Published online Nov 14, 2013. doi: 10.3748/wjg.v19.i42.7476
Revised: August 18, 2013
Accepted: September 15, 2013
Published online: November 14, 2013
Processing time: 165 Days and 16.4 Hours
Helicobacter pylori (H. pylori) is one of the most common chronic bacterial infections in humans, affecting half of world’s population. Therapy for H. pylori infection has proven to be both effective and safe. The one-week triple therapy including proton pump inhibitor, clarithromycin, and amoxicillin or metronidazole is still recommended as a first-line treatment to eradicate H. pylori infection in countries with low clarithromycin resistance. Generally, this therapy is well-tolerated, with only a few and usually minor side effects. However, rare but severe adverse effects such as pseudomembranous colitis have been reported, Clostridium difficile (C. difficile) infection being the main causative factor in all cases. We report the cases of two women who developed pseudomembranous colitis after a 1-wk triple therapy consisting of pantoprazole 20 mg bid, clarithromycin 500 mg bid, and amoxicillin 1 g bid to eradicate H. pylori infection. A limited colonoscopy showed typical appearance of pseudomembranous colitis, and the stool test for C. difficile toxins was positive. Rapid resolution of symptoms and negative C. difficile toxins were obtained in both patients with oral vancomycin. No relapse occurred during a four and eleven-month, respectively, follow up. These cases suggest that physicians should have a high index of suspicion for pseudomembranous colitis when evaluate patients with diarrhea following H. pylori eradication therapy.
Core tip: Herein are described the cases of two elderly women who developed pseudomembranous colitis after one-week triple therapy consisting of pantoprazole (20 mg bid), clarithromycin (500 mg bid), and amoxicillin (1 g bid) to eradicate Helicobacter pylori (H. pylori) infection. After a 10-d treatment with oral vancomycin (125 mg every 6 h) both patients had complete resolution of symptoms and negative stool test for Clostridium difficile toxins. Clinicians should have a high index of suspicion of pseudomembranous colitis as a rare, but severe complication of H. pylori therapy.
-
Citation: Trifan A, Girleanu I, Cojocariu C, Sfarti C, Singeap AM, Dorobat C, Grigore L, Stanciu C. Pseudomembranous colitis associated with a triple therapy for
Helicobacter pylori eradication. World J Gastroenterol 2013; 19(42): 7476-7479 - URL: https://www.wjgnet.com/1007-9327/full/v19/i42/7476.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i42.7476
Helicobacter pylori (H. pylori) is one of the most common chronic bacterial infections in humans, affecting half of the world’s population. Its prevalence is high in developing countries and low in the developed ones[1]. H. pylori eradication therapy is supported by numerous consensus groups around the world, and the treatment of millions of infected subjects has demonstrated that such strategy is both effective and safe. The spectrum of indications for H. pylori eradication therapy has steadily extended over the last decade[2] with a resultant increase in its use. The one-week triple therapy including proton pump inhibitor (PPI), clarithromycin and amoxicillin or metronidazole proposed at the first Maastricht conference[3] to eradicate H. pylori is still recommended as the first-line treatment by the recent Maastricht IV consensus conference[2] in countries with clarithromycin resistance rate under 15%-20% (e.g., Northern European countries)[4]. Eradication rates with standard triple therapy have fallen to 70%-80% over the past few years, mainly due to increasing resistance to clarithromycin[5]. Generally, this therapy is well-tolerated, with only a few and usually minor side effects (e.g., nausea, metallic taste). However, severe adverse effects such as pseudomembranous colitis have been reported[6-13], Clostridium difficile (C. difficile) being the main causative agent in all cases.
We report the cases of two elderly women who developed pseudomembranous colitis after one-week triple therapy with pantoprazole, clarithromycin, and amoxicillin for H. pylori infection.
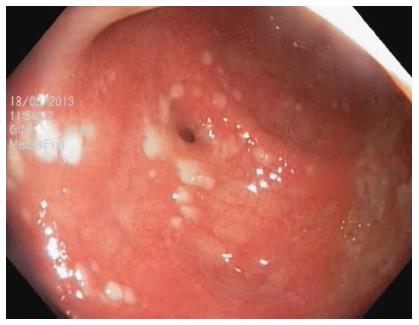
The first case is a 70-year-old woman who was referred to our department with a 10-d history of watery diarrhea (6-12 stools per day) and crampy abdominal pain. Her medical history included hypertension, chronic gastritis H. pylori positive, and colonic diverticulosis (previously diagnosed on colonoscopy). Three weeks before admission she completed a one-week triple therapy (pantoprazole 20 mg bid, clarithromycin 500 mg bid and amoxicillin 1 g bid) for H. pylori infection. On physical examination, she looked unhealthy, and her abdomen was mildly tender, with no masses. Temperature and vital signs were normal. Laboratory investigations revealed leukocytosis (14800/mm3) with neutrophilia, high C-reactive protein (11.5 mg/dL), and low levels of serum albumin (2.4 mg/dL), sodium (133 mEq/L), and potassium (2.7 mEq/L). Two days before admission, stool samples examination excluded enteric bacterial pathogens (Shighella, Salmonella, Yersinia spp.) as well as C. difficile toxins. Without any prior preparation, the patient underwent a limited colonoscopy, which showed diffusely scattered off-white pseudomembranes attached to the hyperemic underlying mucosa and multiple diverticula (Figure 1). Repeated stool sample examination was positive for C. difficile toxins A and B. Treatment with oral metronidazole 500 mg every 8 h was initiated, replaced 72 h later with oral vancomycin 125 mg every 6 h due to unfavorable response. After a 10-d treatment with vancomycin, the patient had a complete resolution of the symptoms and was discharged from hospital with negative results for C. difficile toxins and one stool per day. During a four-month follow-up, patient remained asymptomatic.
The second case concerns a 71-year-old woman who was admitted with profuse watery diarrhea (up to 10 stools daily) and abdominal pain. Her prior medical history was unremarkable. Symptoms occurred 5 d after a one-week triple therapy (pantoprazole 20 mg bid, clarithromycin 500 mg bid and amoxicillin 1 g bid) for H. pylori eradication. Physical examination was normal, except for signs of dehydration. Microbiological examination of stools was negative for Salmonella, Shigella and Yersinia spp., and did not reveal any parasites. The patient had leukocytosis (12400/mm3), hypokalemia (2.8 mEq/L), and mild inflammatory syndrome. Sigmoidoscopy showed typical signs of pseudomembranous colitis, while a stool test for C. difficile toxins A and B proved positive. The patient received 10-d treatment with oral vancomicyn 125 mg every 6 h, followed by prompt improvement in symptoms and negative test for C. difficile toxins. No relapse occurred during an 11-mo follow-up.
Eradication therapy for H. pylori provides enormous benefits and has proved to be both effective and safe. Except from the rare and mild side-effects, eradication therapy is generally well-tolerated. Severe adverse effects such as pseudomembranous colitis following eradication therapy have very rarely been reported[6-13] which is quite surprising, taken into account the immense number of subjects treated worldwide. It is difficult to find a clear explanation why are so rare cases of pseudomembranous colitis after eradication therapy reported in the literature, but some hypotheses were put forward: (1) the use of metronidazole, an efficient drug against C. difficile; however, several cases of pseudomembranous colitis, as published, occurred after a regimen containing metronidazole[8-10]; (2) the short duration of the therapy; (3) almost all treatments are carried out in outpatients (hospitalization is a risk factor for C. difficile infection); and (4) many cases with mild clinical disease were most likely not diagnosed, either because the patients did not consult a physician or the physician did not suspect the development of C. difficile infection[10].
Over the last decade, C. difficile infection rate has increased dramatically worldwide both in incidence and severity[14,15]. In addition to broad-spectrum antibiotic therapy, there have been identified many other potential risk factors for C. difficile infection (advanced age, female gender, comorbidities, admission to ICU, long hospital stay, immunosuppressive therapy, and PPI use)[16-21]. Several studies and recent meta-analyses have shown that PPI therapy is associated with increased risk of C. difficile infection[20-27], and United States Food and Drug Administration even issued a safety announcement to inform the public about this possible risk[28]. Newly published studies have found that C. difficile infection can occur outside the above mentioned well-known risk groups, in the absence of any hospitalization and even in young patients with no comorbidities[29].
All the components of the triple eradication therapy for H. pylori (PPI and two antibiotics: clarithromycin and amoxicillin or metronidazole) are potential risk factors for C. difficile infection. The most responsible for development of pseudomembranous colitis seems to be clarithromycin, used in both our cases and in most of the published reports[6,7,10,13].
The spectrum of C. difficile infection is wide, ranging from mild, self-limiting diarrhea to fulminant pseudomembranous colitis which is associated with significant morbidity and mortality. Most patients with C. difficile infection have a mild-to-moderate disease, but some may develop severe forms of disease such as pseudomembranous colitis, or even complicated by toxic megacolon[7]. Among variables used to define severe disease, the most important are the presence of pseudomembranes at endoscopy and age over 65 years. Both our cases met the criteria for a severe form of disease, and were treated with vancomycin according to current guidelines recommendations[30].
Our cases, in addition to the ones published, demonstrate that pseudomembranous colitis can occur after a usually well-tolerated triple therapy for H. pylori eradication. Despite this very rare complication, it should be underlined that H. pylori eradication therapy provides huge benefits and remains effective and generally safe. However, C.difficile infection may occur with an eradication therapy for H. pylori consisting of two antibiotics and a PPI. Most likely, C. difficile infection cases following H. pylori eradication therapy are not as rare as reported by literature, considering that a significant proportion of mild form of disease does not come to physician’s attention and thus may remain undiagnosed[31]. Clinicians should be aware of such complication when prescribing triple therapy for H. pylori eradication, and should inform the patients that they may have diarrhea during or after treatment, and therefore should seek medical advice.
In conclusion, pseudomembranous colitis should be suspected in any patient with watery diarrhea during or after triple therapy for H. pylori eradication. Awareness of such complication is particularly important in the actual context when both duration and indications for H. pylori eradication therapy have been extended.
P- Reviewers: VetvickaV, Wasko-Czopnik D S- Editor: Zhai HH L- Editor: A E- Editor: Ma S
| 1. | Hunt RH, Xiao SD, Megraud F, Leon-Barua R, Bazzoli F, van der Merwe S, Vaz Coelho LG, Fock M, Fedail S, Cohen H. Helicobacter pylori in developing countries. World Gastroenterology Organisation Global Guideline. J Gastrointestin Liver Dis. 2011;20:299-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 2. | Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1719] [Cited by in RCA: 1588] [Article Influence: 122.2] [Reference Citation Analysis (5)] |
| 3. | Current European concepts in the management of Helicobacter pylori infection. The Maastricht Consensus Report. European Helicobacter Pylori Study Group. Gut. 1997;41:8-13. [PubMed] |
| 4. | Megraud F. Helicobacter pylori and antibiotic resistance. Gut. 2007;56:1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 652] [Cited by in RCA: 735] [Article Influence: 49.0] [Reference Citation Analysis (1)] |
| 6. | Teare JP, Booth JC, Brown JL, Martin J, Thomas HC. Pseudomembranous colitis following clarithromycin therapy. Eur J Gastroenterol Hepatol. 1995;7:275-277. [PubMed] |
| 7. | Schweigart U, Franck H, Schepp W, Lehn N, Becker K, Classen M. Toxic megacolon after Helicobacter pylori eradication therapy. Internist (Berl). 1997;38:352-354. [PubMed] |
| 8. | Archimandritis A, Souyioultzis S, Katsorida M, Tzivras M. Clostridium difficile colitis associated with a ‘triple’ regimen, containing clarithromycin and metronidazole, to eradicate Helicobacter pylori. J Intern Med. 1998;243:251-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Nawaz A, Mohammed I, Ahsan K, Karakurum A, Hadjiyane C, Pellecchia C. Clostridium difficile colitis associated with treatment of Helicobacter pylori infection. Am J Gastroenterol. 1998;93:1175-1176. [PubMed] |
| 10. | Harsch IA, Hahn EG, Konturek PC. Pseudomembranous colitis after eradication of Helicobacter pylori infection with a triple therapy. Med Sci Monit. 2001;7:751-754. [PubMed] |
| 11. | Lau CF, Hui PK, Fung TT, Tung SY, Wong AM, Loo CK, Lam KM. Pseudomembranous colitis without diarrhoea following Helicobacter pylori eradication therapy. Hosp Med. 2001;62:431-433. [PubMed] |
| 12. | Rai R, Rai S. Pseudomembranous colitis requiring surgical intervention following triple therapy for Helicobacter pylori eradication. ANZ J Surg. 2002;72:917-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Kubo N, Kochi S, Ariyama I, Murata M, Furusyo N, Hayashi J. Pseudomembranous colitis after Helicobacter pylori eradication therapy. Kansenshogaku Zasshi. 2006;80:51-55. [PubMed] |
| 14. | Ananthakrishnan AN. Clostridium difficile infection: epidemiology, risk factors and management. Nat Rev Gastroenterol Hepatol. 2011;8:17-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 252] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 15. | O’Donoghue C, Kyne L. Update on Clostridium difficile infection. Curr Opin Gastroenterol. 2011;27:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Khanna S, Pardi DS. The growing incidence and severity of Clostridium difficile infection in inpatient and outpatient settings. Expert Rev Gastroenterol Hepatol. 2010;4:409-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 17. | Morrison RH, Hall NS, Said M, Rice T, Groff H, Brodine SK, Slymen D, Lederman ER. Risk factors associated with complications and mortality in patients with Clostridium difficile infection. Clin Infect Dis. 2011;53:1173-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Dubberke ER, Reske KA, Olsen MA, McMullen KM, Mayfield JL, McDonald LC, Fraser VJ. Evaluation of Clostridium difficile-associated disease pressure as a risk factor for C difficile-associated disease. Arch Intern Med. 2007;167:1092-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Kuntz JL, Chrischilles EA, Pendergast JF, Herwaldt LA, Polgreen PM. Incidence of and risk factors for community-associated Clostridium difficile infection: a nested case-control study. BMC Infect Dis. 2011;11:194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 129] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 20. | Al-Tureihi FI, Hassoun A, Wolf-Klein G, Isenberg H. Albumin, length of stay, and proton pump inhibitors: key factors in Clostridium difficile-associated disease in nursing home patients. J Am Med Dir Assoc. 2005;6:105-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Dalton BR, Lye-Maccannell T, Henderson EA, Maccannell DR, Louie TJ. Proton pump inhibitors increase significantly the risk of Clostridium difficile infection in a low-endemicity, non-outbreak hospital setting. Aliment Pharmacol Ther. 2009;29:626-634. [PubMed] |
| 22. | Aseeri M, Schroeder T, Kramer J, Zackula R. Gastric acid suppression by proton pump inhibitors as a risk factor for clostridium difficile-associated diarrhea in hospitalized patients. Am J Gastroenterol. 2008;103:2308-2313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 168] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 23. | Linney S, Fernandes T, Einarson T, Sengar A, Walker JH, Mills A. Association Between Use of Proton Pump Inhibitors and a Clostridium difficile-Associated Disease Outbreak: Case-Control Study. Can J Hosp Pharm. 2010;63:31-37. [PubMed] |
| 24. | Kwok CS, Arthur AK, Anibueze CI, Singh S, Cavallazzi R, Loke YK. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol. 2012;107:1011-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 408] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 25. | Deshpande A, Pant C, Pasupuleti V, Rolston DD, Jain A, Deshpande N, Thota P, Sferra TJ, Hernandez AV. Association between proton pump inhibitor therapy and Clostridium difficile infection in a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:225-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 154] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 26. | Tleyjeh IM, Bin Abdulhak AA, Riaz M, Alasmari FA, Garbati MA, AlGhamdi M, Khan AR, Al Tannir M, Erwin PJ, Ibrahim T. Association between proton pump inhibitor therapy and clostridium difficile infection: a contemporary systematic review and meta-analysis. PLoS One. 2012;7:e50836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 27. | Howell MD, Novack V, Grgurich P, Soulliard D, Novack L, Pencina M, Talmor D. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010;170:784-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 312] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 29. | Khanna S, Pardi DS, Aronson SL, Kammer PP, Orenstein R, St Sauver JL, Harmsen WS, Zinsmeister AR. The epidemiology of community-acquired Clostridium difficile infection: a population-based study. Am J Gastroenterol. 2012;107:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 480] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 30. | Surawicz CM, Brandt LJ, Binion DG, Ananthakrishnan AN, Curry SR, Gilligan PH, McFarland LV, Mellow M, Zuckerbraun BS. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-98; quiz 499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1179] [Cited by in RCA: 1185] [Article Influence: 98.8] [Reference Citation Analysis (0)] |
| 31. | Alcalá L, Martín A, Marín M, Sánchez-Somolinos M, Catalán P, Peláez T, Bouza E; Spanish Clostridium difficile Study Group. The undiagnosed cases of Clostridium difficile infection in a whole nation: where is the problem? Clin Microbiol Infect. 2012;18:E204-E213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |