Published online Nov 21, 2013. doi: 10.3748/wjg.v19.i43.7766
Revised: September 5, 2013
Accepted: September 29, 2013
Published online: November 21, 2013
Processing time: 150 Days and 11.3 Hours
AIM: To investigate the function of PU.1-silenced semi-mature dendritic cells (DCs) and the possibility of utilizing cell immunity in rat intestinal transplantation.
METHODS: DCs were isolated from the bone marrow of F344 rats and cultured using the adherent method. The PU.1 gene was knocked down in DCs using small interfering RNAs (siRNAs) for 24 h, and the cells were then incubated with lipopolysaccharide for 48 h. The PU.1 siRNA that had the highest silencing efficiency was screened using reverse transcription-polymerase chain reaction and Western blot for further study. The tolerance capacity was analyzed and compared between PU.1-silenced DCs (siRNA PU.1 group), negative control-silenced DCs (siRNA NC group) and immature DCs (control group) both in vitro and in vivo.
RESULTS: Blocking expression of the PU.1 gene in vitro led to a reduction in DC maturation and an increased tolerance capability. PU.1-silenced DCs expressed moderate levels of major histocompatibility complex (MHC)-II and low levels of co-stimulatory molecules, and produced more interleukin (IL)-10, but less IL-12. Compared with the negative control group, surface molecules cluster of differentiation 80 (CD80), CD86 and MHC-II in the siRNA PU.1 group were 27.0% ± 5.6%, 23.6% ± 4.8% and 36.8% ± 6.8%, respectively, and showed a significantly lower trend (P < 0.05). In vivo treatment of recipients with PU.1-silenced DCs injected before intestinal transplantation (siRNA PU.1 group), significantly prolonged allograft survival and resulted in better tissue histopathology compared with the siRNA NC group and control group. Mean survival time after transplantation was 14.3 ± 3.3 d in the siRNA PU.1 group (P < 0.05).
CONCLUSION: PU.1-silenced semi-mature DCs induced partial immune tolerance both in vitro and in vivo, which could be used as a new strategy to promote transplantation tolerance.
Core tip: The inhibition of dendritic cells (DCs) maturation can promote their tolerogenicity in transplantation. PU.1 is a newly discovered transcription factor which is required for the regulation of dendritic cell maturation in all DCs subsets. We silenced the PU.1 gene using siRNA and showed, for the first time, that PU.1-silenced DCs had immune tolerance. This may be a new strategy to prevent graft rejection following intestinal transplantation.
- Citation: Xu XW, Ding BW, Zhu CR, Ji W, Li JS. PU.1-silenced dendritic cells prolong allograft survival in rats receiving intestinal transplantation. World J Gastroenterol 2013; 19(43): 7766-7771
- URL: https://www.wjgnet.com/1007-9327/full/v19/i43/7766.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i43.7766
Dendritic cells (DCs) are key antigen-presenting cells, which play an important role in regulating adaptive immune responses. Studies have shown that whether immune responses are induced or suppressed greatly depends on the degree of DC maturation and specific subsets[1,2]. Immature DCs, which express low levels of major histocompatibility complex (MHC-II) and co-stimulatory molecules, such as cluster of differentiation 80 (CD80), CD86 and CD40, have a lower ability to capture antigens for presentation to specific T cells[3,4]. Therefore, various approaches have been explored to inhibit the maturation of DCs and to promote their tolerogenicity[5].
MicroRNA-155 has emerged as an important regulator in the immune system[6,7]. MicroRNA (mRNA)-155 knockout mice showed aberrant immune functions, such as defective B and T cell immunity, abnormal function of antigen-presenting cells, and a failure in the production of high-affinity Immunoglobulin G (IgG)1 antibodies[8,9]. These phenotypes are related to the impaired ability of mRNA-155 to target the E-twenty six transcription factor PU.1, which was first discovered to have multiple roles in hematopoiesis. PU.1 is an essential regulator of both cDC and pDC lineages[10,11], and can regulate numerous genes within the myeloid and lymphoid lineages[12]. Recent studies have shown that PU.1 can partially direct the important cytokine receptor Flt3 and play a critical role in DC development and function. Therefore, PU.1 is a major and critical regulator of DC maturation.
In this study, we silenced PU.1 expression in rat bone marrow DCs (BM cells) using small interference RNA (siRNA) molecules and stimulated the cells with lipopolysaccharide (LPS) to obtain semi-mature DCs. These semi-mature DCs were then used to determine whether they could induce tolerance and have an effect on intestinal transplantation in rats.
F344 and Wistar rats (weighing 180-220 g) were purchased from the Vital River Corporation (Beijing, China) and kept under specific-pathogen-free conditions. Animal experiments and maintenance were approved and regulated by the Ethics Committee of Jinling Hospital (Nanjing, China).
BM cells of F344 rats were used for DC generation following the method described by Lutz et al[13] and Yang et al[14]. Briefly, the femur and tibia were mechanically obtained, and the marrow cells were flushed out using phosphate-buffered saline (PBS). The obtained single cell suspensions were centrifuged, treated with 0.15 mol/L NH4Cl for 5 min and washed twice. The harvested BM cells were cultured in six-well plates (density, 4 × 106/mL) in RPMI1640 with 5 ng/mL recombinant rat granulocyte-macrophage colony-stimulating factor and 5 ng/mL interleukin (IL)-4 (Peprotech, NJ, United States). Non-adherent granulocytes were removed after 48 h of culture. From day 3, half of the medium was replaced with fresh medium every other day. On day 7, non-adherent and loosely adherent cells were harvested and identified as immature DCs, which were ready for transfection, and the supernatants were used for cytokine detection.
For in vitro studies, siRNAs targeting the PU.1 gene were synthesized by Jima Corporation (Shanghai, China)[10,15]. The siRNAs were transiently transfected into the cells using Lipofectamine 2000 (Invitrogen, United States) for 24 h according to the manufacturer’s instructions. The sequences of a PU.1-specific siRNA were: sense, 5’-AGCGAUCACUAUUGGGAUUTT-3’; and antisense, 5’-AAUCCCAAUAGUGAUCGCUTT-3’. The sequences of a negative control siRNA were: sense, 5’-UUCUCCGAACGUGUCACGUTT-3’; and antisense, 5’-ACGUGACACGUUCGGAGAATT-3’. Transfected DCs were cultured in the presence of 10 μg/mL LPS (Sigma-Aldrich, United States) for a further 48 h. Cells and supernatants were harvested for later use, and the cells were designated as PU.1-silenced-LPS DCs (siRNA PU.1 group), negative control-silenced-LPS DCs (siRNA NC group) or immature DCs (control group).
Total RNA was extracted from cells using Trizol (Invitrogen, United States). RNA (1 pg) was reverse transcribed using an oligo-(dT) primer and reverse transcriptase (Invitrogen). All the measurements were performed in triplicate for each sample and normalized to the β-actin gene. The primer sequences for PU.1 were: forward, 5’-GAGTTTGAGAACTTCCCTGAG-3’; and reverse, 5’-TGGTAGGTCATCTTCTTGCGG-3’. Primer sequences for β-actin were: forward, 5’-ATGGATGACGATATCGCT-3’; and reverse, 5’-ATGAGGTAGTCTGTCAGGT-3’[15].
Cells were homogenized in RIPA lysis buffer and used for Western blot assays. Briefly, equal amounts of protein extracts were boiled in sodium dodecyl sulfate (SDS)-sample buffer for 5 min before being electrophoretically resolved on SDS polyacrylamide gels and transferred to nitrocellulose membranes (Bio-Rad). The membranes were blocked with 5% fat-free dried milk and bovine serum albumin dissolved in Tris Buffered Saline with Tween-20 for 1 h at room temperature and incubated overnight at 4 °C with antibodies raised against PU.1 (Santa Cruz, United States) according to the manufacturer’s instructions. Binding of these primary antibodies was visualized with goat anti-rabbit secondary antibodies (1:2000 dilution; Santa Cruz, Texas, United States). Finally, the membranes were washed and an emitter-coupled logic signal detection kit was used (Amersham, IL, United States) for signal detection.
The following antibodies were purchased from eBioscience Corporation (CA, United States): phycoerythrin (PE)-coupled anti-CD86, PE-coupled anti-CD80 and fluorescein isothiocyanate-coupled anti-MHC-II. OX62-Alexa Fluor was obtained from BioLegend (CA, United States). After 7 d of cultivation, the prepared cells mentioned above were stained using the above antibodies at 4 °C for 30 min in PBS containing 0.1% sodium azide. Phenotypic analysis of DCs was performed on a fluorescence activated cell sorter Calibur flow cytometer equipped with Cell Quest (Becton Dickinson, New Jersey, United States).
T cells (2 × 105) purified from rat splenocytes (responder cells) were plated with immature DCs, PU.1-silenced DCs or negative control-silenced DCs (stimulator cells) at varying ratios. Cells were cultured for 3 d and pulsed with 1 μCi of [3H] thymidine (PerkinElmer, Woodbridge, United States) for the final 18 h. The cells were subsequently harvested onto glass fiber filters, and incorporated radioactivity was quantified using a liquid scintillation counter.
The supernatants from each group as described above were collected and the cytokines IL-12p70 and IL-10 were measured by enzyme-linked immunosorbent assay according to the manufacturer’s instructions (R and D Systems, Minneapolis, United States).
Recipient Wistar rats, six in each group, were treated with PU.1-silenced DCs, negative control-silenced-LPS DCs or immature DCs from donor F344 rats (1 × 106 cells), seven days prior to intestinal transplantation via tail vein injection. Heterotopic intestinal transplantation was performed using the technique described by Zhang et al[16]. The state of intestinal health/rejection was monitored and evaluated daily by examining the color of the graft and secretions from the stoma. Recipient rats that died within three days were regarded as technical failures and excluded from further analysis. The allografts were collected from a location 5 cm from the origin of the jejunum on day 5 after transplantation. Tissues from the three groups were sectioned and subjected to HE staining to evaluate morphologic changes.
Data were reported as mean ± SD. One-way analysis of variance was used for data analysis within groups. P values less than 0.05 were considered significant.
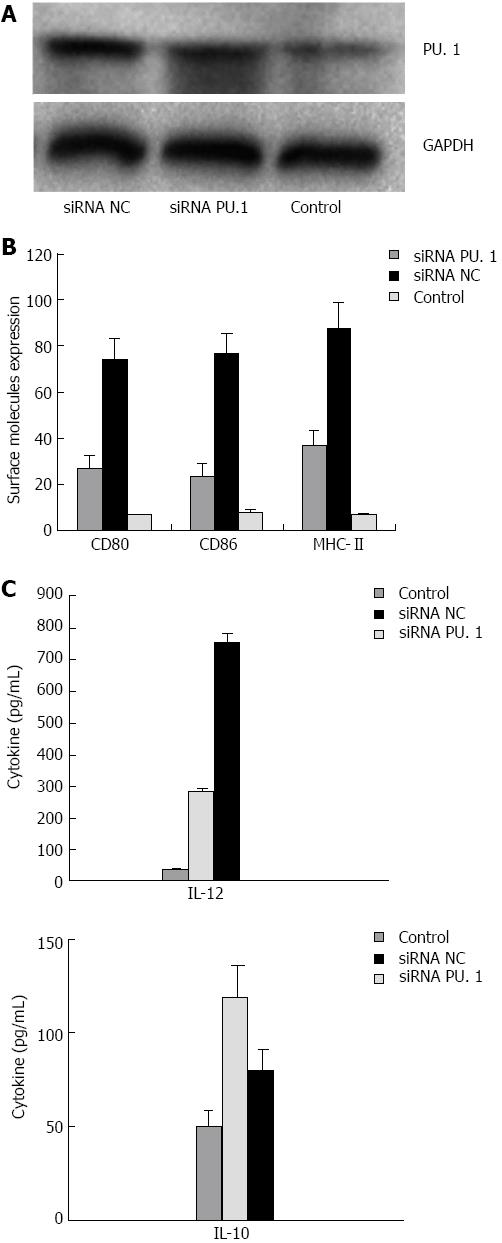
In order to silence the PU.1 gene in DCs, we constructed three pre-siRNA vectors targeting PU.1 and one vector carrying a negative control siRNA. We incubated synthetic siRNAs with DCs which were induced with GM-CSF and IL-4 for 7 d to validate the efficiency of gene silencing. The most efficient plasmid to silence PU.1 was selected by assaying the PU.1 mRNA and protein expression. Forty-eight hours after transfection, PU.1 expression in the siRNA PU.1 group was reduced by approximately 85% at the protein level compared with the siRNA NC group (Figure 1A).
Similar to the characteristics of mature DCs, the DCs in the siRNA NC group also expressed high levels of MHC class II and co-stimulatory molecules. However, in the siRNA PU.1 group, the DCs were semi-mature, with the expression of CD80, CD86 and MHC-II (27.0% ± 5.6%, 23.6% ± 4.8% and 36.8% ± 6.8%, respectively) significantly lower than that in the siRNA NC group (74.0% ± 9.4%, 76.5% ± 8.7% and 87.8% ± 11.3%, respectively) (Figure 1B, P < 0.05). The ability of DCs in the three groups to produce cytokines in cell culture supernatants was also determined, and an opposite trend was noted between IL-12p70 and IL-10 production (P < 0.05) (Figure 1C). These data indicate that the PU.1 silencing partially inhibits DC maturation.
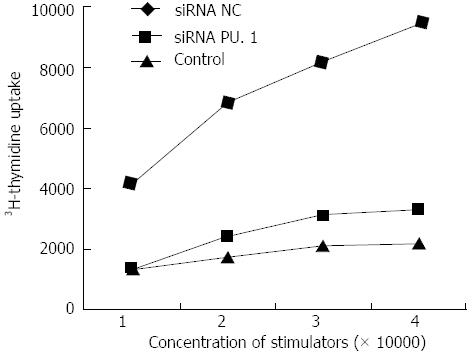
Purification of T cells and MLR analysis were performed to observe the in vitro activity of DCs. The proliferation of Wistar rat splenic T cells in a primary mixed lymphocyte reaction (MLR) in response to stimulation with DCs in the siRNA PU.1 group was significantly reduced compared with that in the siRNA NC group (P < 0.05, Figure 2), suggesting that PU.1-silenced DCs have an impaired capacity to stimulate T cell proliferation.
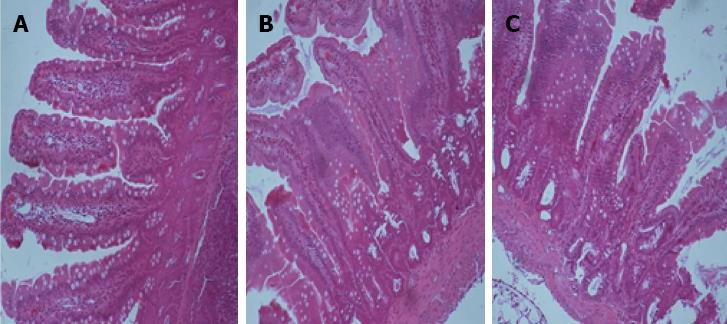
Since the results of the in vitro study showed that silencing of PU.1 reduced DC maturation and inhibited allogeneic T cell proliferation, we postulated that knockdown of this key transcription factor might prevent graft rejection. To determine this, we treated Wistar recipients with different groups of DCs 7 d before performing intestinal transplantation. While recipient survival was short in the siRNA NC group (7.8 ± 1.5 d, n = 6, P < 0.05) and the control group (8.0 ± 2.5 d, n = 6, P < 0.05), the infusion of siRNA PU.1 DCs significantly prolonged survival (14.3 ± 3.3 d). Consistent with our surmise, morphological features of acute rejection were prominent in the siRNA NC group and in the control group. Histological examination showed different degrees of lymphocyte infiltration and villous edema. In contrast, PU.1-silenced DCs delayed and reduced the immune response and injury, with mild lymphocyte infiltration and reduced inflammation observed in the allograft intestine (Figure 3).
Recently, the role of innate immunity in shaping the adaptive response has been focused in transplantation research, and studies have shown that the infusion of donor immature DCs can prolong graft survival after organ transplantation[17,18], mainly because they are capable of inducing tolerance by inducing T cell anergy or apoptosis[19,20]. Immature DCs express low levels of MHC II and co-stimulatory molecules and fail to elicit naïve T cells to modulate the adaptive immune response. However, they are not stable in vivo and can easily be stimulated to transform into mature DCs through several signaling pathways[21], which limits their preservation and utilization. Recent studies show that by controlling ambient conditions in vitro, semi-mature DCs are obtained from immature DCs following LPS stimulation. These cells are phenotypically stable and hard to differentiate or mature. Yang et al[14] found that silencing of MyD88, a proximal component of nuclear factor-kappaB (NF-κB) signaling, affected the maturation of immature DCs by increasing the secretion of IL-10 and decreasing the secretion of IL-12p70. The NF-κB signaling pathway plays a critical role in DC maturation, and IL-10 is regarded as an immunosuppressive cytokine which can downregulate the synthesis of a broad range of inflammatory cytokines and inhibit allogeneic T cell proliferation[22]. Therefore, the silencing of key factors involved in DC maturation may lead to a stunted capacity to prime the immune response and better stability[23,24]. As PU.1 is highly expressed and plays a critical role in DC maturation, suppression of this gene may result in interruption of DC maturation. Unsurprisingly, we found that PU.1-silenced semi-mature DCs[25] had a better effect in reducing the inflammatory response than immature DCs in an intestinal allograft model.
It is difficult to perform rat intestinal transplantation due to complex microvascular techniques and high mortality. Many animals died of immune rejection within several days. Although immature DCs express low levels of MHC II and determine tolerogenicity, current evidence for the application of immature DCs in rodent transplantation models is equivocal. In our experiment, rat survival, cytokine production and intestinal histological changes were evaluated to test the immunosuppressive function of semi-mature DCs. We found that acute rejection was significantly alleviated on day 5 compared to the controls, along with prolonged survival time and better condition in these rats. Rats in the siRNA PU.1 group showed slowed neointima formation and reduced inflammation and fibrosis in the allograft intestine. These results can be explained by increased secretion of IL-10 and decreased IL-12p70. The increase in IL-10 may play a crucial role in mediating the functions of semi-mature DCs. The surface expression of co-stimulatory molecules, such as CD86, CD80 and CD40, also showed a reduced trend, which is consistent with the results of T-cell proliferation. Thus, our experiments demonstrated that PU.1 gene silencing induced partial tolerance in this animal transplantation model.
However, we do not know whether the number of semi-mature DCs fluctuated or changed in recipient rats, and whether these DCs reduced the number and maturation of native DCs at the time of small bowel transplantation. More in vivo studies in both donors and recipients are required to identify the mechanism related to better graft survival.
In conclusion, we have provided evidence that silencing of the PU.1 gene can impair DC maturation, inhibit allogeneic T cell proliferation, and induce immunosuppressive activity. Since PU.1 silencing can prolong intestinal transplant survival in rats, it may be used as a new strategy and viable therapeutic option to prevent graft rejection following intestinal transplantation.
We thank Yao Nie, Jian Wang, Bo Shen, Chun Tang, Qin He, Chen-Yang Wang for their encouragement and technical support in the laboratory tests; and Yu-Qin Xu for managing the supplies of the animal room for the research in Jinling Hospital, Nanjing, China.
As key antigen-presenting cells, whether dendritic cells (DCs) induce or suppress immune responses greatly depends on the degree of DC maturation and specific subsets. MicroRNA-155 has emerged as an important regulator in the immune system, and the transcription factor PU.1 is a direct target of miR-155, which has recently been found to play multiple critical roles in DC maturation and function.
PU.1 is a major and critical regulator of DC maturation. However, the mechanism of action of PU.1 in DC maturation and tolerogenicity has not been unequivocally addressed. In this study, the authors silenced the PU.1 gene using siRNA and demonstrated that PU.1 silencing impaired DC maturation and inhibited allogeneic T cell proliferation. PU.1-silenced DCs also prolonged intestinal transplant survival and improved the general state of the graft.
Recent reports have highlighted the important role of PU.1 in DC maturation and function. This is the first study to report that PU.1-silenced DCs can induce tolerogenicity, which can be applied in rat intestinal transplantation. Furthermore, the authors’in vitro studies suggest that inhibiting a key factor in a given signaling pathway is an effective way of inducing DC tolerogenicity.
The authors’ finding that PU.1-silenced dendritic cells can prolong intestinal transplant survival in rats suggest that PU.1 silencing may be used as a new strategy and viable therapeutic option to prevent graft rejection following intestinal transplantation.
PU.1 is an EPS transcription factor which was first discovered to play multiple roles in hematopoiesis. Recent studies have shown that PU.1 is an essential regulator of both cDC and pDC lineages and can regulate numerous genes within the myeloid and lymphoid lineages. Such a mechanism is thought to be crucial in inducing stable immature DCs. Not surprisingly, the DCs the authors cultivated in the study showed tolerogenicity both in vivo and in vitro.
The authors examined the surface molecule expression of PU.1-silenced DCs, T cell proliferation and cytokines of cell culture supernatants. The cells were injected to recipient rats to test the immunogenicity in vivo. It revealed that they induced immunosuppressive activity; and the increased interleukin-10 expression may play a crucial role in the semi-mature DC functions and induce immunogenicity. The results are interesting and may supplement molecular mechanism of PU.1.
P- Reviewers: Detry Olivier, Pei ZH S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Wu HL
| 1. | Santiago-Schwarz F. Positive and negative regulation of the myeloid dendritic cell lineage. J Leukoc Biol. 1999;66:209-216. [PubMed] |
| 2. | Chaussabel D, Banchereau J. Dendritic cells, therapeutic vectors of immunity and tolerance. Am J Transplant. 2005;5:205-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5:296-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 902] [Cited by in RCA: 902] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 4. | Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213-1222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1190] [Cited by in RCA: 1151] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 5. | van Duivenvoorde LM, van Mierlo GJ, Boonman ZF, Toes RE. Dendritic cells: vehicles for tolerance induction and prevention of autoimmune diseases. Immunobiology. 2006;211:627-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | O’Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci USA. 2007;104:1604-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1365] [Cited by in RCA: 1484] [Article Influence: 82.4] [Reference Citation Analysis (0)] |
| 7. | Thai TH, Calado DP, Casola S, Ansel KM, Xiao C, Xue Y, Murphy A, Frendewey D, Valenzuela D, Kutok JL. Regulation of the germinal center response by microRNA-155. Science. 2007;316:604-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1145] [Cited by in RCA: 1199] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 8. | Rodriguez A, Vigorito E, Clare S, Warren MV, Couttet P, Soond DR, van Dongen S, Grocock RJ, Das PP, Miska EA. Requirement of bic/microRNA-155 for normal immune function. Science. 2007;316:608-611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1554] [Cited by in RCA: 1552] [Article Influence: 86.2] [Reference Citation Analysis (0)] |
| 9. | Vigorito E, Perks KL, Abreu-Goodger C, Bunting S, Xiang Z, Kohlhaas S, Das PP, Miska EA, Rodriguez A, Bradley A. microRNA-155 regulates the generation of immunoglobulin class-switched plasma cells. Immunity. 2007;27:847-859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 652] [Cited by in RCA: 637] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 10. | Wu L, Liu YJ. Development of dendritic-cell lineages. Immunity. 2007;26:741-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 264] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 11. | Carotta S, Dakic A, D’Amico A, Pang SH, Greig KT, Nutt SL, Wu L. The transcription factor PU.1 controls dendritic cell development and Flt3 cytokine receptor expression in a dose-dependent manner. Immunity. 2010;32:628-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 219] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 12. | McKercher SR, Torbett BE, Anderson KL, Henkel GW, Vestal DJ, Baribault H, Klemsz M, Feeney AJ, Wu GE, Paige CJ. Targeted disruption of the PU.1 gene results in multiple hematopoietic abnormalities. EMBO J. 1996;15:5647-5658. [PubMed] |
| 13. | Lutz MB, Suri RM, Niimi M, Ogilvie AL, Kukutsch NA, Rössner S, Schuler G, Austyn JM. Immature dendritic cells generated with low doses of GM-CSF in the absence of IL-4 are maturation resistant and prolong allograft survival in vivo. Eur J Immunol. 2000;30:1813-1822. [PubMed] |
| 14. | Yang XJ, Meng S, Zhu CF, Jiang H, Wu WX. Semi-mature MyD88-silenced bone marrow dendritic cells prolong the allograft survival in a rat model of intestinal transplantation. Chin Med J (Engl). 2011;124:268-272. [PubMed] |
| 15. | Hirose S, Nishizumi H, Sakano H. Pub, a novel PU.1 binding protein, regulates the transcriptional activity of PU.1. Biochem Biophys Res Commun. 2003;311:351-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Zhang X, Li J, Li N, Shi X, Li Y. Safety of enteral rehabilitative therapy in rat small bowel transplantation. Chin Med J (Engl). 2003;116:703-707. [PubMed] |
| 17. | McCurry KR, Colvin BL, Zahorchak AF, Thomson AW. Regulatory dendritic cell therapy in organ transplantation. Transpl Int. 2006;19:525-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Casiraghi F, Aiello S, Remuzzi G. Transplant tolerance: progress and challenges. J Nephrol. 2010;23:263-270. [PubMed] |
| 19. | Vosters O, Nève J, De Wit D, Willems F, Goldman M, Verhasselt V. Dendritic cells exposed to nacystelyn are refractory to maturation and promote the emergence of alloreactive regulatory t cells. Transplantation. 2003;75:383-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Mahnke K, Schmitt E, Bonifaz L, Enk AH, Jonuleit H. Immature, but not inactive: the tolerogenic function of immature dendritic cells. Immunol Cell Biol. 2002;80:477-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 273] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 21. | Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1013] [Cited by in RCA: 983] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 22. | Akdis CA, Blaser K. Mechanisms of interleukin-10-mediated immune suppression. Immunology. 2001;103:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 263] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 23. | Miller G, Pillarisetty VG, Shah AB, Lahrs S, DeMatteo RP. Murine Flt3 ligand expands distinct dendritic cells with both tolerogenic and immunogenic properties. J Immunol. 2003;170:3554-3564. [PubMed] |
| 24. | Yamazaki S, Inaba K, Tarbell KV, Steinman RM. Dendritic cells expand antigen-specific Foxp3+ CD25+ CD4+ regulatory T cells including suppressors of alloreactivity. Immunol Rev. 2006;212:314-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 201] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 25. | Rössner S, Voigtländer C, Wiethe C, Hänig J, Seifarth C, Lutz MB. Myeloid dendritic cell precursors generated from bone marrow suppress T cell responses via cell contact and nitric oxide production in vitro. Eur J Immunol. 2005;35:3533-3544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 113] [Article Influence: 5.9] [Reference Citation Analysis (0)] |