Published online Nov 28, 2013. doi: 10.3748/wjg.v19.i44.8141
Revised: September 12, 2013
Accepted: September 16, 2013
Published online: November 28, 2013
Processing time: 127 Days and 21.8 Hours
An 85 year male patient complaining epigastric discomfort was admitted. From the esophagogastroduodenoscopy, three early gastric cancer (EGCa) lesions had been identified and these were diagnosed as adenocarcinoma with poorly differentiated cell type. The patient underwent operation. From the post-operative mapping, however, additional 4 EGCa lesions were found, and the patient was diagnosed with 7 synchronous EGCa. Out of the 7 EGCa lesions, 6 had shown invasion only to the mucosal layer and one had shown invasion into the 1/3 layer of submucosa. In spite of such superficial invasions, 28 of 48 lymph nodes had been identified as metastases. The multiple lesions of EGCa do not increase the risk of lymph node metastasis, but if their differentiations are poor or if they have lympho-vascular invasion, multiple lymph node metastases could incur even if the depth of invasion is limited to the mucosal layer or the upper portion of the submucosal layer.
Core tip: Early gastric cancer is often found synchronously in 2 to 3 lesions. However, this case reports on an unprecedented case of 7 lesions of early gastric cancer. Furthermore, this case deserves more attention because 28 out of 48 lymph nodes showed post-operative metastasis, even though there was only 1 invasion to the 1/3 of the submucosal layer and the remaining 6 invading only up to the mucosal layer. This report speaks to the necessity of extra caution in diagnosing multiple synchronous lesions of early gastric cancer with esophagogastro-duodenoscopy.
- Citation: Seong H, Kim JI, Lee HJ, Kim HJ, Cho HJ, Kim HK, Cheung DY, Kim DJ, Kim W, Kim TJ. Seven synchronous early gastric cancer with 28 lymph nodes metastasis. World J Gastroenterol 2013; 19(44): 8141-8145
- URL: https://www.wjgnet.com/1007-9327/full/v19/i44/8141.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i44.8141
Owing to the recent development of diagnostic technology through esophagogastroduodenoscopy (EGD), the prevalence of early gastric cancer (EGCa) is increasing. Also, reports of multiple synchronous EGCa lesions are increasing as well. The prevalence of multiple EGCa does not differ between advanced gastric cancer patients as 6%-14%[1] and EGCa patients as 8.3%-17%[2]. Multiple EGCa show a high level of prevalence in elder patients or in male patients, and also when the cancer is well differentiated or invasion is limited to the mucosal layer[3]. And most accessory lesions have been known to occur adjacent to the main lesion with same or even better differentiation[4]. It was found that there was no difference in lymph node metastasis when comparing multiple EGCa with single EGCa in general, but if the invasion depth was deep, the possibility of lymph node metastasis was even higher[3]. If the indications of endoscopic treatment are expanded, since even the surgical treatment tends to orient towards less invasive methods to preserve the normal part of stomach as much as possible, accurate pretreatment diagnosis is important for the multiple lesions of EGCa. In our case, 7 EGCa had been found in an 85 year old male patient and there were multiple lymph node metastases identified post-operatively even though the cancer had shown invasion into the upper portion of the submucosal layer.
An 85-year-old male patient complaining of epigastric pain for 3 mo was admitted to our hospital. He had no special medical, family and social history, not to mention cancer, and was found nonspecific in his physical examination and initial laboratory finding. From the results of his physical examination, we found that his blood pressure was 135/87 mmHg, pulse rate was 70 times/min, respiratory rate was 20 times/min and body temperature was 36.5 °C. The conjunctivae were not pale and no jaundice was observed from the sclerae. There were no palpable lymph nodes from the neck examination, and the auscultation had a normal respiratory sound from the thorax. There was no palpable mass, no tenderness or no rebound tenderness found from the abdominal examination.
From the complete blood count of the laboratory findings, hemoglobin was 12.4 g/dL, white blood cell count was 5670/mm3 and the platelet count was 212000/mm3, whereas biochemistry examination revealed, fasting blood glucose as 93 mg/dL, urea nitrogen as 14.5 mg/dL, creatinine as 1.19 mg/dL, aspartate aminotransferase as 30 IU/L and alanine aminotransferase as 22 IU/L, total bilirubin was 0.66 mg/dL, direct bilirubin was 0.22 mg/dL, total protein was 6.7 g/dL and albumin 3.56 g/dL, presenting that all the results were in the normal range. Also, tumor markers such as carcinoembryonic antigen and cancer antigen 19-9 were within normal limit as 2.59 ηg/mL and 11.13 U/mL.
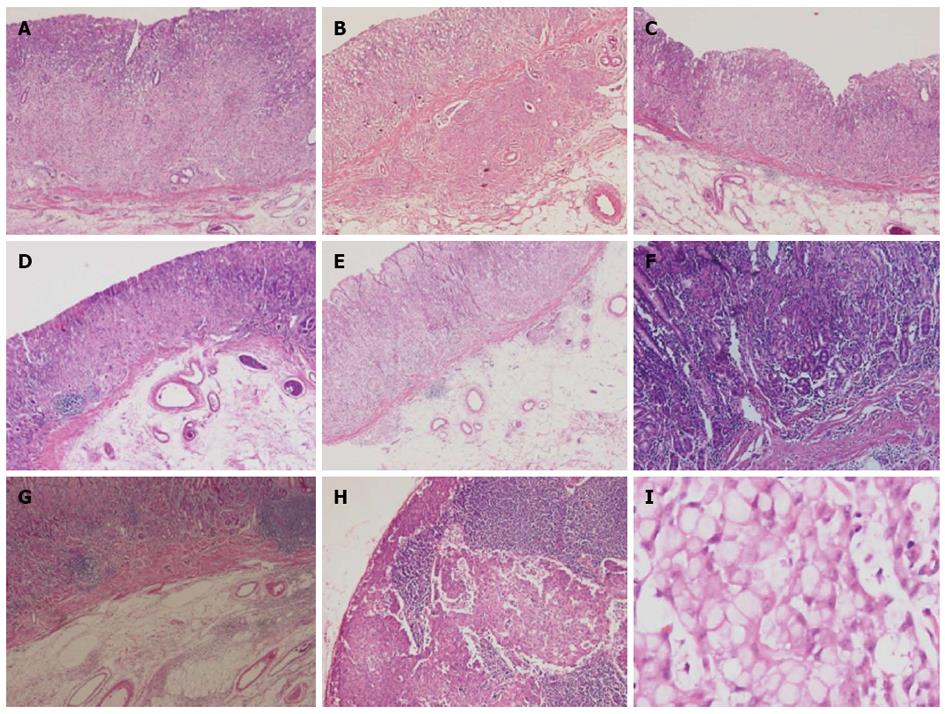
From the EGD, the whole stomach had atrophic mucosal change from antrum to cardia, open type III atrophic gastritis and had no Helicobacter pylori infection in Warthin-Starry silver stain. There were findings of a well demarcated erythematous depressed erosion in the sized of 8 mm on the posterior wall of proximal antrum (Figure 1A) and a depressed mucosal lesion with a red colored center, pale boundary and clear margin in the size of 15 mm on the posterior wall of lower body (Figure 1B), as well as a depressed erosion in the sized of 7 mm on the posterior wall of lower body (Figure 1C). The lesions were diagnosed as EGCa IIc and the biopsy revealed an adenocarcinoma, poorly differentiated cell type. Although the lesions occurred in multiple regions showing reddish depression with surrounding white rim, the main lesion was very small and in its early stage, suggesting that they are multiple EGCa rather than metastasis. From the abdominal computerized tomography, the locations of lesions could not be identified. Furthermore there was neither any finding of metastasis to neighboring organs such as the liver or pancreas nor any finding of lymph node enlargement in the neighboring areas.
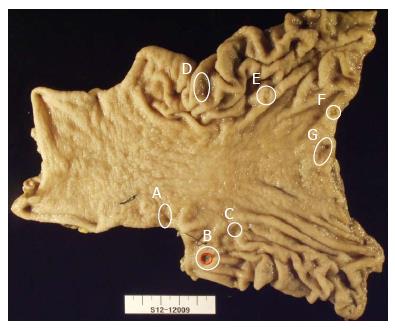
As the patient had shown three lesions of EGCa, poorly differentiated cell type cell type, we did not perform endoscopic submucosal dissection but instead performed subtotal gastrectomy. After the surgery, we also performed a mapping of the subtotal gastrectomy specimen and were able to diagnose additional lesions (Figure 2). The lesions were flat erosions 6 mm in diameter on the anterior wall of proximal antrum (Figure 1D), depressed mucosal lesion 10 mm in diameter on the anterior wall of lower body (Figure 1E), depressed mucosal lesion 7 mm in diameter on the anterior wall of mid body (Figure 1F) and squamous mucosal lesion in the sized 2 mm on the lesser curvature of mid body (Figure 1G). We had reviewed pictures taken during EGD, but could not find any definite lesion and there were no ulcer findings of each lesions (Figure 1).
In histopathological findings, each lesion was identified as adenocarcinoma, poorly differentiated cell type which was the diffuse type and the growth pattern was infiltrative type in accordance with Lauren’s classification. All lesions were composed of poorly differentiated adenocarcinoma. There were no lesions with well or moderate differentiated cancer component and the back ground was atrophic gastritis, marked grade (Figure 3). On the invasion depth, the lesion (Figure 3A, C-G) had invaded into the mucosal layer, while the lesion (Figure 3B), which was the largest, had shown invasion into 1/3 of the submucosal layer, SM1, 1000 μm (Figure 1H and I) (Table 1). There was no perineural invasion but vascular and lymphatic invasion were found from the subtotal gastrectomy specimen. The grade of lymphatic invasion was marked (Figure 1I) and out of the 48 resected lymph nodes, 28 lymph nodes had shown metastasis (Figure 3H). Lymph node metastasis was as follow: 1 (4/4), 3 (4/6), 4 (3/10), 5 (5/6), 6 (12/15), 8a (0/7). According to American Joint Committee on Cancer TNM staging classification for gastric cancer, the final pathologic stage was T1bN3bM0.
| A | B | C | D | E | F | G | |
| Type | IIc | IIc | IIc | IIb | IIc | IIc | IIb |
| Size (mm2) | 6 × 6 | 15 × 12 | 7 × 7 | 6 × 5 | 10 × 8 | 7 × 7 | 2 × 2 |
| Depth | M | SM1 | M | M | M | M | M |
The diagnosis of EGCa is increasing as the performance of upper endoscopy-related equipments advances and as pathological diagnosis techniques became more developed in recent days. In South Korea, health screening EGD is performed biannually to the entire public nationwide and this led to increase in diagnosis of early stage stomach cancer, thereby leading to increase in diagnosis of multiple EGCa. However, in spite of much effort to find multiple EGCa lesions, the rate of lesions unidentified prior to surgical intervention are as high as 20% to 25%[4].
In the past, gastrectomy was the major treatment of gastric cancer even if multiple gastric cancers were not identified, but because the multiple gastric cancers were included in the resected portion of stomach there was no significant difference in prognosis in some cases. However, in recent days, as endoscopic submucosal dissection is being used as treatment of EGCa, the finding of multiple EGCa is considered important. Moreover, as more non-invasive procedures are preferred for elderly patients for the treatment of EGCa, the importance of finding multiple lesions became more significant.
According to Moertel et al[5], diagnosis of multiple EGCa requires evidence of pathological malignancy for each lesion, which should be present at independent locations without the possibility of metastasis from the other organs. Multiple EGCa are more prevalent in male or older patients. In many cases, the area of occurrence is located at middle third portion and lower third portion of the stomach, whereas most accessory lesions incur at adjacent locations to the main lesion, usually the distal part[3,4]. Most multiple EGCa have shapes of the elevated type or flat type rather than the depressed type, and most are associated with well differentiated lesions rather than poorly differentiated lesion, and majority of them show invasion only to the mucosal layer[3,4]. In addition, when there is adenoma or atrophic gastritis, and when the patient has a family history of stomach cancer, multiple EGCa is more likely to be prevalent[1,6].
The prognosis and 5 year survival rate of multiple EGCa as well as single EGCa are both similarly over 90%[7]. The recurrence rate is also similarly 11.2% in both types, and a substantial number of them are considered to be caused by multiple synchronous EGCa which had been overlooked during prior EGD[8]. Therefore, it is important to keep in mind the possible existence of synchronous lesion when establishing plans for examination and treatment.
There are arguments that total gastrectomy should be performed for the treatment of multiple EGCa due to the risk of recurrence but subtotal gastrectomy can also be performed as the treatment of multiple EGCa as they mainly occur at the distal part and not much different in prognosis. In consideration of the post-operative quality of life, even if subtotal gastrectomy is performed when possible with accurate diagnosis, the results are not different from the cases performed with total gastrectomy.
Recently, endoscopic submucosal dissection is increasing trend as the treatment method of EGCa, and lymph node metastasis becomes an important factor in deciding endoscopic therapy over surgical treatment. Other important factors for prediction of lymph node metastasis include presence/absence of ulcer lesion, tumor size, and invasion depth. Due to the increased accuracy of pre-operative CT scan and endoscopic ultrasonography, it is easier to find lymph node metastasis, therefore the identification of depth of invasion as major risk factors of lymph node metastasis becomes important[9]. In our case, although the depth of invasion is limited to the mucosal layer or the 1/3 part of the submucosa layer, numerous lymph node metastasis had occurred. Such outcomes were considered to be caused not from the presence of multiple lesions but from the lympho-vascular invasion.
This case was finally diagnosed as a very rare case of EGCa with 7 multiple synchronous EGCa in a male patient of old age. In addition, the patient had shown metastasis of 28 lymph nodes out of 48 resected lymph nodes although its depth of invasion was limited to the mucosal and the 1/3 part of the submucosal layer. Thereby, we report this very rare case with literature review in order to inform the importance of accurate diagnosis on multiple EGCa.
We appreciate Ka Young Kim from Cornell University who provided great support in data analysis and English proofreading, as well as secretarial assistance.
P- Reviewers: Franceschi F, Guo JM, Jiang CP S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN
| 1. | Lee IS, Park YS, Kim KC, Kim TH, Kim HS, Choi KD, Lee GH, Yook JH, Oh ST, Kim BS. Multiple synchronous early gastric cancers: high-risk group and proper management. Surg Oncol. 2012;21:269-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Hirasaki S, Moriwaki T, Hyodo I. Multiple synchronous early gastric carcinoma with seven lesions. J Gastroenterol. 2003;38:1194. [PubMed] |
| 3. | Seto Y, Nagawa H, Muto T. Treatment of multiple early gastric cancer. Jpn J Clin Oncol. 1996;26:134-138. [PubMed] |
| 4. | Lee HL, Eun CS, Lee OY, Han DS, Yoon BC, Choi HS, Hahm JS, Koh DH. When do we miss synchronous gastric neoplasms with endoscopy? Gastrointest Endosc. 2010;71:1159-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Moertel CG, Bargen JA, Soule EH. Multiple gastric cancers; review of the literature and study of 42 cases. Gastroenterology. 1957;32:1095-1103. [PubMed] |
| 6. | Yoo JH, Shin SJ, Lee KM, Choi JM, Wi JO, Kim DH, Lim SG, Hwang JC, Cheong JY, Yoo BM. How can we predict the presence of missed synchronous lesions after endoscopic submucosal dissection for early gastric cancers or gastric adenomas? J Clin Gastroenterol. 2013;47:e17-e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Borie F, Plaisant N, Millat B, Hay JM, Fagniez PL, De Saxce B. Treatment and prognosis of early multiple gastric cancer. Eur J Surg Oncol. 2003;29:511-514. [PubMed] |
| 8. | Kim HJ, Lee JH, Lee JS, Moon TG, Kim JJ, Rhee JC, Noh JH, Sohn TS, Kim S. Clinicopathologic characteristics of multiple synchronous early gastric cancers. Korean J Med. 2007;72:360-367. |
| 9. | Lee JH, Choi IJ, Kook MC, Nam BH, Kim YW, Ryu KW. Risk factors for lymph node metastasis in patients with early gastric cancer and signet ring cell histology. Br J Surg. 2010;97:732-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |