Published online Nov 21, 2013. doi: 10.3748/wjg.v19.i43.7711
Revised: July 30, 2013
Accepted: August 4, 2013
Published online: November 21, 2013
Processing time: 235 Days and 7.7 Hours
AIM: To assess co-stimulatory and co-inhibitory markers of dendritic cells (DCs) in hepatitis C virus (HCV) infected subjects with and without uremia.
METHODS: Three subject groups were included in the study: group 1 involved 50 control subjects, group 2 involved 50 patients with chronic HCV infection and group 3 involved 50 HCV uremic subjects undergoing hemodialysis. CD83, CD86 and CD40 as co-stimulatory markers and PD-L1 as a co-inhibitory marker were assessed in peripheral blood mononuclear cells by real-time polymerase chain reaction. Interleukin-10 (IL-10) and hyaluronic acid (HA) levels were also assessed. All findings were correlated with disease activity, viral load and fibrogenesis.
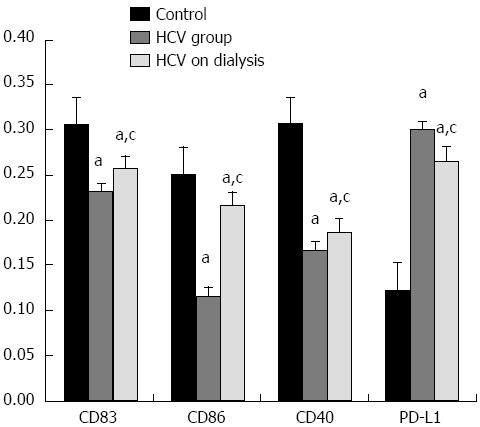
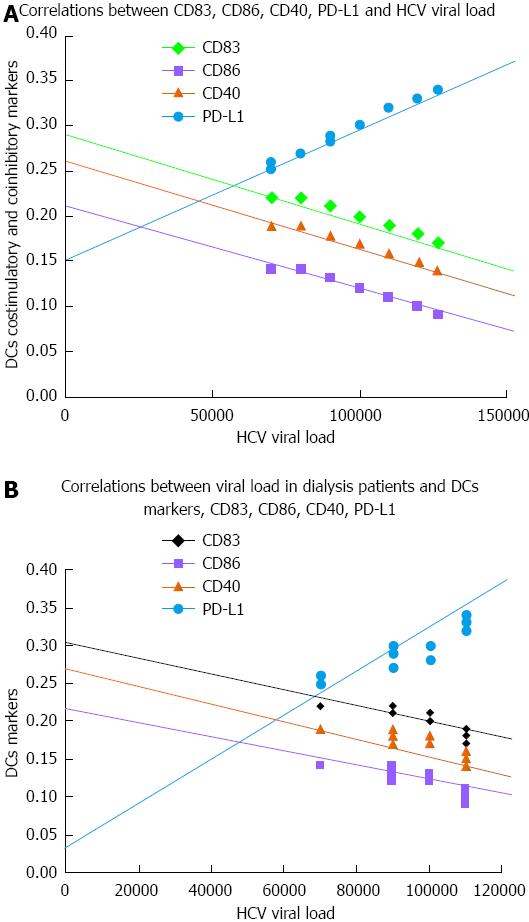
RESULTS: There was a significant decrease in co-stimulatory markers; CD83, CD86 and CD40 in groups 2 and 3 vs the control group. Co-stimulatory markers were significantly higher in group 3 vs group 2. There was a significant elevation in PD-L1 in both HCV groups vs the control group. PD-L1 was significantly lower in group 3 vs group 2. There was a significant elevation in IL-10 and HA levels in groups 2 and 3, where IL-10 was higher in group 3 and HA was lower in group 3 vs group 2. HA level was significantly correlated with disease activity and fibrosis grade in group 2. IL-10 was significantly correlated with fibrosis grade in group 2. There were significant negative correlations between co-stimulatory markers and viral load in groups 2 and 3, except CD83 in dialysis patients. There was a significant positive correlation between PD-L1 and viral load in both HCV groups.
CONCLUSION: A significant decrease in DC co-stimulatory markers and a significant increase in a DC co-inhibitory marker were observed in HCV subjects and to a lesser extent in dialysis patients.
Core tip: An assessment of the gene expression of co-stimulatory and a co-inhibitory marker (CD83, CD86, CD40, PD-L1) was conducted in patients with hepatitis C virus (HCV) infection and their correlations with viral load, hepatitis activity score and fibrosis grade were determined. There was a significant decrease in dendritic cell (DC) co-stimulatory markers in HCV infected subjects, where HCV uremic subjects exhibited a lower degree of reduced co-stimulatory markers. There was a significant increase in the DC co-inhibitory marker in HCV infected subjects, where HCV uremic subjects exhibited a lower degree of increased co-inhibitory marker. All DC markers were significantly correlated with HCV viral load, hepatitis activity index and fibrosis score.
- Citation: Fouad H, Raziky MSE, Aziz RAA, Sabry D, Aziz GMA, Ewais M, Sayed AR. Dendritic cell co-stimulatory and co-inhibitory markers in chronic HCV: An Egyptian study. World J Gastroenterol 2013; 19(43): 7711-7718
- URL: https://www.wjgnet.com/1007-9327/full/v19/i43/7711.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i43.7711
Hepatitis C virus (HCV) infection is a major public health problem, with an estimated global prevalence of 3% occurring in about 180 million carriers and approximately 4 million people are newly infected annually[1]. It is estimated that up to 70% of individuals exposed to HCV develop viral persistence[2,3]. On average, over half a million people in Egypt are infected by HCV annually, far more than any other country in the world, according to a new study published in 2010[4].
A high prevalence of HCV has been reported among hemodialysis (HD) patients worldwide. The prevalence of HCV infection among HD patients is significantly higher than healthy blood donors and the general population[5]. HD patients may be at risk for HCV due to the involvement of multiple routes of infection, especially poor blood screening of transfused blood, low standard of dialysis procedures and the need to apply infection control practices.
Patients who spontaneously clear HCV infection have strong and broad T cell responses, while patients with chronic HCV have weak and functionally impaired responses characterized by poor proliferation, impaired cytotoxicity and reduced cytokine secretion after antigen exposure[6,7]. Dendritic cells (DCs) are efficient and potent antigen presenters and activators of antigen-specific T cells and adaptive immunity[8]. Defective DC activation of T cells may underlie poor T cell responsiveness in HCV infection, and may, in part, determine the response to therapy[9,10].
Human peripheral blood DCs are currently categorized into two major subsets: myeloid DCs (mDCs) and plasmacytoid DCs (pDCs). mDCs are effective antigen presenters to T cells and secrete interleukin 12, while pDCs are the most potent secretors of antiviral type-I interferons such as interferon α (IFN-α)[11]. DCs migrate to sites of inflammation, sample antigens, and integrate generic microbial danger signals via innate immune receptors, named pathogen recognition receptors (PRRs) that recognize pathogen-associated molecular patterns[12]. Signals from PRRs combine with signals from inflammatory cytokines to activate DCs, causing up-regulation of co-stimulatory molecules such as CD40 and CD86. DCs then migrate to lymphoid tissue where they activate antigen-specific CD4 and CD8 T cells by presenting antigens on major histocompatibility complex (MHC) class I and II molecules[13,14].
Reports of global immune dysfunction in HCV infection are controversial; some authors have found faulty responses to general PRRs stimulation including decreased IFNα and IL12 secretion, reduced CD86 expression, decreased HLA-DR (MHC class II) and impaired stimulation of T cells in mixed lymphocyte reaction compared with normal controls[13]. Specific HCV proteins such as core and E2 can cause DC dysfunction in tissue culture models[14]. Other authors, including those using direct ex vivo human samples or a chimpanzee model of HCV have found no defects[15,16]. It has been consistently shown in HCV infection that pDC and mDC numbers are reduced in the peripheral compartment compared with normal controls, whereas reports have described increased numbers of DCs in the livers of HCV patients, suggesting hepatic DC sequestration[17-20]. The unresolved controversies listed above highlight the need for further study of DCs in HCV infection.
With regard to HD patients with HCV, some researchers reported altered monocyte-derived DC function in patients on HD[21]. However, reports on the natural history of hepatitis C in HD patients vary. Several studies stated that HCV disease activity in HD patients is mild, and is not progressive, perhaps due to immunological abnormalities in these patients[22].
The present study was conducted to assess DC response to HCV infection via assessment of the gene expression of co-stimulatory markers (CD83, CD86, and CD40) and a co-inhibitory marker (PD-L1) in pDCs and mDCs, and to study the correlations between DC functions and viral load, hepatitis activity score and fibrosis grade.
The present study was conducted in the Hepatic Virology Center, Kasr Al-Ainy, Faculty of Medicine, Cairo University. The study involved Group I which included 50 healthy subjects of both genders aged 18-40 years representing the control group and 100 adult age- and sex-matched patients with HCV-related chronic liver disease (CLD).
The patients selected had to comply with the following inclusion criteria: HCV antibody-positive serum and HCV RNA-positive serum by reverse transcription polymerase chain reaction (RT/PCR) for more than 6 months. All patients had to comply with the following exclusion criteria: coinfection with HBV and HCV, hepatocellular carcinoma, severe psychiatric disease, serious co-morbid conditions, HIV-positive patients defined as having a positive reaction to anti-HIV-1/2 (EIA), auto-immune hepatitis (positive reaction to antinuclear, anti-smooth muscle, anti-mitochondrial and anti-liver-kidney microsomal antibodies), schistosomiasis mansoni (patients with no previous history and negative stool examination), no previous history of regular use of hepatotoxic drugs or alcohol abuse (> 40 g of alcohol/d).
HCV patients were categorized into two groups: Group 2 included 50 HCV subjects with related CLD who were candidates for interferon therapy, and Group 3 included 50 HCV uremic subjects undergoing HD.
Patients were subjected to full clinical examination and abdominal ultrasonography. The following parameters were assessed in all subjects: serum levels of IL-10 and hyaluronic acid (HA) to assess fibrosis, as these parameters have been shown to be accurate in predicting significant fibrosis, severe fibrosis, and cirrhosis with area under characteristic curves (AUCs) of 0.73, 0.77 and 0.97, respectively. Moreover, accurate HA level cut-offs were defined for predicting significant fibrosis, severe fibrosis, and cirrhosis[23]. In addition, HA was an accurate noninvasive marker in predicting significant fibrosis in patients with hepatitis C on HD.
Quantitative gene expression of CD83, CD86, CD40 and PD-L1 in peripheral blood mononuclear cells was assessed by real-time PCR[24-27]. Histopathological examination of liver biopsy was performed using the Metavir scoring system for grading inflammation and staging fibrosis[28].
Whole blood samples were collected from all subjects. Serum was separated for assessment of HA and IL-10 levels by ELISA kits supplied by Corgenix Inc. (Westminster, CO, United States) according to the manufacturer’s recommendations.
The peripheral blood mononuclear cell layer (buffy coat) was isolated using Histopaque-1077 (Sigma, St. Louis, MO, United States) and centrifuged at 400 g for 30 min. Total RNA was isolated from the buffy coat using RNeasy purification reagent (Qiagen, Valencia, CA, United States). cDNA was generated from 5 μg of total RNA extracted with 1 μL (20 pmol) antisense primer and 0.8 μL superscript AMV reverse transcriptase for 60 min at 37 °C. The relative abundance of mRNA species was assessed using the SYBR® Green method on an ABI prism 7700 sequence detector system (Applied Biosystems, Foster City, CA, United States). PCR primers were designed with Primer-BLAST Software[29], (http://www.ncbi.nlm.nih.gov/tools/primer-blast) for RNA sequences from GenBank (Table 1). All primer sets had a calculated annealing temperature of 60 °C. Quantitative RT-PCR was performed in duplicate in a 25 μL reaction volume consisting of 2X SYBR Green PCR Master Mix (Applied Biosystems, United States), 900 nmol/L of each primer and 2-3 μL of cDNA. Amplification conditions were 2 min at 50 °C, 10 min at 95 °C and 40 cycles of denaturation for 15 s and annealing/extension at 60 °C for 10 min. Data from real-time assays were calculated using the v1.7 Sequence Detection Software from PE Biosystems (Foster City, CA, United States). Relative gene expression of CD83, CD86, CD40 and PD-L1 mRNA was calculated using the comparative Ct method as previously described. All values were normalized to the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene and reported as fold change over background levels detected in the control group.
| Homo sapiens CD40 | 5'-CCA AAA CGG GCC CTG CTC CA-3' |
| 5'-GAG CCT GGC CCC CTC CAA CA-3' | |
| Gene Bank accession number NM_000074.2 | |
| Homo sapiens CD86 | 5'-TAG GAG GTA CGG GGA GCT CGC AA-3' |
| 5'-TTG GCA TGG CAG GTC TGC AGT C-3' | |
| Gene Bank accession number 006889.3 | |
| Homo sapiens CD83 | 5'-CGA CGC CGG AGG TGA AGG TG-3' |
| 5'-TCC GGG TCC TGC AGA GTG CA-3' | |
| Gene Bank accession number 001040280.1 | |
| Homo sapiens PDL1 | 5'-ACA GAG GGC CCG GCT GTT GA-3' |
| 5'-CTT CGG CCT TGG GGT AGC CC-3' | |
| Gene Bank accession number AY254342.1 | |
| Homo sapiens GAPDH | 5'-GAAGGTGAAGGTCGGAGTCA-3' |
| 5'-GAAGATGGTGATGGGATTTC-3' | |
| Gene Bank accession number NC_000019.9 |
The SPSS program version 16.0.1 (SPSS Inc., Chicago, IL, United States) was used. Numerical data were expressed as mean ± SD. For comparisons between treatment groups, the null hypothesis was tested by a single-factor ANOVA for multiple groups or unpaired t test for two groups. Comparisons were considered statistically significant if P < 0.05. Spearman correlations were assessed between certain studied parameters.
The results showed that there was a significant decrease in all co-stimulatory markers; CD83, CD86 and CD40 in group 2 (HCV subjects) and in group 3 (HCV subjects on HD) in comparison to control subjects. Co-stimulatory markers were significantly higher in group 3 in comparison to group 2. With regard to PD-L1, there was a significant increase in groups 2 and 3 in comparison to control subjects. PD-L1 was significantly lower in group 3 as compared to group 2 (Figure 1). This was reflected in viral load, where significant negative correlations were observed between all co-stimulatory markers and viral load in groups 2 and 3, except CD83 in dialysis patients (Figure 2)
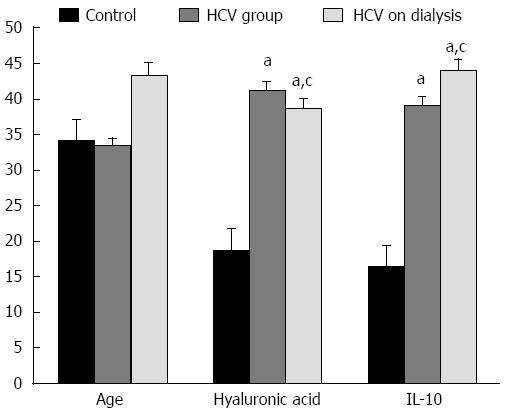
The findings in the present study showed a significant elevation in IL-10 and in HA levels in groups 2 and 3, where IL-10 was more significantly elevated in group 3 and HA was significantly lower in group 3 in comparison to group 2 (Figure 3).
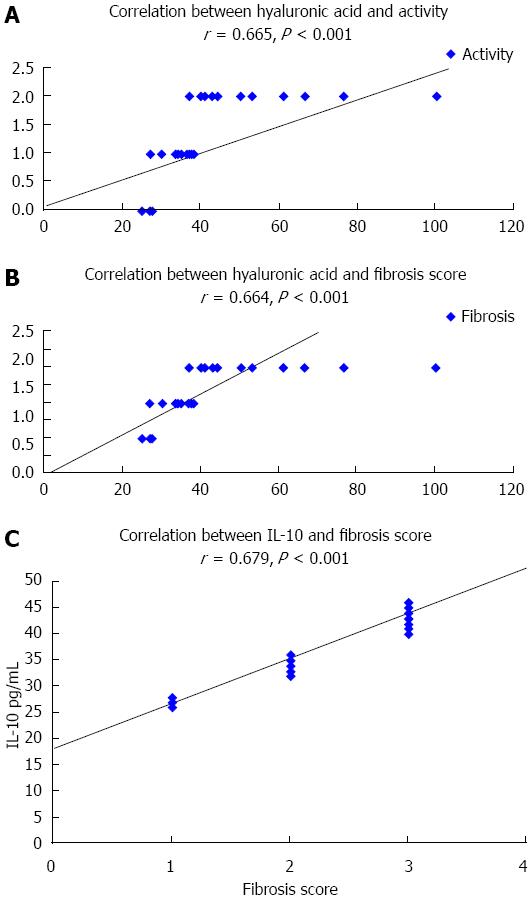
In group 2, the results showed that there was a significant correlation between HA and hepatitis activity score as well as grade of fibrosis; P < 0.001. No correlation between IL-10 levels and hepatitis activity score was observed, whereas, there was a significant positive correlation between IL-10 and fibrosis grade, P < 0.001 (Figure 4). Biopsy samples were not taken from group 3 patients (HCV on dialysis).
The results showed that there were significant negative correlations between all co-stimulatory markers and viral load in groups 2 and 3, except CD83 in dialysis patients. There was a significant positive correlation between PD-L1 and viral load in both HCV groups (Figure 2).
Of the 50 non-uremic patients who were candidates for interferon therapy only 4 remained PCR positive for HCV after treatment.
The results of the present study showed that in HCV infected subjects peripheral blood mononuclear cells exhibited lower expression of co-stimulatory markers; CD83, CD86, and CD40 and higher expression of a co-inhibitory marker; PD-L1 in comparison to healthy control subjects. Various studies have assessed DC function in HCV infection. Some have reported that DC function was impaired in HCV infection, which was identified by impaired allostimulatory capacity, decreased DC frequencies, increased mDC IL-10 secretion and decreased IL-12 secretion, as well as decreased pDC IFN-α secretion, while others did not[15,17,30].
Our findings coincide with the results reported by MacDonald et al[12], who stated that monocyte-derived DCs from patients with chronic HCV infection were significantly defective in their capacity to up-regulate the expression of surface molecules involved in antigen presentation (HLA-DR, CD86, CD40) and a classical marker of DC activation (CD83) and at physiological ratios of DCs to T cells, and decreased their ability to present antigen in allogeneic mixed lymphocyte reaction (MLR) assays. More recently, Shen et al[24] reported that impaired HCV-specific T cell immunity was associated with the persistence of HCV infection. DC dysfunction was believed to be involved in impaired T cell immunity, but the mechanisms are not understood. The results showed that the expression of both co-stimulatory markers (CD83, CD86, and CD40) and a co-inhibitory marker (PD-L1) was imbalanced in HCV-infected patients compared with healthy controls. The PD-L1/CD86 ratio was increased and positively correlated with PD-L1 expression on DCs in HCV-infected patients. The allostimulatory capacity of DCs was impaired and inversely correlated with PD-L1 expression and the PD-L1/CD86 ratio. These findings agree with our study and suggest that the effect of inhibitory marker PD-L1 overwhelmed the effect of co-stimulatory markers and down-regulated DC-T activation in HCV-infected patients.
HCV may inhibit the immune response of DCs, hindering the adaptive response from T cells[31]. It was hypothesized that DC dysfunction may determine the response to PEG-IFN/ribavirin therapy. In the study by Mengshol et al[32], the authors found that pDCs and mDCs were decreased compared to normal controls, consistent with prior studies in HCV and similar to prior studies of patients with HIV[33].
In the present study, the results of HA assessment revealed a significant elevation in HCV infected subjects in comparison to the healthy control group. Moreover, HA levels were positively correlated with hepatitis activity score and grade of fibrosis. These findings can be explained by a recent study conducted on another virus by Spahn et al[34], who stated that ineffective CD8 (+) T cell immunity to adeno-associated virus can result in prolonged liver injury and fibrogenesis. More recently, Jiao et al[35] determined whether liver DCs play a role in enhancing regression of liver fibrosis in murine carbon tetrachloride-induced liver injury. They found that conditional DC depletion soon after discontinuation of the liver insult led to delayed regression of fibrosis and reduced clearance of activated hepatic stellate cells, the key fibrogenic cells in the liver. Conversely, DC expansion induced either by Flt3L (fms-like tyrosine kinase-3 ligand) or adoptive transfer of purified DCs accelerated liver fibrosis regression. DC modulation of fibrosis was partially dependent on matrix metalloproteinase (MMP)-9, as MMP-9 inhibition abolished the Flt3L-mediated effect and the ability of transferred DCs to accelerate regression of fibrosis. In contrast, transfer of DCs from MMP-9-deficient mice failed to improve fibrosis regression. Another study conducted by Ryan et al[36] proved that in HCV chronically infected subjects, a single nucleotide polymorphism in a c-type lectin expressed by DCs, CD209, was associated with more advanced liver disease and with significantly higher liver fibrosis scores.
With regard to IL-10 levels in our study, the findings revealed significantly elevated IL-10 levels in HCV infected subjects in comparison to healthy controls. Moreover, IL-10 levels were positively correlated with fibrosis grade. These findings agree with results reported by Díaz-Valdés et al[37], who stated that high levels of IL-10 present in chronic HCV infection are associated with the poor antiviral cellular immune responses found in these patients. To overcome the immunosuppressive effect of IL-10 on antigen-presenting cells such as DCs, they developed peptide inhibitors of IL-10 and found that IL-10 inhibiting peptides have important applications in enhancing anti-HCV immune responses by restoring the immunostimulatory capabilities of DCs.
With regard to HD patients, Choi et al[21] found that surface expression of major histocompatibility complex class II, CD83, and CD86, and chemokine receptor CCR7 in monocyte derived dendritic cells (moDCs) was not different between HD patients and healthy controls. Furthermore, moDCs from HD patients produced significantly higher amounts of IL-6, IL-8, IL-1b, and TNF-α when stimulated by cytokine cocktails compared to healthy controls. Abnormalities in cytokine production by moDCs in ESRD patients have also been reported in several previous studies[38,39]. Verkade et al[39] also demonstrated a marked increase in IL-15 production, a known stimulatory cytokine for DCs, while IL-10 and IL-12p70 levels were not different. In addition, mature moDCs from HD patients showed significantly enhanced allogeneic T cell proliferation compared to healthy controls. These findings could explain our results which showed a significantly lower degree of reduced co-stimulatory markers as well as a significantly lower degree of elevated co-inhibitory marker in HD patients as compared to HCV subjects without uremia.
In conclusion, a significant decrease in DC co-stimulatory markers in HCV infected subjects was observed, where HCV uremic subjects exhibited a lower degree of reduced co-stimulatory markers. There was a significant increase in the DC co-inhibitory marker in HCV infected subjects, where HCV uremic subjects exhibited a lower degree of elevated co-inhibitory marker. All DC markers were significantly correlated with HCV viral load, hepatitis activity index and fibrosis score.
Defective dendritic cell (DC) activation of T cells may underlie poor T cell responsiveness in hepatitis C virus (HCV) infection, and may, in part, determine the response to therapy. It has been consistently shown in HCV infection that plasmacytoid DCs (pDCs) and myeloid DCs (mDCs) numbers are reduced in the peripheral compartment compared with normal controls. Other reports have described increased numbers of DCs in the livers of HCV patients, suggesting hepatic DC sequestration. The unresolved controversies listed above highlight the need for further study of DCs in HCV infection. With regard to hemodialysis (HD) patients with HCV, some researchers have reported altered monocyte-derived dendritic cell function in patients on HD. However, reports on the natural history of hepatitis C in HD patients vary. Several studies stated that HCV disease activity in HD patients is mild, and is not progressive, perhaps due to immunological abnormalities in these patients. The present study was conducted to assess DC response to HCV infection with and without uremia via assessment of the gene expression of co-stimulatory markers (CD83, CD86, and CD40) and a co-inhibitory marker (PD-L1) in pDCs and mDCs, and to study the correlations between DC functions and viral load, hepatitis activity score and fibrosis grade.
Researchers have recently explored the mechanisms by which DC function is regulated during HCV infection, leading to impaired antiviral T cell responses and so to persistent viral infection. Recently, DC-based vaccines against HCV have been developed. Several studies describe the current understanding of DC function during HCV infection and explore the prospects of DC-based HCV vaccines. In particular, they describe the biology of DC, the phenotype of DC in HCV-infected patients, the effect of HCV on DC development and function, studies on new DC-based vaccines against HCV infection, and strategies to improve the efficacy of DC-based vaccines.
A recent study stated that the immature pDC phenotype and sustained pDC and mDC hyperresponsiveness are associated with spontaneous resolution of acute HCV infection. Several investigators found that injection of DCs presenting viral proteins constitutes a promising approach to stimulate T cell immunity against HCV. They also describe the strategy implemented to enhance antigen loading and immunostimulatory functions of DCs used in the preparation of therapeutic vaccines.
Assessment of DC functions in HCV patients may be applicable to anticipate the response to HCV standard of care therapy and to assign patients to specific therapeutic protocols. DC-based immunotherapy may be used in selected HCV cases with poor therapeutic response, high fibrosis index and high hepatitis activity score.
Plasmacytoid DCs (pDCs), myeloid DCs (mDCs), interferon α (IFNα), pathogen recognition receptors (PRRs), pathogen-associated molecular patterns (PAMPs), co-stimulatory markers (CD83, CD86, and CD40), a co-inhibitory marker (PD-L1), matrix metalloproteinase (MMP).
Interesting subject with sufficiently large cohorts to detect difference in response to Hepatitis C infection in those with liver disease vs others under dialysis treatment for chronic kidney failure. The key finding that there is “a significant decrease in dendritic cell co-stimulatory markers in HCV infected subjects, where HCV uremic subjects exhibited lower degree of the decrease. There was a significant increase in dendritic cell co-inhibitory marker in HCV infected subjects, where HCV uremic subjects exhibited lower degree of the elevation.” If proved in an individual HCV patient may prove important in directing management of HCV infected persons.
P- Reviewers: Madalinski KZ, Friedman EA S- Editor: Wen LL L- Editor: Webster JR E- Editor: Ma S
| 1. | Khan S, Attaullah S, Ali I, Ayaz S, Naseemullah SN, Siraj S, Khan J. Rising burden of Hepatitis C Virus in hemodialysis patients. Virol J. 2011;8:438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Guerra J, Garenne M, Mohamed MK, Fontanet A. HCV burden of infection in Egypt: results from a nationwide survey. J Viral Hepat. 2012;19:560-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 141] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 3. | Sievert W, Altraif I, Razavi HA, Abdo A, Ahmed EA, Alomair A, Amarapurkar D, Chen CH, Dou X, El Khayat H. A systematic review of hepatitis C virus epidemiology in Asia, Australia and Egypt. Liver Int. 2011;31 Suppl 2:61-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 395] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 4. | Miller FD, Abu-Raddad LJ. Evidence of intense ongoing endemic transmission of hepatitis C virus in Egypt. Proc Natl Acad Sci USA. 2010;107:14757-14762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 5. | Khodir SA, Alghateb M, Okasha KM, Shalaby Sel-S. Prevalence of HCV infections among hemodialysis patients in Al Gharbiyah Governorate, Egypt. Arab J Nephrol Transplant. 2012;5:145-147. [PubMed] |
| 6. | Centers for Disease Control and Prevention (CDC). Progress toward prevention and control of hepatitis C virus infection--Egypt, 2001-2012. MMWR Morb Mortal Wkly Rep. 2012;61:545-549. [PubMed] |
| 7. | Bowen DG, Walker CM. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature. 2005;436:946-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 578] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 8. | Pembroke T, Rees I, Gallagher K, Jones E, Mizen P, Navruzov T, Freedman A, Fielding C, Humphreys IR, Wang EC. Rapid early innate control of hepatitis C virus during IFN-α treatment compromises adaptive CD4+ T-cell immunity. Eur J Immunol. 2012;42:2383-2394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Gordon FD, Kwo P, Ghalib R, Crippin J, Vargas HE, Brown KA, Schiano T, Chaudhri E, Pedicone LD, Brown RS. Peginterferon-α-2b and ribavirin for hepatitis C recurrence postorthotopic liver transplantation. J Clin Gastroenterol. 2012;46:700-708. [PubMed] |
| 10. | Gruener NH, Lechner F, Jung MC, Diepolder H, Gerlach T, Lauer G, Walker B, Sullivan J, Phillips R, Pape GR. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J Virol. 2001;75:5550-5558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Rehermann B. Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J Clin Invest. 2009;119:1745-1754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 419] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 12. | MacDonald AJ, Semper AE, Libri NA, Rosenberg WM. Monocyte-derived dendritic cell function in chronic hepatitis C is impaired at physiological numbers of dendritic cells. Clin Exp Immunol. 2007;148:494-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Dolganiuc A, Chang S, Kodys K, Mandrekar P, Bakis G, Cormier M, Szabo G. Hepatitis C virus (HCV) core protein-induced, monocyte-mediated mechanisms of reduced IFN-alpha and plasmacytoid dendritic cell loss in chronic HCV infection. J Immunol. 2006;177:6758-6768. [PubMed] |
| 14. | Kanto T, Hayashi N, Takehara T, Tatsumi T, Kuzushita N, Ito A, Sasaki Y, Kasahara A, Hori M. Impaired allostimulatory capacity of peripheral blood dendritic cells recovered from hepatitis C virus-infected individuals. J Immunol. 1999;162:5584-5591. [PubMed] |
| 15. | Szabo G, Chang S, Dolganiuc A. Altered innate immunity in chronic hepatitis C infection: cause or effect? Hepatology. 2007;46:1279-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Velazquez VM, Hon H, Ibegbu C, Knechtle SJ, Kirk AD, Grakoui A. Hepatic enrichment and activation of myeloid dendritic cells during chronic hepatitis C virus infection. Hepatology. 2012;56:2071-2081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Martinet J, Leroy V, Dufeu-Duchesne T, Larrat S, Richard MJ, Zoulim F, Plumas J, Aspord C. Plasmacytoid dendritic cells induce efficient stimulation of antiviral immunity in the context of chronic hepatitis B virus infection. Hepatology. 2012;56:1706-1718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2469] [Cited by in RCA: 2553] [Article Influence: 121.6] [Reference Citation Analysis (0)] |
| 19. | Otsuka M, Kato N, Moriyama M, Taniguchi H, Wang Y, Dharel N, Kawabe T, Omata M. Interaction between the HCV NS3 protein and the host TBK1 protein leads to inhibition of cellular antiviral responses. Hepatology. 2005;41:1004-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 95] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Sioud M. Innate sensing of self and non-self RNAs by Toll-like receptors. Trends Mol Med. 2006;12:167-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 136] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 21. | Choi HM, Woo YS, Kim MG, Jo SK, Cho WY, Kim HK. Altered monocyte-derived dendritic cell function in patients on hemodialysis: a culprit for underlying impaired immune responses. Clin Exp Nephrol. 2011;15:546-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Bahadi A, Maoujoud O, Zejjari Y, Alayoud A, Hassani K, Elkabbaj D, Benyahia M. [Diagnosis and evaluation of hepatitis C virus among haemodialysis patients]. East Mediterr Health J. 2013;19:192-199. [PubMed] |
| 23. | Avila RE, Carmo RA, Farah Kde P, Teixeira AL, Coimbra LV, Antunes CM, Lambertucci JR. Hyaluronic acid in the evaluation of liver fibrosis in patients with hepatitis C on haemodialysis. Braz J Infect Dis. 2010;14:335-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 24. | Shen T, Chen X, Chen Y, Xu Q, Lu F, Liu S. Increased PD-L1 expression and PD-L1/CD86 ratio on dendritic cells were associated with impaired dendritic cells function in HCV infection. J Med Virol. 2010;82:1152-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Pachiadakis I, Chokshi S, Cooksley H, Farmakiotis D, Sarrazin C, Zeuzem S, Michalak TI, Naoumov NV. Early viraemia clearance during antiviral therapy of chronic hepatitis C improves dendritic cell functions. Clin Immunol. 2009;131:415-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Szabo G, Dolganiuc A. Subversion of plasmacytoid and myeloid dendritic cell functions in chronic HCV infection. Immunobiology. 2005;210:237-247. [PubMed] |
| 27. | Barnes E, Salio M, Cerundolo V, Francesco L, Pardoll D, Klenerman P, Cox A. Monocyte derived dendritic cells retain their functional capacity in patients following infection with hepatitis C virus. J Viral Hepat. 2008;15:219-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Theise ND. Liver biopsy assessment in chronic viral hepatitis: a personal, practical approach. Mod Pathol. 2007;20 Suppl 1:S3-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 29. | Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics. 2012;13:134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3185] [Cited by in RCA: 3904] [Article Influence: 300.3] [Reference Citation Analysis (0)] |
| 30. | Kanto T, Inoue M, Miyazaki M, Itose I, Miyatake H, Sakakibara M, Yakushijin T, Kaimori A, Oki C, Hiramatsu N. Impaired function of dendritic cells circulating in patients infected with hepatitis C virus who have persistently normal alanine aminotransferase levels. Intervirology. 2006;49:58-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Shiina M, Rehermann B. Cell culture-produced hepatitis C virus impairs plasmacytoid dendritic cell function. Hepatology. 2008;47:385-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 32. | Mengshol JA, Golden-Mason L, Castelblanco N, Im KA, Dillon SM, Wilson CC, Rosen HR. Impaired plasmacytoid dendritic cell maturation and differential chemotaxis in chronic hepatitis C virus: associations with antiviral treatment outcomes. Gut. 2009;58:964-973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Barron MA, Blyveis N, Palmer BE, MaWhinney S, Wilson CC. Influence of plasma viremia on defects in number and immunophenotype of blood dendritic cell subsets in human immunodeficiency virus 1-infected individuals. J Infect Dis. 2003;187:26-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 169] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 34. | Spahn J, Pierce RH, Crispe IN. Ineffective CD8(+) T-cell immunity to adeno-associated virus can result in prolonged liver injury and fibrogenesis. Am J Pathol. 2011;179:2370-2381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Jiao J, Sastre D, Fiel MI, Lee UE, Ghiassi-Nejad Z, Ginhoux F, Vivier E, Friedman SL, Merad M, Aloman C. Dendritic cell regulation of carbon tetrachloride-induced murine liver fibrosis regression. Hepatology. 2012;55:244-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 36. | Ryan EJ, Dring M, Ryan CM, McNulty C, Stevenson NJ, Lawless MW, Crowe J, Nolan N, Hegarty JE, O’Farrelly C. Variant in CD209 promoter is associated with severity of liver disease in chronic hepatitis C virus infection. Hum Immunol. 2010;71:829-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Díaz-Valdés N, Manterola L, Belsúe V, Riezu-Boj JI, Larrea E, Echeverria I, Llópiz D, López-Sagaseta J, Lerat H, Pawlotsky JM. Improved dendritic cell-based immunization against hepatitis C virus using peptide inhibitors of interleukin 10. Hepatology. 2011;53:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Agrawal S, Gollapudi P, Elahimehr R, Pahl MV, Vaziri ND. Effects of end-stage renal disease and haemodialysis on dendritic cell subsets and basal and LPS-stimulated cytokine production. Nephrol Dial Transplant. 2010;25:737-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 39. | Verkade MA, van Druningen CJ, Vaessen LM, Hesselink DA, Weimar W, Betjes MG. Functional impairment of monocyte-derived dendritic cells in patients with severe chronic kidney disease. Nephrol Dial Transplant. 2007;22:128-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |