Published online Nov 21, 2013. doi: 10.3748/wjg.v19.i43.7778
Revised: August 28, 2013
Accepted: September 15, 2013
Published online: November 21, 2013
Processing time: 170 Days and 10.3 Hours
AIM: To investigate whether a virus constitutively expressing active Akt is useful to prevent cirrhosis induced by carbon tetrachloride (CCl4).
METHODS: Using cre-loxp technique, we created an Ad-myr-HA-Akt virus, in which Akt is labeled by a HA tag and its expression is driven by myr promoter. Further, through measuring enzyme levels and histological structure, we determined the efficacy of this Ad-myr-HA-Akt virus in inhibiting the development of cirrhosis induced by CCl4 in rats. Lastly, using western blotting, we examined the expression levels and/or phosphorylation status of Akt, apoptotic mediators, endothelial nitric oxide synthase (eNOS), and markers for hepatic stellate cells activation to understand the underlying mechanisms of protective role of this virus.
RESULTS: The Ad-myr-HA-Akt virus was confirmed using polymerase chain reaction amplification of inserted Akt gene and sequencing for full length of inserted fragment, which was consistent with the sequence reported in the GenBank. The concentrations of Ad-myr-HA-Akt and adenoviral enhanced green fluorescent protein (Ad-EGFP) virus used in the current study were 5.5 × 1011 vp/mL. The portal vein diameter, peak velocity of blood flow, portal blood flow and congestion index were significantly increased in untreated, saline and Ad-EGFP cirrhosis groups when compared to normal control after the virus was introduced to animal through tail veil injection. In contrast, these parameters in the Akt cirrhosis group were comparable to normal control group. Compared to the normal control, the liver function (Alanine aminotransferase, Aspartate aminotransferase and Albumin) was significantly impaired in the untreated, saline and Ad-EGFP cirrhosis groups. The Akt cirrhosis group showed significant improvement of liver function when compared to the untreated, saline and Ad-EGFP cirrhosis groups. The Hyp level and portal vein pressure in Akt cirrhosis groups were also significantly lower than other cirrhosis groups. The results of HE and Van Gieson staining indicated that Akt group has better preservation of histological structure and less fibrosis than other cirrhosis groups. The percentage of apoptotic cell was greatly less in Akt cirrhosis group than in other cirrhosis groups. Akt group showed positive HA tag and an increased level of phosphorylated Akt as well as decreased levels of Fas. In contrast, Caspase-3 and Caspase-9 levels in Akt group were significantly lower than other cirrhosis groups. Noticeable decrease of DR5 and α-SMA and increase of phosphorylated eNOS were observed in the Akt group when compared to other cirrhosis groups. The NO level in liver was significantly higher in Akt group than other cirrhosis groups, which was consistent with the level of phosphorylated eNOS in these groups.
CONCLUSION: This study suggest that Ad-myr-HA-Akt virus is a useful tool to prevent CCl4-induced cirrhosis in rat model and Akt pathway may be a therapeutic target for human cirrhosis.
Core tip: In the present study, we have demonstrated for the first time that Ad-myr-HA-Akt virus was a useful tool to prevent carbon tetrachloride-induced cirrhosis in rat model. Our data obtained at different levels, from function to histological changes, apoptosis rate of hepatocytes, activation of hepatic stellate cells, deposition of collagen, portal vein pressure and NO level, which were all consistently and collectively supported the hypothesis that introduction of Ad-myr-HA-Akt virus inhibits the development of cirrhosis.
- Citation: Deng G, Huang XJ, Luo HW, Huang FZ, Liu XY, Wang YH. Amelioration of carbon tetrachloride-induced cirrhosis and portal hypertension in rat using adenoviral gene transfer of Akt. World J Gastroenterol 2013; 19(43): 7778-7787
- URL: https://www.wjgnet.com/1007-9327/full/v19/i43/7778.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i43.7778
It has been found that massive apoptosis of hepatocytes resulted in activation of hepatic stellate cells (HSC), which produce collagen and ultimately lead to the fibrosis and cirrhosis[1-5]. Accordingly, inhibiting hepatic apoptosis is considered as an important strategy to prevent cirrhosis[6-10]. Previous research showed that Akt plays a crucial role in preventing Fas signaling-mediated hepatic apoptosis, and that over-expression of Akt was capable of preventing hepatic apoptosis[11,12]. Therefore, we intended to establish a recombinant vector carrying Akt gene to inhibit cirrhosis in a rat model.
Adenovirus can effectively express genes of interest. Accordingly it has been widely used as vector for gene therapy[13-18]. Recently, Cre-loxp system has been used to create replication-defective virus and successfully used in creating therapeutic virus with recombinant gene[19-22]. In the present study, using cre-loxp technique, we created an Ad-myr-HA-Akt virus, in which Akt is labeled by a Hemagglutinin (HA) tag and its expression is driven by myr promoter. We further determined that this Ad-myr-HA-Akt virus was capable of inhibiting the development of cirrhosis induced by carbon tetrachloride (CCl4). Lastly, we examined the expression level and/or phosphorylation status of Akt, apoptotic mediators, endothelial nitric oxide synthase (eNOS), and markers for HSC activation. Our results indicated that introduction of Ad-myr-HA-Akt virus was able to prevent hepatocyte apoptosis and subsequent cirrhosis in a rat CCl4-induced cirrhosis model.
According to the method that has been published[23], a replication-defective adenovirus vector was constructed in HEK293 cell with co-transfection of pCD316 plasmid, pBHGloxΔE1 and 3Cre (Invitrogen, United States). The details have been previously published. The vector of interest was selected with polymerase chain reaction (PCR) amplification of inserted Akt gene using the following primer pair as show: F: 5’-GGGAATTCATGGGATGCGTGTGTAGC-3’; R: 5’-GGGGATCCTCAGGCCGTGCCGCTGGCCGAGTA-3’. The PCR thermal condition consisted of 94 °C 5 min, 30 cycles of 94 °C 30 s, 58 °C 30 s, 72 °C 1 min, and a final extension at 72 °C 5 min. The amplified gene was further confirmed with DNA sequencing (Sunbiotech Co., Ltd, ABi 3730, Beijing). adenoviral enhanced green fluorescent protein (Ad-EGFP) was purchased from Stratagene (CA, United States) and used as a control vector for tracking the distribution of virus after introduced to animal. The concentration of recombinant adenovirus was measured with tissue culture infective dose method as previously reported[24].
Plasmids were amplified in DH5a competent cells and purified using a commercial kit (Qiagen). Viruses were prepared in HEK293 cells (Qiagen, GmbH, Hilden, German), which contain the necessary gene for the virus package. Specifically, one day prior to the transfection, HEK293 cells were seeded at a concentration of 10 × 106 in 10 mL complete dulbecco’s modified eagle medium and cultured overnight. Upon transfection, 1 mg/mL plasmid stock was taken and adjusted into a volume of 1600 μL, 150 μL calcium phosphate was added to the same tube, mixed well and incubated at room temperature for 20 min. The resultant mixture was slowly added into HEK293 cell culture. The dish was gently moved and swirled to allow the even distribution of virus in culture. Cells were further cultured for 16 h. The supernatant was discarded and replaced with fresh medium. After a 2-d culture, cells were examined under a fluorescence microscope (E80i, Nikon, Japan) for the green fluorescent protein (GFP+) cells. Cells were harvested and lysed using freezing (-70 °C) and thawing (37 °C) twice. The resultant mixture was centrifuged at 10000 g for 10 min. The supernatant with virus was collected and stored at -70 °C for future infection of target cells.
Fifty male rats (weight 220 ± 20 g) were purchased from Animal Center of Hunan Agriculture University (Hunan, China). Rats were housed under 25 °C ± 2 °C and a 12-h light/dark cycle in microisolater cages. Ten rats were randomly selected and used for the normal control. The remaining 40 rats were subjected to the induction of cirrhosis using CCl4 as previously reported[25]. These rats were further randomly divided into 4 groups (n = 10 per group). One group did not receive additional treatment, the other three groups received saline, Ad-EGFP, Ad-myr-HA-Akt (3.0 × 1011 vp in 1 mL saline), respectively, via tail vein injection at 2 wk after cirrhosis induction. The treatment was repeated at 6 wk. Accordingly, the experiment groups of rats were defined as follows: normal control, untreated cirrhosis, saline cirrhosis, EGFP cirrhosis and Akt cirrhosis. All surgical procedures were completed in accordance with the guidelines on the care and use of laboratory animals for research purposes by the Central South University Xiangya Medical School’s Animal Care and Use Committee. Mice were anesthetized with chloral hydrate (iv) for all surgical procedures.
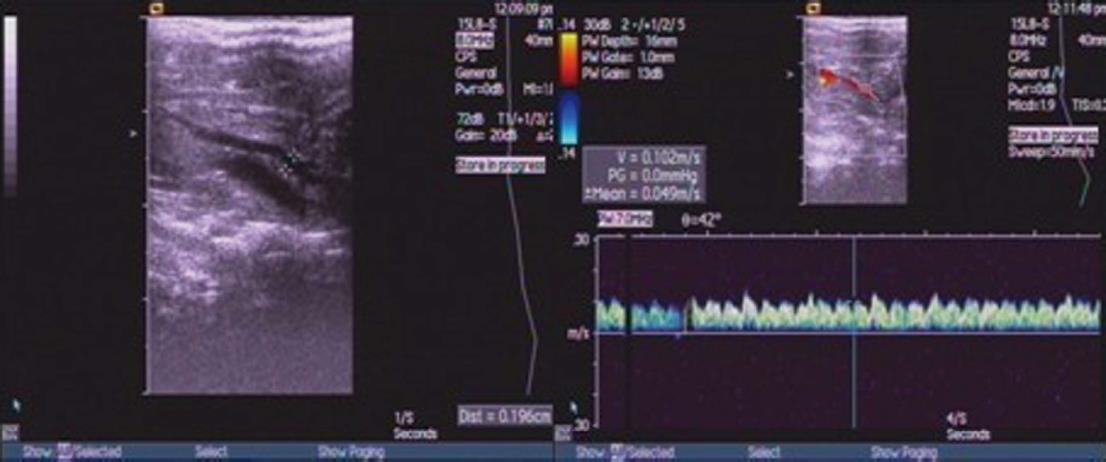
Three days after treatment, five rats from each experimental groups (normal control group, untreated cirrhosis, saline cirrhosis, Ad-EGFP and Akt cirrhosis) were restricted from food with free access to water for 12 h. Rats were anesthetized by iv infusion of 2.5% chloral hydrate (50 gtt/min). Then, diameter (D) and peak velocity of blood flow (V) in portal was measured using ultrasound system (SIMENS Co., Ltd, Acuson Seguoia 512, Germany). The blood flow (Q) and congestion index (CI) was calculated using the following formulas: Q = 0.57πD2/4V × 60, and CI = πD2/4V, respectively. Using a blood pressure device (RBP-1B, Sino-Japan friendship clinical medicine institute), the tail mean arterial pressure was recorded.
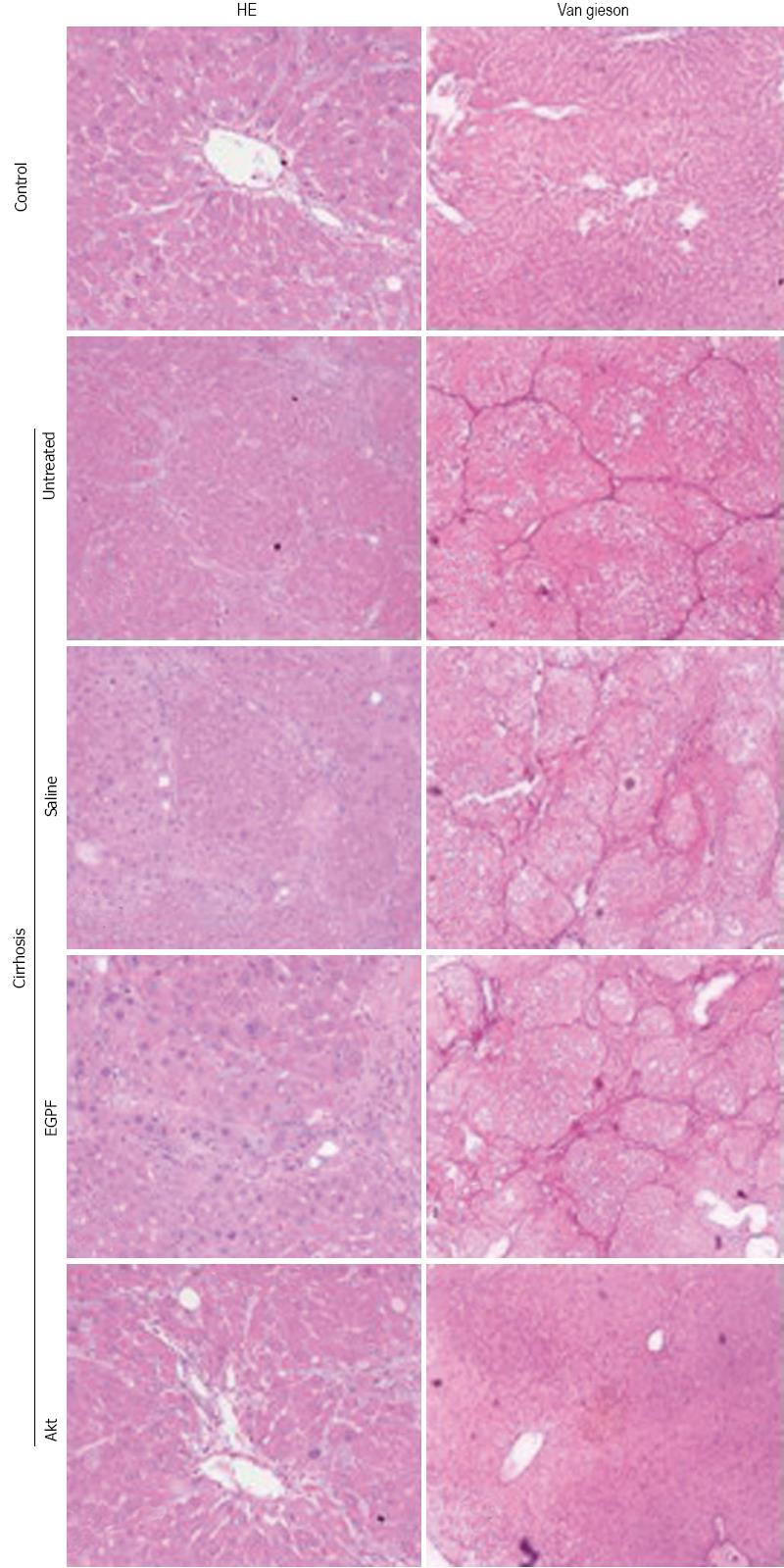
Eight weeks after cirrhosis induction, 1 mL of blood from each rat was collected from portal vein and subjected to analysis for alanine aminotransferase (ALT); aspartate aminotransferase (AST) and albumin (ALB) levels using a automatic biochemical analyzer (Spotchem SP4430, Arkray, Kyoto, Japan). Using aseptic techniques, laparotomy was performed, the portal vein was exposed, and portal vein pressure was measured with catheterization. Then rats were sacrificed and the right lobe of the liver was removed and stored in liquid nitrogen for future analysis. Liver tissue sections were subjected to HE staining for cellular and tissue structure as well as Van Gieson (VG) staining for collagen deposition. Hepatic hydroxyproline (Hyp), which is the main constituent of collagen protein, was used to estimate the degree of hepatic fibrosis, was measured with a commercial kit following manufacture’s protocol (Jiancheng Biological product institute, Nanjing, China). The stained sections were examined and photographed under a microscope equipped with a digital photograph acquiring system (E80i, Nikon, Japan). Hyp and liver function markers were measured with an automatic machine (Beckman LX20, United States).
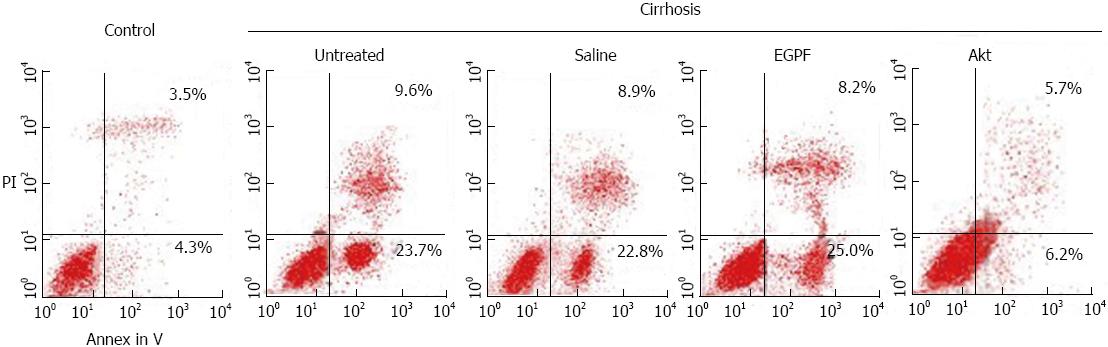
Apoptosis of hepatic cells was detected using Annexin-V-fluorescein isothiocyanate (FITC)/Propidium iodide (PI) double staining and flow cytometry analysis. The cells were harvested and resuspended in Annexin-V binding buffer and further incubated with 5 μL of Annexin V-FITC and 10 μL of PI for 10 min at room temperature in the dark, followed by cytometric analysis (EPICS XL, Beckman Coulter, United States) within 30 min of staining. All experiments were performed in triplicate.
Using a protein extraction kit (Biovision, CA, United States), whole-cell extracts were prepared from frozen liver tissue. Protein concentration of the extracts was determined by the Bradford method with a kit purchased from Biovision (United States). Forty micrograms of protein were separated on 10% sodium dodecyl sulfate-polyacrylamide gels and were electronically transferred onto a polyvinylidene difluoride membrane (Roche Diagnostics, Mannheim, Germany). The membrane was blocked in a standard western blotting procedure. Briefly, the membrane was blocked with 7.5% milk in tris-buffered-saline with tween (TBST) buffer [20 mmol/L Tris-HCl (pH 7.6), 137 mmol/L NaCl, 0.05% Tween 20], then probed with a primary antibody [anti-Akt, anti-phospho-Akt-Ser-473 (p-Akt) Akt, p-Akt, Fas, DR5 and HA, purchased from Cell Signaling Technology (Beverly, MA, United States). After washing with TBST buffer, the membrane was further incubated with HRP-conjugated goat anti-rabbit secondary antibody (1:20000, KPL, United States). The protein bands were visualized using an enhanced chemiluminescence detection system, LumiGLO (KPL, United States). β-actin (NeoMarkers, Fremont, CA, United States) was used as a loading control and normalization reference for quantification.
Nitric acid reductase method was used to measure hepatic NO using a commercial kit (CST, United States), following the manufacture manual. Briefly, 1 g of rat liver tissue stored in the liquid nitrogen was thawed and homogenized at 4 °C in the saline at a concentration of 100 g/L. Following centrifuge (1000 r/min for 5 min), the supernatant was used for the nitric acid reductase reaction to measure product of NO2- and NO3-, which indirectly represent the level of NO.
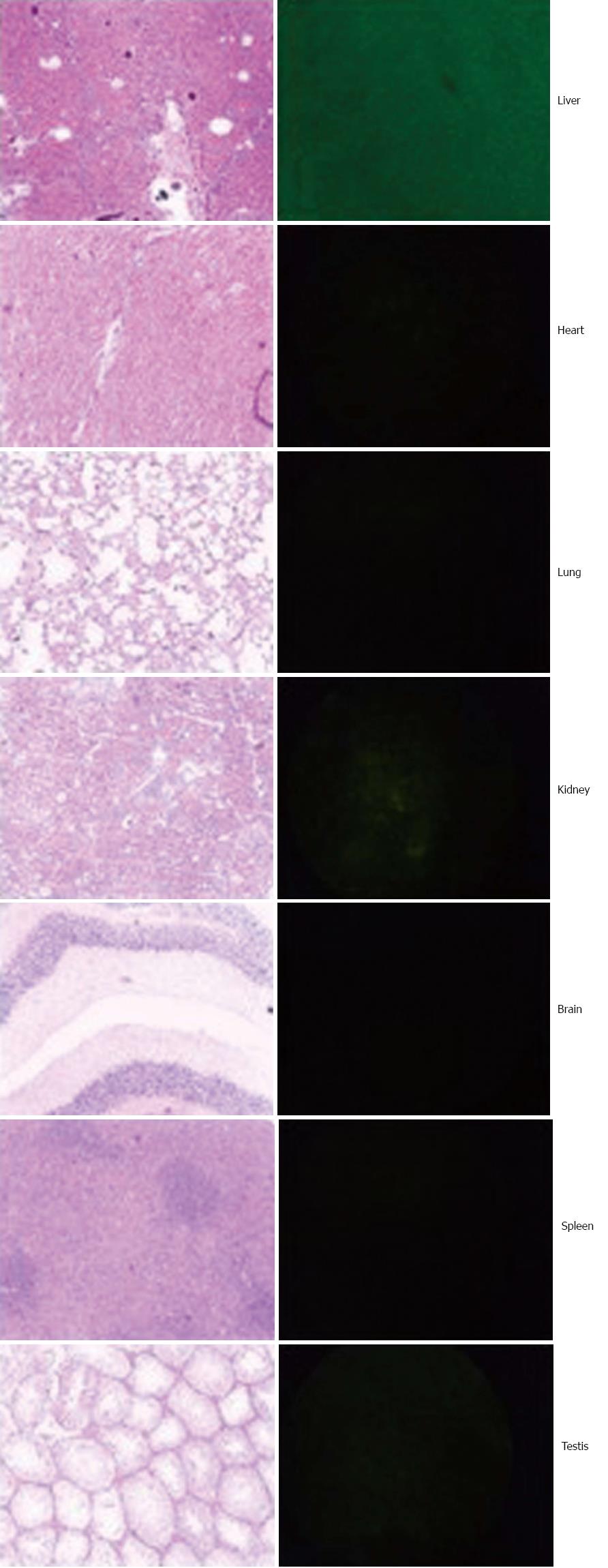
GFP+ cells in the host were examined at 8 wk after induction of cirrhosis. Mice was euthanized and infused with normal saline until the liver became pale. The liver, spleen, heart, lung, brain and kidney were collected, cryostat sectioned at a thickness of 2 μm, and examined for GFP+ cells under a fluorescence microscope (E80i, Nikon, Japan). A fraction of the tissues from the above organs were also formalin-fixed, paraffin-embedded, continuously sectioned for H and E (hematoxylin and eosin) staining and histological analysis.
The SPSS program (version 12.0, SPSS Inc., United States) was used for statistical analysis. Quantitative data were expressed as mean ± SD. Student t test and/or one-way Analysis of variance was used for group comparisons. The differences were considered significant when P < 0.05.
As detailed in the method, the Ad-myr-HA-Akt virus was confirmed using PCR amplification of inserted Akt gene and sequencing for full length of inserted fragment, which was consistent with the sequence reported in the GenBank. The concentrations of Ad-myr-HA-Akt and Ad-EGFP virus used in the current study were 5.5 × 1011 vp/mL. The virus was introduced to animal through tail veil injection. According to the control vector with expression of green fluorescent protein (GFP), the distribution of virus was mainly in the liver (Figure 1).
Five rats from each group (normal control group, untreated cirrhosis, saline cirrhosis, Ad-EGFP, and Akt cirrhosis groups) were subjected to measure portal vein diameter (D) and peak velocity of blood flow (V) and calculation of portal Q and CI (Figure 2). As shown in the Table 1, these parameters were significantly increased in untreated, saline and Ad-EGFP cirrhosis groups when compared to normal control. In contrast, these parameters in the Akt cirrhosis group were comparable to normal control group.
| Group | n | D (mm) | V (cm/s) | CI | Q (mL/min) |
| Normal | 5 | 1.13 ± 0.24 | 12.67 ± 0.64 | 0.0010 ± 0.0003 | 4.56 ± 1.86 |
| Cirrhosis | 20 | ||||
| Untreated | 5 | 1.81 ± 0.19a | 10.13 ± 0.68a | 0.0021 ± 0.0007a | 9.69 ± 2.58a |
| Saline | 5 | 1.83 ± 0.29a | 10.06 ± 0.72a | 0.0024 ± 0.0008a | 9.32 ± 2.83a |
| EGFP | 5 | 1.82 ± 0.27a | 9.98 ± 0.77a | 0.0020 ± 0.0006a | 8.97 ± 2.45a |
| Akt | 5 | 1.28 ± 0.32ceg | 11.39 ± 0.63ceg | 0.0013 ± 0.0004ceg | 5.11 ± 2.30ceg |
Eight weeks after cirrhosis induction, blood samples from portal vein were collected and subjected to the measurement of ALT, AST and ALB. As shown in the Table 2, compared to the normal control, the liver function was significantly impaired in the untreated, saline and Ad-EGFP cirrhosis groups. However, the Akt cirrhosis group showed significant improvement of liver function (lower levels of the four parameters) when compared to the untreated, saline and Ad-EGFP cirrhosis groups. Consistently, the Hyp level (Table 2) and portal vein pressure (Table 3) in Akt cirrhosis groups were also significantly lower than other cirrhosis groups (untreated, saline and Ad-EGFP). Of note, Mean arterial pressur and heart rate did not showed significant differences among the groups.
| Group | n | ALT (U/L) | AST (U/L) | ALB (g/L) | Hyp (μg/g) |
| Normal | 5 | 23.5 ± 6.3 | 109.3 ± 6.1 | 33.1 ± 2.6 | 180.5 ± 12.5 |
| Cirrhosis | 20 | ||||
| Untreated | 5 | 277.6 ± 25.8a | 380.5 ± 16.9a | 22.7 ± 3.5a | 375.2 ± 17.3a |
| Saline | 5 | 290.7 ± 22.9a | 368.9 ± 23.8a | 24.7 ± 3.7a | 393.8 ± 22.3a |
| EGFP | 5 | 285.9 ± 27.3a | 374.4 ± 26.7a | 23.9 ± 2.9a | 388.5 ± 9.8a |
| Akt | 5 | 126.4 ± 5.8aceg | 202.5 ± 9.5aceg | 30.7 ± 4.8aceg | 245.9 ± 15.6aceg |
To determine whether the above observations were derived from the histological changes of liver, we examined cellular and tissue structure and collagen deposition using HE and VG staining, respectively (Figure 3). Our results indicated that Akt group has better preservation of histological structure and less fibrosis than other cirrhosis groups. Collectively, these data supported that Ad-myr-HA-AKT virus was efficient in inhibiting the development of cirrhosis induced by CCl4.
To confirm the reduction of liver fibrosis was related to the reduction of apoptosis of hepatocytes, we doubly stained hepatocytes with PI and Annenin V to detect the apoptosis rate in each experimental group. As shown in Figure 4, the percentage of apoptotic cell was greatly less in Akt cirrhosis group than in other cirrhosis groups (2.5%-3.9% reduction). These results suggested that amelioration of liver function and fibrosis in Akt may be involved in the reduction of apoptosis of hepatocytes.
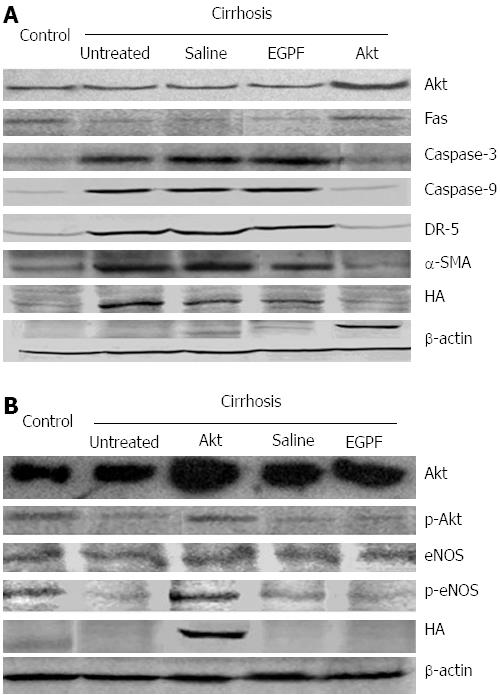
Next, to confirm the apoptosis, we measured the expression levels of apoptotic mediators using Western blotting. As shown in Figure 5A. Akt group showed positive HA tag and an increased level of phosphorylated Akt (p-Akt) as well as decreased levels of Fas. The levels of p-Akt and Fas are comparable in Akt cirrhosis group and normal control group. In contrast, Caspase-3 and Caspase-9 levels in Akt group were significantly lower than other cirrhosis groups (untreated, saline and Ad-EGFP groups). These results suggested that introduction of Ad-myr-HA-Akt virus resulted in the inhibition of Fas-mediated apoptotic pathway, which presumably led to the reduction of the apoptosis of hepatocytes.
To understand the mechanism underlying the hepatocyte apoptosis and fibrosis of liver, we further measured two markers (DR5 and α-SMA) for the activation of HSC and the levels of eNOS and its phosphorylation status that is correlated to the NO production. Noticeable decrease of DR5 and α-SMA (Figure 5A) and increase of phosphorylated eNOS (Figure 5B) were observed in the Akt group when compared to other cirrhosis groups (untreated, saline and Ad-EGFP). As shown in Table 4, the NO level in liver was significantly higher in Akt group than other cirrhosis groups (untreated, saline and Ad-EGFP), which was consistent with the level of phosphorylated eNOS in these group. Collectively, these results suggested that introduction of Ad-myr-HA-Akt virus blocked the activation of HSC and maintained NO level, which may subsequently reduced liver fibrosis and blood vessel resistance following the damage from CCl4.
Fas is one of the most important receptors on cell surface to mediate apoptosis[26-29]. It has been shown that FasL-Fas pathway is an important cascade leading to hepatocyte apoptosis, which in turn activates HSC that produce collagen[1-5]. Song et al[30] showed that block of Fas signaling pathway could inhibit the development of cirrhosis. Akt plays important roles in regulating cell survival through inhibiting Fas-mediated apoptosis[11,12]. In the current study, we utilized the constitutive expression of active form of Akt to block cirrhosis induced by CCl4. The efficacy of this virus was firstly confirmed at the level of liver function. Furthermore, using multiple approaches, we examined whether the transfer of Akt via this virus in liver could result in the molecular alterations that favor the survival of hepatocyte and/or disfavor the fibrosis. Our data indicated that Ad-myr-HA-Akt virus was a useful tool to prevent CCl4-induced cirrhosis in rat model.
Encouragingly, the data obtained at different levels, from function to histological changes, apoptosis rate of hepatocytes, activation of HSC, deposition of collagen, portal vein pressure and NO level, which were all consistently and collectively supported the hypothesis that introduction of Ad-myr-HA-Akt virus inhibits the development of cirrhosis. First, all measured parameters for liver function were consistent with reduced hepatocyte apoptosis: the introduction of Akt virus led to increased expression of Akt and its phosphorylation, decreased expression of apoptotic mediators (Caspase-9 and Caspase-3) and ultimately preserved liver functions (enzyme levels). Second, the portal vein pressure was consistent with the histological structure as well as NO levels. The introduction of Akt virus led to reduction of formation of liver nodules, portal vein pressures and increased level of NO, which is directly correlated with vasodilation. Third, the level of specific marker (Hyp) for the liver fibrosis was consistent with the amount of deposition of collagen and the expression of α-SMA and DR5, markers for activated HSC.
While the introduction of Ad-myr-HA-Akt virus led to the rescue of phosphorylated Akt level as well as inhibition of apoptotic pathway in the liver of rat cirrhosis model. However, several issues remain to be addressed regarding the application of this virus for the treatment for cirrhosis. First, the efficacy of this form of virus in other type of cirrhosis, especially those chronically developed remains to be determined. In addition, regarding the development of the cirrhosis, it was thought that HSC activation plays a crucial role in producing collagen[1-5]. While we speculate that reduced HSC activation in Akt group may be a subsequent outcome of reduced hepatocyte apoptosis, it is unknown whether the introduction of Akt-virus has a direct effect on HSC activation. Furthermore, we observed that rats in the cirrhosis groups without treatment or received saline or Ad-EGFP treatment showed a noticeable reduction of phosphorylated Akt level, suggesting that the damage of hepatocytes following CCl4 treatment may also be involved in the disruption of Akt signaling pathway. Lastly, one of the risks of the constitutive activation of Akt is the increased susceptibility to tumorgenesis. We could not conduct a long-term study to determine the time of virus clearance and evaluate the risk of tumor development. As future directions, we will further determine the application of this virus in other forms of cirrhosis and assess its side effects.
We thank Dr. Liwu Gou (Buffloa Roswell Park institute, New York State University, United States) for providing pcDNA3.1-myr-HA-Akt.
Massive apoptosis of hepatocytes result in activation of hepatic stellate cells (HSC), which produce collagen and ultimately lead to the fibrosis and cirrhosis. Inhibiting hepatic apoptosis is considered as an important strategy to prevent cirrhosis. Akt plays a crucial role in preventing Fas signaling-mediated hepatic apoptosis, and that over-expression of Akt was capable of preventing hepatic apoptosis.
The progress in understanding mechanisms of cirrhosis brings the development of effective therapies closer to reality. Points of therapeutic intervention may include: (1) removing the injurious stimuli; (2) suppressing hepatic inflammation; (3) down-regulating stellate cell activation; and (4) promoting matrix degradation. The future prospects for effective treatment are more promising than ever for the millions of patients with chronic liver disease worldwide.
To date, there have been a number of studies regarding the therapeutic implication of hepatic cirrhosis. However, the research about the strategy to block apoptotic signaling pathway are limited. In this study, the authors created an Ad-myr-HA-Akt virus and determined the efficacy of this virus in inhibiting the development of cirrhosis induced by CCl4 in rats. The authors confirmed that that introduction of Ad-myr-HA-Akt virus was not only able to ameliorate the liver cirrhosis but also to reduce the portal vein pressure.
These results could be the basis for further studies to understand the pathogenesis of hepatic cirrhosis. The conclusion suggest that Akt pathway may be a therapeutic target for human cirrhosis.
HSC, also known as perisinusoidal cells or Ito cells are pericytes found in the perisinusoidal space of the liver. Substantial evidence now exists to recognize HSCs as the main matrix-producing cells in the process of liver cirrhosis. Liver injury of any etiology will ultimately lead to activation of HSCs, which undergo transdifferentiation to fibrogenic myofibroblast-like cells.
Authors demonstrated that Akt improved liver histology and function and reduced portal venous pressure, liver apoptosis and collagen levels is useful for accurate understanding the progress of hepatic cirrhosis and also give a feasible target for the therapy of cirrhosis.
P- Reviewers: Abdel-Raheem IT, Trinder D S- Editor: Qi Y L- Editor: A E- Editor: Wu HL
| 1. | Guicciardi ME, Gores GJ. Apoptosis: a mechanism of acute and chronic liver injury. Gut. 2005;54:1024-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 390] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 2. | Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655-1669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2139] [Cited by in RCA: 2161] [Article Influence: 127.1] [Reference Citation Analysis (0)] |
| 3. | Mehal W, Imaeda A. Cell death and fibrogenesis. Semin Liver Dis. 2010;30:226-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Forbes SJ, Parola M. Liver fibrogenic cells. Best Pract Res Clin Gastroenterol. 2011;25:207-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 5. | Iwaisako K, Brenner DA, Kisseleva T. What's new in liver fibrosis? The origin of myofibroblasts in liver fibrosis. J Gastroenterol Hepatol. 2012;27 Suppl 2:65-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 179] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 6. | Li JT, Liao ZX, Ping J, Xu D, Wang H. Molecular mechanism of hepatic stellate cell activation and antifibrotic therapeutic strategies. J Gastroenterol. 2008;43:419-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 123] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 7. | Kisseleva T, Brenner DA. Anti-fibrogenic strategies and the regression of fibrosis. Best Pract Res Clin Gastroenterol. 2011;25:305-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 8. | Rockey DC. Current and future anti-fibrotic therapies for chronic liver disease. Clin Liver Dis. 2008;12:939-962, xi. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 132] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Ruehl M, Erben U, Kim K, Freise C, Dagdelen T, Eisele S, Trowitzsch-Kienast W, Zeitz M, Jia J, Stickel F. Extracts of Lindera obtusiloba induce antifibrotic effects in hepatic stellate cells via suppression of a TGF-beta-mediated profibrotic gene expression pattern. J Nutr Biochem. 2009;20:597-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Lin YL, Lin CY, Chi CW, Huang YT. Study on antifibrotic effects of curcumin in rat hepatic stellate cells. Phytother Res. 2009;23:927-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Moumen A, Ieraci A, Patané S, Solé C, Comella JX, Dono R, Maina F. Met signals hepatocyte survival by preventing Fas-triggered FLIP degradation in a PI3k-Akt-dependent manner. Hepatology. 2007;45:1210-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Hatano E, Brenner DA. Akt protects mouse hepatocytes from TNF-alpha- and Fas-mediated apoptosis through NK-kappa B activation. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1357-G1368. [PubMed] |
| 13. | Imamura Y, Ishikawa S, Sato N, Karashima R, Hirashima K, Hiyoshi Y, Nagai Y, Koga Y, Hayashi N, Watanabe M. Adenoviral oncolytic suicide gene therapy for a peritoneal dissemination model of gastric cancer in mice. Ann Surg Oncol. 2010;17:643-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Liu RY, Zhu YH, Zhou L, Zhao P, Li HL, Zhu LC, Han HY, Lin HX, Kang L, Wu JX. Adenovirus-mediated delivery of interferon-γ gene inhibits the growth of nasopharyngeal carcinoma. J Transl Med. 2012;10:256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Nie ZL, Pan YQ, He BS, Gu L, Chen LP, Li R, Xu YQ, Gao TY, Song GQ, Hoffman AR. Gene therapy for colorectal cancer by an oncolytic adenovirus that targets loss of the insulin-like growth factor 2 imprinting system. Mol Cancer. 2012;11:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Zhu H, Zhou W, Hu J, Huang Z, Lao W, Huang X, He C. Suppressing the growth of rectal cancer xenografts derived from patient tumors by an adenovector expressing small hairpin RNA targeting Bcl-XL. J Gene Med. 2012;14:761-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Yamanaka M, Tada Y, Kawamura K, Li Q, Okamoto S, Chai K, Yokoi S, Liang M, Fukamachi T, Kobayashi H. E1B-55 kDa-defective adenoviruses activate p53 in mesothelioma and enhance cytotoxicity of anticancer agents. J Thorac Oncol. 2012;7:1850-1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Fukui H, Wong HT, Beyer LA, Case BG, Swiderski DL, Di Polo A, Ryan AF, Raphael Y. BDNF gene therapy induces auditory nerve survival and fiber sprouting in deaf Pou4f3 mutant mice. Sci Rep. 2012;2:838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Kinoshita K, Iimuro Y, Fujimoto J, Inagaki Y, Namikawa K, Kiyama H, Nakajima Y, Otogawa K, Kawada N, Friedman SL. Targeted and regulable expression of transgenes in hepatic stellate cells and myofibroblasts in culture and in vivo using an adenoviral Cre/loxP system to antagonise hepatic fibrosis. Gut. 2007;56:396-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Ahn M, Gamble A, Witting SR, Magrisso J, Surendran S, Obici S, Morral N. Vector and helper genome rearrangements occur during production of helper-dependent adenoviral vectors. Hum Gene Ther Methods. 2013;24:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Pei Z, Kondo S, Kanegae Y, Saito I. Copy number of adenoviral vector genome transduced into target cells can be measured using quantitative PCR: application to vector titration. Biochem Biophys Res Commun. 2012;417:945-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Chiyo T, Sekiguchi S, Hayashi M, Tobita Y, Kanegae Y, Saito I, Kohara M. Conditional gene expression in hepatitis C virus transgenic mice without induction of severe liver injury using a non-inflammatory Cre-expressing adenovirus. Virus Res. 2011;160:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Winter LE, Barenkamp SJ. Construction and immunogenicity of recombinant adenovirus vaccines expressing the HMW1, HMW2, or Hia adhesion protein of nontypeable Haemophilus influenzae. Clin Vaccine Immunol. 2010;17:1567-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Segura MM, Monfar M, Puig M, Mennechet F, Ibanes S, Chillón M. A real-time PCR assay for quantification of canine adenoviral vectors. J Virol Methods. 2010;163:129-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Hua J, Qiu de K, Li JQ, Li EL, Chen XY, Peng YS. Expression of Toll-like receptor 4 in rat liver during the course of carbon tetrachloride-induced liver injury. J Gastroenterol Hepatol. 2007;22:862-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Akazawa Y, Gores GJ. Death receptor-mediated liver injury. Semin. Liver Dis. 2007;27:327-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Okano H, Shiraki K, Inoue H, Kawakita T, Yamanaka T, Deguchi M, Sugimoto K, Sakai T, Ohmori S, Fujikawa K. Cellular FLICE/caspase-8-inhibitory protein as a principal regulator of cell death and survival in human hepatocellular carcinoma. Lab Invest. 2003;83:1033-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 106] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Reinehr R, Häussinger D. CD95 death receptor and epidermal growth factor receptor (EGFR) in liver cell apoptosis and regeneration. Arch Biochem Biophys. 2012;518:2-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Guicciardi ME, Gores GJ. Apoptosis as a mechanism for liver disease progression. Semin Liver Dis. 2010;30:402-410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 30. | Song E, Lee SK, Wang J, Ince N, Ouyang N, Min J, Chen J, Shankar P, Lieberman J. RNA interference targeting Fas protects mice from fulminant hepatitis. Nat Med. 2003;9:347-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 813] [Article Influence: 37.0] [Reference Citation Analysis (0)] |