mutations in pancreatitis. J Cyst Fibros. 2012;11:355-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 48. | Ooi CY, Dorfman R, Cipolli M, Gonska T, Castellani C, Keenan K, Freedman SD, Zielenski J, Berthiaume Y, Corey M. Type of CFTR mutation determines risk of pancreatitis in patients with cystic fibrosis. Gastroenterology. 2011;140:153-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 177] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 49. | Chaves CRM, Cunha ALP. Nutritional assessment and recommendations for children and adolescents with cystic fibrosis. Rev Paul Pediatr. 2012;30:131-138. [DOI] [Full Text] |
| 50. | Park RW, Grand RJ. Gastrointestinal manifestations of cystic fibrosis: a review. Gastroenterology. 1981;81:1143-1161. [PubMed] |
| 51. | Colombo C, Battezzati A. Growth failure in cystic fibrosis: a true need for anabolic agents? J Pediatr. 2005;146:303-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 52. | Giglio L, Candusso M, D’Orazio C, Mastella G, Faraguna D. Failure to thrive: the earliest feature of cystic fibrosis in infants diagnosed by neonatal screening. Acta Paediatr. 1997;86:1162-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 53. | Bronstein MN, Sokol RJ, Abman SH, Chatfield BA, Hammond KB, Hambidge KM, Stall CD, Accurso FJ. Pancreatic insufficiency, growth, and nutrition in infants identified by newborn screening as having cystic fibrosis. J Pediatr. 1992;120:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 111] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 54. | Walkowiak J, Sands D, Nowakowska A, Piotrowski R, Zybert K, Herzig KH, Milanowski A. Early decline of pancreatic function in cystic fibrosis patients with class 1 or 2 CFTR mutations. J Pediatr Gastroenterol Nutr. 2005;40:199-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 55. | Cipolli M, Castellani C, Wilcken B, Massie J, McKay K, Gruca M, Tamanini A, Assael MB, Gaskin K. Pancreatic phenotype in infants with cystic fibrosis identified by mutation screening. Arch Dis Child. 2007;92:842-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 56. | Dalcin PTR, Silva FA. Fibrose cística no adulto: aspectos diagnósticos e terapêuticos. J Bras Pneumol. 2008;34:107-117. [DOI] [Full Text] |
| 57. | Borowitz D, Baker RD, Stallings V. Consensus report on nutrition for pediatric patients with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2002;35:246-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 367] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 58. | Wilson DC, Pencharz PB. Nutrition and cystic fibrosis. Nutrition. 1998;14:792-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 59. | Smith BA, Cogswell A, Garcia G. Vitamin D and Depressive Symptoms in Children with Cystic Fibrosis. Psychosomatics. 2013;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 60. | Grossmann RE, Zughaier SM, Kumari M, Seydafkan S, Lyles RH, Liu S, Sueblinvong V, Schechter MS, Stecenko AA, Ziegler TR. Pilot study of vitamin D supplementation in adults with cystic fibrosis pulmonary exacerbation: A randomized, controlled trial. Dermatoendocrinol. 2012;4:191-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 61. | Ambroszkiewicz J, Gajewska J, Sands D, Chełchowska M, Ołtarzewski M, Laskowska-Klita T. [Assessment of selected bone metabolism marker concentrations in children with cystic fibrosis]. Med Wieku Rozwoj. 2012;16:117-123. [PubMed] |
| 62. | Robinson C, Chiang M, Thompson SN, Sondike SB. Occurrence of vitamin D deficiency in pediatric patients at high risk in West Virginia. South Med J. 2012;105:504-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 63. | Colombo C. Liver disease in cystic fibrosis. Curr Opin Pulm Med. 2007;13:529-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 64. | Feranchak AP, Sokol RJ. Cholangiocyte biology and cystic fibrosis liver disease. Semin Liver Dis. 2001;21:471-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 77] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 65. | Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1686] [Cited by in RCA: 1560] [Article Influence: 91.8] [Reference Citation Analysis (0)] |
| 66. | Tsukada S, Parsons CJ, Rippe RA. Mechanisms of liver fibrosis. Clin Chim Acta. 2006;364:33-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 286] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 67. | Poustie VJ, Russell JE, Watling RM, Ashby D, Smyth RL. Oral protein energy supplements for children with cystic fibrosis: CALICO multicentre randomised controlled trial. BMJ. 2006;332:632-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 68. | Debray D, Kelly D, Houwen R, Strandvik B, Colombo C. Best practice guidance for the diagnosis and management of cystic fibrosis-associated liver disease. J Cyst Fibros. 2011;10 Suppl 2:S29-S36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 286] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 69. | Sokol RJ, Durie PR. Recommendations for management of liver and biliary tract disease in cystic fibrosis. Cystic Fibrosis Foundation Hepatobiliary Disease Consensus Group. J Pediatr Gastroenterol Nutr. 1999;28 Suppl 1:S1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 146] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 70. | Ng SM, Jones AP. Drug therapies for reducing gastric acidity in people with cystic fibrosis. Cochrane Database Syst Rev. 2003;CD003424. [PubMed] |
| 71. | Colombo C, Fredella C, Russo MC, Faelli N, Motta V, Valmarana L, Longo L, D’Orazio C. Efficacy and tolerability of Creon for Children in infants and toddlers with pancreatic exocrine insufficiency caused by cystic fibrosis: an open-label, single-arm, multicenter study. Pancreas. 2009;38:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 72. | FitzSimmons SC, Burkhart GA, Borowitz D, Grand RJ, Hammerstrom T, Durie PR, Lloyd-Still JD, Lowenfels AB. High-dose pancreatic-enzyme supplements and fibrosing colonopathy in children with cystic fibrosis. N Engl J Med. 1997;336:1283-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 179] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 73. | Borowitz DS, Grand RJ, Durie PR. Use of pancreatic enzyme supplements for patients with cystic fibrosis in the context of fibrosing colonopathy. Consensus Committee. J Pediatr. 1995;127:681-684. [PubMed] |
| 74. | Mehta A. Further comments on fibrosing colonpathy study. Lancet. 2001;358:1546-1547; author reply 1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 75. | Littlewood JM, Connett GJ, Sander-Struckmeier S, Henniges F. A 2-year post-authorization safety study of high-strength pancreatic enzyme replacement therapy (pancreatin 40,000) in cystic fibrosis. Expert Opin Drug Saf. 2011;10:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 76. | Markowitz JE, Spergel JM, Ruchelli E, Liacouras CA. Elemental diet is an effective treatment for eosinophilic esophagitis in children and adolescents. Am J Gastroenterol. 2003;98:777-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 372] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 77. | Kagalwalla AF, Sentongo TA, Ritz S, Hess T, Nelson SP, Emerick KM, Melin-Aldana H, Li BU. Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2006;4:1097-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 498] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 78. | Gonsalves N, Yang GY, Doerfler B, Ritz S, Ditto AM, Hirano I. Elimination diet effectively treats eosinophilic esophagitis in adults; food reintroduction identifies causative factors. Gastroenterology. 2012;142:1451-1459.e1; quiz e14-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 453] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 79. | Adde FV, Dolce P, Tanikawa CE, Uehara DY, Cardoso AL, Rozov T. [Nutritional supplementation in patients with cystic fibrosis]. J Pediatr (Rio J). 1997;73:317-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 80. | Widhalm K, Götz M. [Long-term use of medium chain triglycerides in cystic fibrosis (author’s transl)]. Wien Klin Wochenschr. 1976;88:557-561. [PubMed] |
| 81. | Morais MB, Jacob CM. The role of probiotics and prebiotics in pediatric practice. J Pediatr (Rio J). 2006;82:S189-S197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 82. | Infante Pina D, Redecillas Ferreiro S, Torrent Vernetta A, Segarra Cantón O, Maldonado Smith M, Gartner Tizziano L, Hidalgo Albert E. [Improvement of intestinal function in cystic fibrosis patients using probiotics]. An Pediatr (Barc). 2008;69:501-505. [PubMed] |
| 83. | Weiss B, Bujanover Y, Yahav Y, Vilozni D, Fireman E, Efrati O. Probiotic supplementation affects pulmonary exacerbations in patients with cystic fibrosis: a pilot study. Pediatr Pulmonol. 2010;45:536-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 84. | Bruzzese E, Raia V, Gaudiello G, Polito G, Buccigrossi V, Formicola V, Guarino A. Intestinal inflammation is a frequent feature of cystic fibrosis and is reduced by probiotic administration. Aliment Pharmacol Ther. 2004;20:813-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 134] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 85. | Saad SMI. Probióticos e prebióticos: o estado da arte. Rev Bras Cienc Farm. 2006;42:1-16. [RCA] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 86. | White H, Morton AM, Conway SP, Peckham DG. Enteral tube feeding in adults with cystic fibrosis; patient choice and impact on long term outcomes. J Cyst Fibros. 2013;12:616-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 87. | Conway SP, Morton A, Wolfe S. Enteral tube feeding for cystic fibrosis. Cochrane Database Syst Rev. 2008;CD001198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 88. | Leggieri E, De Biase RV, Savi D, Zullo S, Halili I, Quattrucci S. Clinical effects of diet supplementation with DHA in pediatric patients suffering from cystic fibrosis. Minerva Pediatr. 2013;65:389-398. [PubMed] |
| 89. | Katrangi W, Lawrenz J, Seegmiller AC, Laposata M. Interactions of linoleic and alpha-linolenic acids in the development of fatty acid alterations in cystic fibrosis. Lipids. 2013;48:333-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 90. | Njoroge SW, Laposata M, Katrangi W, Seegmiller AC. DHA and EPA reverse cystic fibrosis-related FA abnormalities by suppressing FA desaturase expression and activity. J Lipid Res. 2012;53:257-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 91. | Henderson WR, Astley SJ, McCready MM, Kushmerick P, Casey S, Becker JW, Ramsey BW. Oral absorption of omega-3 fatty acids in patients with cystic fibrosis who have pancreatic insufficiency and in healthy control subjects. J Pediatr. 1994;124:400-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 92. | Keen C, Olin AC, Eriksson S, Ekman A, Lindblad A, Basu S, Beermann C, Strandvik B. Supplementation with fatty acids influences the airway nitric oxide and inflammatory markers in patients with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2010;50:537-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 93. | Panchaud A, Sauty A, Kernen Y, Decosterd LA, Buclin T, Boulat O, Hug C, Pilet M, Roulet M. Biological effects of a dietary omega-3 polyunsaturated fatty acids supplementation in cystic fibrosis patients: a randomized, crossover placebo-controlled trial. Clin Nutr. 2006;25:418-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 94. | Smith LJ, Lacaille F, Lepage G, Ronco N, Lamarre A, Roy CC. Taurine decreases fecal fatty acid and sterol excretion in cystic fibrosis. A randomized double-blind trial. Am J Dis Child. 1991;145:1401-1404. [PubMed] |
| 95. | Belli DC, Levy E, Darling P, Leroy C, Lepage G, Giguère R, Roy CC. Taurine improves the absorption of a fat meal in patients with cystic fibrosis. Pediatrics. 1987;80:517-523. [PubMed] |
| 96. | Kappler M, Espach C, Schweiger-Kabesch A, Lang T, Hartl D, Hector A, Glasmacher C, Griese M. Ursodeoxycholic acid therapy in cystic fibrosis liver disease--a retrospective long-term follow-up case-control study. Aliment Pharmacol Ther. 2012;36:266-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 97. | Cheng K, Ashby D, Smyth RL. Ursodeoxycholic acid for cystic fibrosis-related liver disease. Cochrane Database Syst Rev. 2012;10:CD000222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 98. | Melzi ML, Kelly DA, Colombo C, Jara P, Manzanares J, Colledan M, Strazzabosco M, DeLorenzo P, Valsecchi MG, Adam R. Liver transplant in cystic fibrosis: a poll among European centers. A study from the European Liver Transplant Registry. Transpl Int. 2006;19:726-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 99. | Lamireau T, Martin S, Lallier M, Marcotte JE, Alvarez F. Liver transplantation for cirrhosis in cystic fibrosis. Can J Gastroenterol. 2006;20:475-478. [PubMed] |
| 100. | Vandeleur M, Massie J, Oliver M. Gastrostomy in children with cystic fibrosis and portal hypertension. J Pediatr Gastroenterol Nutr. 2013;57:245-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |