Published online Sep 7, 2013. doi: 10.3748/wjg.v19.i33.5464
Revised: June 14, 2013
Accepted: June 28, 2013
Published online: September 7, 2013
Processing time: 196 Days and 18.1 Hours
AIM: To investigate the effect of plasmapheresis via the portal vein for “small-for-size” syndrome (SFSS) aided by extracorporeal continuous portal diversion (ECPD).
METHODS: Extensive or total hepatectomy in the pig is usually adopted as a postoperative liver failure (PLF) or SFSS model. In this study, animals which underwent 85%-90% hepatectomy were randomized into either the Systemic group (n = 7) or the Portal group (n = 7). In the Systemic group, all pigs received temporal plasmapheresis (PP) via the extracorporeal catheter circuit (systemic to systemic circulation) from 24 to 30 h post-hepatectomy (PH); in the Portal group, all pigs received ECPD to divert partial portal vein flow (PVF) to the systemic circulation after hepatectomy, then converted to temporal PP from 24 to 30 h PH, and subsequently converted to ECPD again until 48 h PH. In the Portal group, the PVF was preserved at 3.0-3.3 times that of the baseline value, similar to that following 70% hepatectomy, which was regarded as the optimal PVF to the hypertrophic liver remnant. At 48 h PH, all pigs were re-opened and the portal vein pressure (PVP), PVF, and HAF (hepatic artery flow) were measured, and then diversion of the portal venous flow was terminated. After 1 h the PVP, PVF, and HAF were re-measured. The portal hemodynamic changes, liver injury, liver regeneration and bacterial/lipopolysaccharide (LPS) translocation were evaluated in the two groups.
RESULTS: The PVP in the Portal group was significantly lower than that in the Systemic group during the time period from 2 to 49 h PH (P < 0.05). Serum alanine aminotransferase (ALT), total bilirubin (TB) and ammonia were significantly reduced in the Portal group compared with the Systemic group from 24 to 48 h PH (P < 0.05). The Portal group may have attenuated sinusoidal endothelial injury and decreased the level of HA compared with the Systemic group. In the Systemic group, there was significant sinusoidal dilation, hydropic changes in hepatocytes and hemorrhage into the hepatic parenchyma, and the sinusoidal endothelial lining was partially destroyed and detached into the sinusoidal space. CD31 immunostaining revealed significant destruction of the endothelial lining. In the Portal group, there was no intraparenchymal hemorrhage and the sinusoidal endothelial cells and hepatocytes were well preserved. CD31 immunostaining was mild which indicated less destruction of the endothelial lining. HA was significantly decreased in the Portal group compared with the Systemic group from 2 to 48 h PH. The rate of liver remnant regeneration was elevated, while apoptosis was attenuated in the Portal group compared with the Systemic group. Thymidine kinase activity was much higher in the Portal group than in the Systemic group at 48 h PH. The PCNA index was significantly increased and the apoptotic index was significantly decreased in the Portal group compared with the Systemic group. Bacterial translocation and endotoxin, as well as the inflammatory response, were significantly attenuated in the Portal group compared with the Systemic group. LPS, tumor necrosis factor-α and interleukin-6 levels were all significantly decreased in the Portal group compared with the Systemic group from 24 to 48 h PH, while bacterial DNA level was significantly decreased from 2 to 48 h PH.
CONCLUSION: PP plus ECPD via the portal vein can attenuate toxic load and hyperperfusion injury, and should be undertaken instead of PP via the systemic circulation in SFSS or PLF.
Core tip: Plasmapheresis (PP) and other artificial liver support (ALS) modalities have been used to treat postoperative liver failure (PLF) and “small-for-size” syndrome (SFSS). However, these modalities did not result in a significant improvement in survival. It is thought that these modalities cannot relieve portal hypertension, thus are inefficacious. This study demonstrated that ECPD plus temporal PP via the portal vein can not only dynamically turn the portal flow to the systematic circulation and attenuate portal overflow injury, but also reduces toxic load. This technique should be undertaken instead of PP or ALS via the systemic circulation in SFSS or PLF, and shows potential for clinical application.
- Citation: Hou P, Chen C, Tu YL, Zhu ZM, Tan JW. Extracorporeal continuous portal diversion plus temporal plasmapheresis for “small-for-size” syndrome. World J Gastroenterol 2013; 19(33): 5464-5472
- URL: https://www.wjgnet.com/1007-9327/full/v19/i33/5464.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i33.5464
Plasmapheresis (PP) and other plasma purification modalities have been used in the past to treat postoperative liver failure (PLF) and “small-for-size” syndrome (SFSS) following extensive liver resection and living donor liver transplantation (LDLT)[1-4]. However, none of these modalities has resulted in a significant improvement in survival. Currently, it is deemed that PP and other modalities via systemic circulation access adopted in clinical practice can decrease toxin load and improve serum biochemistry, but do not relieve portal hypertension or hyperperfusion, which results in significant harm to sinusoidal endothelial cells, liver function and intestinal barrier function and is regarded as the determining pathogenesis of PLF and SFSS following subtotal or critical hepatectomy[5-8]. This study aims to investigate the effects of temporal PP via portal vein access with the aid of extracorporeal continuous portal diversion (ECPD) in SFSS and PLF compared with temporal PP via systemic circulation access in a porcine model.
Fourteen male Bama miniature pigs (15-20 kg) were obtained from the Pig and Poultry Production Institute (Guangxi Autonomous Region, China). The pigs were raised from a closed herd and kept under strict quarantine. All experiments were conducted according to the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH). All animals in this study were treated humanely in accordance with institutional and national guidelines for the ethical treatment of animals.
All 14 animals, were anesthetized by initial sedation with a deep intramuscular injection of ketamine (15-20 mg/kg) and chlorpromazine (6-8 mg/kg) 15 min after the administration of atropine (0.01 mg/kg), then underwent 85%-90% hepatectomy (left tri-lobe and partial right posterior lobe resection) with less than 60 mL blood loss and no hepatic pedicle occlusion, according to a previously described protocol[6,9]. First, ultrasonic flow probes were connected to a flow meter (Transonic Systems INC. TS420, NY, United States) to measure hepatic artery flow (HAF) and portal vein flow (PVF). Second, two 11.5-Fr dual-lumen catheters (Hanahao, Tyco Healthcare, Tianjin, China) were introduced into the upper vena cava through the internal jugular vein and the portal vein before hepatectomy.
Extensive or total hepatectomy in the pig is usually adopted as a model of acute liver failure[10]. All animals which underwent 85%-90% hepatectomy were randomized into either the Systemic group (n = 7) or the Portal group (n = 7). In the Systemic group, all pigs received temporal PP via the extracorporeal catheter circuit (systemic to systemic circulation) from 24 to 30 h post-hepatectomy (PH); in the Portal group, all pigs received ECPD to divert partial PVF to the systemic circulation after hepatectomy, then converted to temporal PP from 24 to 30 h PH, and subsequently converted to ECPD again until 48 h PH. In the Portal group, the PVF was preserved at 3.0-3.3 times that of the baseline value, similar to that following 70% hepatectomy, which was regarded as the optimal PVF to the hypertrophic liver remnant[5]. At 48 h PH, all pigs were re-opened and the portal vein pressure (PVP), PVF, and HAF were measured, then diversion of the portal venous flow was terminated. After 1 h, the PVP, PVF, and HAF were re-measured. The portal hemodynamic changes, liver injury, liver regeneration and bacterial/lipopolysaccharide (LPS) translocation were evaluated in the two groups.
At the end of surgery, one dose of 375 mg penicillin/streptomycin was given intramuscularly to all pigs. This dose was repeated daily every morning until the pigs were euthanized. Each pig was allowed access to food and water ad libitum in the postoperative phase, and they were monitored postoperatively until euthanized at 49 h PH. The pigs’ systemic arterial pressure was monitored throughout the experiment. Food and water intake and serum glucose levels were evaluated at each postoperative assessment. The liver remnant was removed, weighed, and sampled, and the animals were euthanized.
During each extraction, 200-300 mL plasma was obtained from 6 donor pigs via the internal jugular vein catheter and immediately frozen at -20 °C.
PVF, HAF, and PVP were measured in both groups at several time points during the procedure: at laparotomy, at 1 h PH, 24 h PH, and 48 h PH. At 48 h PH, the pigs were re-opened, the ECPD was stopped, and the PVP, HAF and PVF were re-measured.
Blood samples at pre-operation, 2, 24, 30, and 48 h PH were collected and analyzed. The serum levels of alanine aminotransferase (ALT), total bilirubin (TB) and ammonia were determined in these samples. Increased serum level of hyaluronic acid (HA), which is chiefly eliminated in the hepatic sinusoidal endothelium, indicated sinusoidal endothelial damage[11,12]. HA in serum samples was measured by radiometric assay using the Pharmacia HA test (Shanghai Hua Yi Scientific, Inc., Shanghai, China) at pre-operation, 2 h PH, 24 h PH and 48 h PH. Thymidine kinase (TK) activity is the index of hepatic regeneration. Serum TK activity was measured with the Liaison TK assay (DiaSorin, Inc., Stillwater, MN, United States) at pre-operation, 2 h PH, 24 h PH and 48 h PH[13,14].
Hepatic tissue was sampled at 1 h PH. Each biopsy sample was divided into 2 specimens. The tissue specimens for electron microscopy were fixed in 2.5% glutaraldehyde and 2% paraformaldehyde in 0.1 mol/L sodium cacodylate buffer (pH 7.3). The other set of samples were preserved in 10% neutral buffered formalin, embedded in paraffin, and stained with hematoxylin and eosin. Then, 4-μm-thick sections were immunostained with porcine anti-CD31 antibody (Serotec, Oxford, United Kingdom) to evaluate the microstructural integrity of the hepatic sinusoid[15,16]. The animals were sacrificed at 49-50 h PH. The liver was excised after laparotomy, then weighed and processed. The hepatic tissue was sampled again, preserved in 10% neutral-buffered formalin and embedded in paraffin for proliferating cell nuclear antigen (PCNA) immunostaining and in situ terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) examination.
The PCNA expression was detected by immunostaining using monoclonal anti-PCN-antibody (DAKO) (Shanghai Hua Yi Scientific, Inc., Shanghai, China). The rate of increase of liver volume was evaluated by the following equation: The rate of increase = liver volume at sacrifice⁄estimated remnant liver volume at operation × 100%.
Liver samples at 48 h PH were stained for PCNA. PCNA is a stable cell-cycle nuclear protein. The rate of DNA synthesis correlates with proliferation of the cells. Data were expressed as the percentage of hepatocytes stained with PCNA. The percent of PCNA-stained hepatocytes in the total cells per 10 high-power fields was calculated and compared between the two groups.
Three-micrometer-thick sections were stained with hematoxylin and eosin and analyzed by TUNEL using an in situ apoptosis-detection kit (Jiamei Biotech Co. Ltd., Shenzhen, China) following the manufacturer’s instructions. The percent of apoptotic cells in the total cells in each high-power field was measured and compared between the Portal group and the Systemic group. Ten consecutive high-power fields were calculated at × 400 magnification for each pig.
LPS levels in serum samples were measured by the quantitative chromogenic limulus amebocyte lysate test (Yihua BioScience Ltd. Shanghai, China) according to the manufacturer’s instructions. All samples were tested in duplicate and read at 405 nm[17].
Total bacterial quantification was accomplished by DNA isolation and real-time polymerase chain reaction (PCR). DNA was isolated from blood using the Fast DNA Spin Kit (Cat. 69506; Qiagen, United States) according to the manufacturer’s instructions. Subsequently, total bacterial quantification was performed with 16S rRNA gene-targeted primers. The sequences of the universal primers were as follows: 5’TTCCGGTTGATCCTGCCGGA3’ forward and 5’GGTTACCTTGTTACGACTT-3’ reverse[18,19]. The serially diluted genomic DNA of selected bacterial isolates was used as a real-time PCR positive control for total bacterial quantification. Bacterial counts are expressed as Log10 cells per gram tissue (cells/g).
Tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1) and IL-6 levels in serum were measured using enzyme-linked immunosorbent assays (ELISAs) following the manufacturer’s instructions (Jingmei Biotech Co. Ltd., Shenzhen, China). All samples were tested in triplicate and read at 490 nm
in a thermomax microplate reader.
All variables were expressed as mean ± SD and compared with the Student’s t test using PASW Statistics 18 software (SPSS Inc., Chicago, IL, United States). P values of less than 0.05 were considered significant.
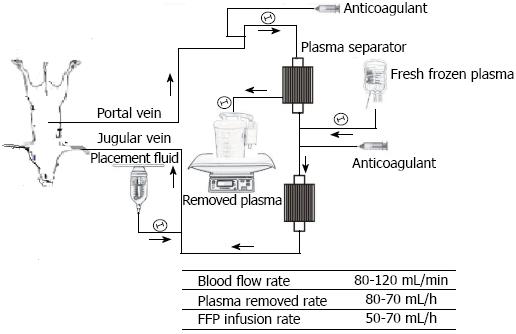
Extracorporeal continuous portal diversion (ECPD) plus temporal plasmapheresis (PP) by the extracorporeal catheter circuit was established (Figure 1). In the Portal group, portal venous blood was aspirated through the portal catheter and into tubing connected to a centrifugal pump immediately PH. The portal venous blood first passed through the centrifugal pump and then through plasma-separation cartridges with a blood flow of 90-110 mL/min to preserve the pigs’ PVF per unit volume range of 3.0-3.3 times that of the baseline. From 24 h PH, these pigs were converted to plasma exchange or PP for 6 h. After plasma exchange, the blood was returned to the pig through a double-lumen catheter inserted into the internal jugular or subclavian vein, and then continued on ECPD until 48 h PH. In the Systemic group, the extracorporeal catheter circuit was established with systemic circulation to systemic circulation at 24 h PH. The systemic circulation blood was aspirated through the systemic circulation catheter and into tubing connected to a centrifugal pump, and the same plasma exchange as the above group was performed for 6 h in all pigs from 24 to 30 h PH, and then stopped after the blood was returned to the pig.
The infusion plasma volume was equal to 1.3 times the plasma volume that had been removed per hour in each pig. The total exchanged plasma volume was 4000-5000 mL each time. The adequacy of anticoagulation was monitored by activated clotting time (ACT), and heparin was administered as required to maintain ACT levels greater than 250 s. Standard monitoring (ECG and arterial line for blood pressure, and Foley catheter for urine output) was performed for all pigs.
The characteristics of the hemodynamic studies are shown in Table 1. The evolution of hemodynamic parameters was measured at baseline, immediately PH, 48 h PH, and euthanasia (49 h PH). All flow values are reported in mL/min per 100 g hepatic tissue. The results showed that hemodynamic parameters such as PVF, HAF and P/A gradually reduced with time in the Systemic group and the Portal group. These hemodynamic parameters were significantly decreased in the Portal group compared with the Systemic group.
| Systemic group | Portal group | P value | |
| Body weight (kg) | 22.4 ± 3.4 | 23.6 ± 3.6 | 0.910 |
| Left trilobes (g) | 381.2 ± 14.9 | 390.5 ± 15.8 | 0.840 |
| ETL (g) | 476.8 ± 18.4 | 487.0 ± 19.7 | 0.860 |
| WRL (g) | 412.1 ± 15.6 | 413.1 ± 20.4 | 0.790 |
| ERL (g) | 61.7 ± 3.8 | 63.8 ± 4.1 | 0.760 |
| Proportion of ERL | 12.8% ± 2.3% | 13.1% ± 3.5% | 0.870 |
| Operation time (min) | 110 ± 23 | 126 ± 28 | 0.450 |
| Blood loss (mL) | 41.7 ± 13.8 | 49.1 ± 16.1 | 0.730 |
| PVF (mL/min per 100 g) | |||
| BAS | 61.9 ± 9.6 | 64.1 ± 10.6 | 0.945 |
| 2 h PH | 431.8 ± 36.6 | 238.8 ± 29.3 | 0.002 |
| 48 h PH | 220.3 ± 21.1 | 152.3 ± 21.6 | 0.014 |
| 49 h PH | 214.3 ± 26.1 | 227.4 ± 27.61 | 0.674 |
| HAF (mL/min per 100 g) | |||
| BAS | 19.4 ± 4.5 | 19.9 ± 4.1 | 0.921 |
| 2 h PH | 6.1 ± 2.5 | 14.9 ± 2.5 | 0.003 |
| 48 h PH | 7.9 ± 2.1 | 13.2 ± 4.2 | 0.002 |
| EUT (49 h PH) | 8.2 ± 2.4 | 11.6 ± 3.5 | 0.003 |
| P/A | |||
| BAS | 3.1 ± 0.2 | 3.2 ± 0.2 | 0.843 |
| 2 h PH | 70.8 ± 8.1 | 16.0 ± 3.1 | 0.000 |
| 48 h PH | 27.8 ± 6.6 | 11.5 ± 1.8 | 0.002 |
| EUT (49 h PH) | 26.1 ± 4.9 | 19.4 ± 4.61 | 0.001 |
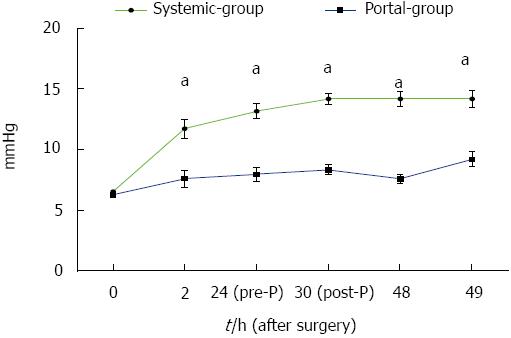
The changes in PVP in both groups are shown in Figure 2. The results showed that PVP in the Portal group was significantly lower than that in the Systemic group during the time period from 2 to 49 h PH (P < 0.05). These results suggested that PVP in the Portal group reduced much more than in the Systemic group. Thus, the Portal group had fewer complications in terms of portal hypertension compared with the Systemic group.
Levels of serum ammonia, ALT and TB collected serially during the follow-up period are shown in Table 2. The results showed that serum ALT, TB and ammonia were significantly reduced in the Portal group compared with the Systemic group from 24 to 48 h PH (P < 0.05). In addition, serum ALT and ammonia in the Portal group were significantly improved, while TB remained the same after PP from 24 to 30 h PH (P < 0.05). These results indicated that the Portal group may be better than the Systemic group in improving liver function in PLF, and PP in the Portal group might enhance this protection of liver function.
| ALT (U/L) | TB (mmol/L) | Ammonia (μmol/L) | ||||
| Systemic | Portal | Systemic | Portal | Systemic | Portal | |
| Pre | 45.2 ± 12.1 | 51.3 ± 15.5 | 17.3 ± 4.1 | 16.4 ± 5.5 | 158.4 ± 57.5 | 164.3 ± 46.2 |
| 2 h | 67.2 ± 23.4 | 61.7 ± 26.1 | 19.5 ± 6.1 | 18.6 ± 6.3 | 239.6 ± 61.8 | 193.7 ± 47.0 |
| 24 h | 129.7 ± 35.2 | 78.6 ± 24.51 | 45.9 ± 8.5 | 28.4 ± 5.71 | 345.2 ± 59.5 | 210.3 ± 67.71 |
| 30 h | 101.5 ± 23.2 | 67.2 ± 16.412 | 36.4 ± 7.4 | 25.8 ± 5.11 | 217.4 ± 51.8 | 131.7 ± 37.412 |
| 48 h | 118.6 ± 31.4 | 74 ± 291 | 58.4 ± 9.0 | 38.3 ± 7.11 | 254.3 ± 49.7 | 180.1 ± 54.51 |
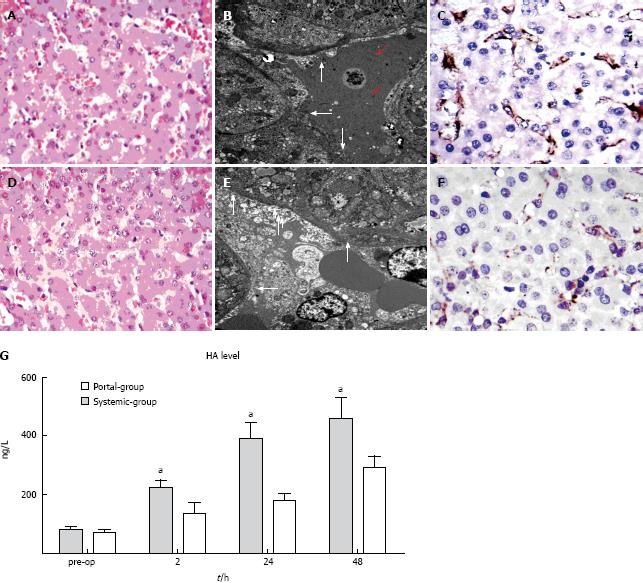
In the Systemic group, there was significant sinusoidal dilation, hydropic changes in hepatocytes and hemorrhage into the hepatic parenchyma (Figure 3A). The sinusoidal endothelial lining was partially destroyed and detached into the sinusoidal space, accompanied by enlargement of the Disse’s spaces (red arrows) (Figure 3B). CD31 immunostaining revealed significant destruction of the endothelial lining (Figure 3C); whereas in the Portal group, no intraparenchymal hemorrhage was observed (Figure 3D), the sinusoidal endothelial cells and hepatocytes were well preserved (arrow, Figure 3E), and CD31 immunostaining was mild which indicated less destruction of the endothelial lining (Figure 3F). Serial changes in the levels of HA in the two groups are shown in Figure 3G. HA was significantly decreased in the Portal group compared with the Systemic group from 2 to 48 h PH (P < 0.05). These results suggested that the Portal group may have attenuated sinusoidal endothelial injury and decreased HA level compared with the Systemic group.
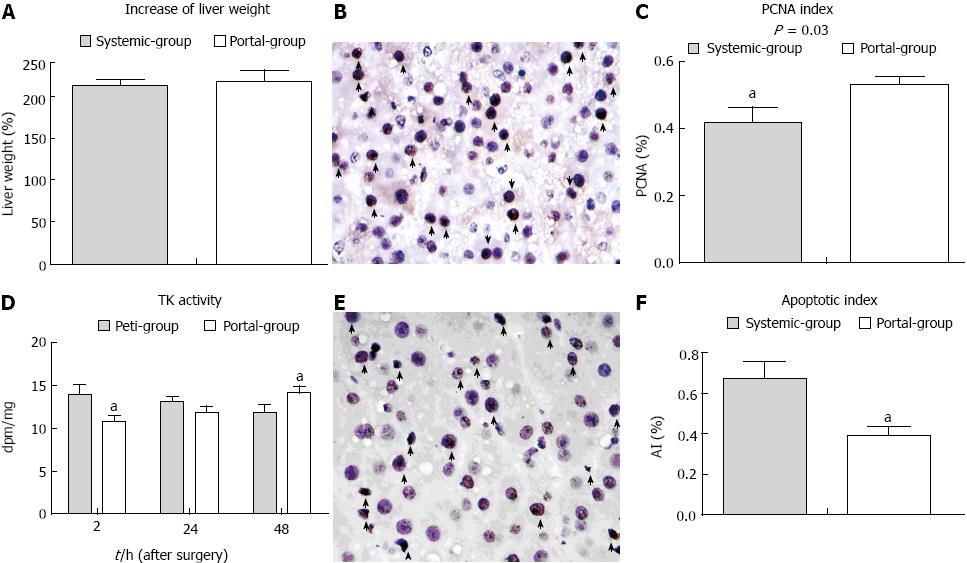
The rate of increase of liver volume in the Portal group was slightly increased compared with the Systemic group, although there was no statistical difference (P > 0.05) (Figure 4A). Thymidine kinase activity was initially lower in the Portal group than the Systemic group immediately PH, and was subsequently higher at 48 h PH (Figure 4D). The PCNA index (PI) was significantly increased and the apoptotic index (AI) was significantly decreased in the Portal group compared with the Systemic group (Figure 4C, F). These results suggested that the rate of liver remnant regeneration was elevated, while the rate of apoptosis was attenuated in the Portal group compared with the Systemic group.
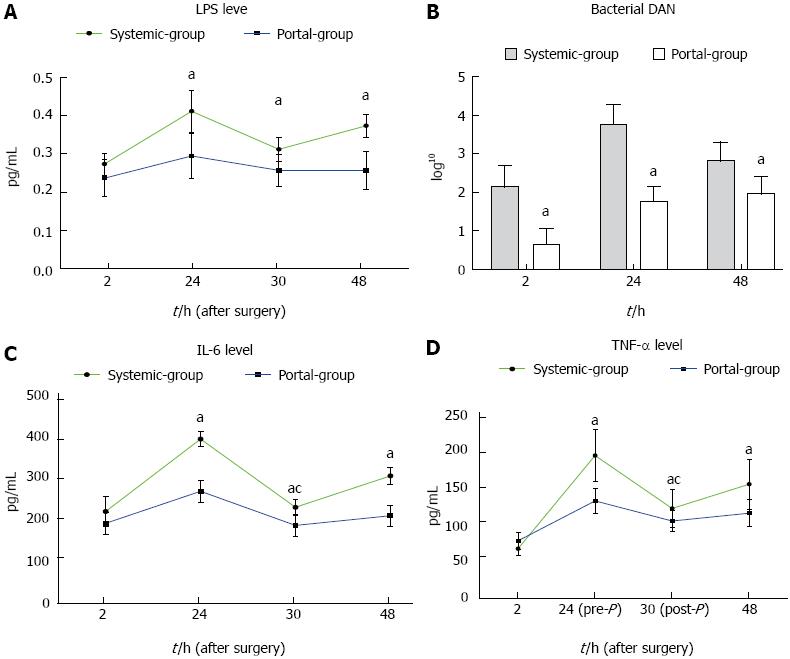
Serial changes in serum LPS levels, bacterial DNA levels, TNF-α and IL-6 levels in the Portal group compared with the Systemic group are shown in Figure 5. The results showed that LPS, TNF-α and IL-6 levels were all significantly decreased in the Portal group compared with the Systemic group from 24 to 48 h PH, while bacterial DNA level was decreased from 2 to 48 h PH (Figure 5). In addition, LPS, bacterial DNA, TNF-α and IL-6 levels were all significantly decreased after plasma exchange from 24 to 30 h PH. These results suggested that bacterial translocation and endotoxin as well as the inflammatory response were significantly attenuated in the Portal group compared with the Systemic group.
Following extensive hepatectomy when the remnant liver mass is low, it is unable to sustain synthetic, metabolic and detoxifying functions, and SFSS or PLF may ensue[5,6]. SFSS is a recognizable clinical syndrome, which is characterized by postoperative liver dysfunction with prolonged cholestasis, coagulopathy and portal hypertension. The mortality in severe SFSS or PLF after hepatectomy and LDLT is very high and ranges from 80% to 100%[5,20].
PP or plasma exchange, a type of plasma purification, is usually performed in acute liver failure. During this process, the patient’s blood is introduced into the plasma separator and the plasma is replaced. The harmful substances or protein-binding toxins are eliminated from the blood, and the blood cells and fresh frozen plasma are re-infused. PP can eliminate toxic soluble materials and small-molecule toxins, as well as coagulation factors, opsonins, and albumin among other factors[21]. In previous research[1,2,22,23], it was demonstrated that these modalities can temporarily support the metabolic and excretory functions of the liver, and help to remove potentially hepatotoxic substances and maintain the patient’s clinical stability. The present study demonstrated that PP via both the systemic circulation and the portal vein can reduce toxic load and the inflammatory response and improve liver function, blood coagulation status, and LPS translocation. However, we were unable to find literature reports on the successful treatment of PLF[24-26].
In SFSS or PLF following massive hepatectomy or LDLT, the toxic load is not “solely” pathogenic, as portal hypertension and splanchnic pooling have been reported to greatly contribute to the high postoperative morbidity and mortality of SFSS or PLF[6,24]. Severe damage to the sinusoidal endothelial cells (SECs) of the remnant liver is one of the main factors responsible for high mortality[5,16,27]. Sinusoidal overperfusion seems to be a significant factor impairing liver function following liver resection. PP via systemic circulation access, which is currently universally adopted, did not improve survival rate and relieve portal hyperperfusion[2,25,28]. In the present study, the Portal group undergoing ECPD plus PP via the portal vein not only demonstrated removal of the toxic load, but also diversion of portal flow to the systemic circulation, thus relieving portal hypertension. This method attenuated sinusoidal endothelial injury and hepatocyte injury, and significantly decreased the serum endotoxin/bacterial DNA level, IL-6, and TNF-α level compared with the Systemic group without portal decompression. These results also indicated that portal hypertension not only damages the sinusoidal endothelium, but also aggravates endotoxin absorption/bacterial translocation[7,27,29]. Therefore, ECPD plus PP via the portal vein, which relieved both toxic load and portal hyperperfusion injury, has an advantage over PP via the systemic circulation. In a recent report, it was identified that PP combined with surgical modulation of the portal vein inflow was an effective treatment for SFSS after LDLT[26].
Currently, the portacaval or mesocaval shunt is usually adopted to relieve portal hyperperfusion in both the clinic and in animal experiments. However, these techniques have many shortcomings, including surgical procedure-related complications and, long-lasting and excessive diversion of portal flow which could retard liver regeneration[16,30,31]. Fortunately, dynamic adjustment of the diverting flow between the portal and systemic circulation is characteristic of ECPD, which was able to halt the portal diversion, while the liver remnant underwent hypertrophy. This showed a potential advantage over the modalities presently adopted. In this study, the PVF per unit volume in the Portal group was preserved at more than 3 times the baseline value, and the increased rate of the liver remnant in the Portal group at 48 h PH was similar to that in the Systemic group with the presence of higher portal hyperperfusion. This indicated that PVF preserved at more than 3 times the baseline value was adequate and a good stimulus for liver regeneration. In addition, injury to the liver in the Portal group was milder and the AI was significantly lower than that in the Systemic group. To the best of our knowledge, this is the first study to investigate the feasibility and effectiveness of ECPD plus PP in relieving portal hyperperfusion in PLF or SFSS. As residual liver increases rapidly after major hepatectomy and within two days, portal hypertension will be relieved rapidly. Thus, ECPD is usually only needed for a short time. In this study, even when ECPD was stopped, the PVP only rose slightly compared with the baseline value, indicating that portal hypertension was relieved after a short time.
In general, ECPD plus temporal PP or plasma purification via the portal vein does not only dynamically turn the portal flow to the systematic circulation, attenuate portal overflow injury, and preserve the optimum portal flow for liver regeneration, but also reduces toxic load and improves biochemistry parameters. This technique should be undertaken instead of PP or ALS via the systemic circulation in SFSS or PLF.
Plasmapheresis (PP) and other plasma purification modalities have been used in the past to treat postoperative liver failure (PLF) and “small-for-size” syndrome (SFSS). However, these modalities have not resulted in a significant improvement in survival. It is thought that these modalities were unable to relieve portal hypertension, thus are inefficacious.
In SFSS or PLF after hepatectomy and living donor liver transplantation, the rationale for using PP or other plasma purification modalities is support for the patient, however, these modalities do not relieve portal hyperperfusion. The portacaval or mesocaval shunt (PCS/MCS) is usually adopted to relieve portal hyperperfusion, however, the long-lasting diversion of portal flow and the potential risk of excessive diversion of portal flow to the systemic circulation could retard liver regeneration.
In SFSS and PLF, extracorporeal continuous portal diversion (ECPD) plus PP via the portal vein not only removes the toxic load, but continuously diverts the portal flow to the systemic circulation and relieves portal hypertension, attenuates sinusoidal endothelial injury and has an advantage over PP via the systemic circulation. In addition, ECPD via the portal vein can dynamically adjust the diverting flow to the “functional competition” between the portal and systemic circulation in the case of PCS/MCS, and is available to halt the portal diversion, while the remnant liver underwent hypertrophy. This showed a potential advantage of this technique over the modalities presently adopted.
ECPD plus temporal PP via the portal vein does not only attenuate portal overflow injury, but also reduces toxic load and should be undertaken instead of PP or ALS via the systemic circulation in SFSS or PLF, and shows potential for application in the clinic.
It was supposed that PP and other artificial liver support (ALS) modalities did not relieve portal hypertension, resulting in inefficacious treatment. This study firstly demonstrated that the ECPD plus temporal PP via the portal vein can not only attenuate portal overflow injury, but also reduce toxic load, and should be undertaken instead of PP or ALS via the systemic circulation in SFSS or PLF.
P- Reviewers Dang SC, Han T S- Editor Wen LL L- Editor A E- Editor Lu YJ
| 1. | Kantola T, Koivusalo AM, Höckerstedt K, Isoniemi H. The effect of molecular adsorbent recirculating system treatment on survival, native liver recovery, and need for liver transplantation in acute liver failure patients. Transpl Int. 2008;21:857-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | van de Kerkhove MP, de Jong KP, Rijken AM, de Pont AC, van Gulik TM. MARS treatment in posthepatectomy liver failure. Liver Int. 2003;23 Suppl 3:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Kjaergard LL, Liu J, Als-Nielsen B, Gluud C. Artificial and bioartificial support systems for acute and acute-on-chronic liver failure: a systematic review. JAMA. 2003;289:217-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 240] [Article Influence: 10.9] [Reference Citation Analysis (1)] |
| 4. | Saliba F, Camus C, Durand F. Randomized controlled multicenter trial evaluating the efficacy and safety of albumin dialysis with MARSs in patients with fulminant and subfulminant hepatic failure. Hepatology. 2008;48:377A. |
| 5. | Palmes D, Budny TB, Dietl KH, Herbst H, Stratmann U, Spiegel HU. Detrimental effect of sinusoidal overperfusion after liver resection and partial liver transplantation. Transpl Int. 2005;17:862-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Wang H, Ohkohchi N, Enomoto Y, Usuda M, Miyagi S, Masuoka H, Sekiguchi S, Kawagishi N, Fujimori K, Sato A. Effect of portocaval shunt on residual extreme small liver after extended hepatectomy in porcine. World J Surg. 2006;30:2014-2022; discussion 2014-2022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Yagi S, Iida T, Hori T, Taniguchi K, Nagahama M, Isaji S, Uemoto S. Effect of portal haemodynamics on liver graft and intestinal mucosa after small-for-size liver transplantation in swine. Eur Surg Res. 2012;48:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Court FG, Laws PE, Morrison CP, Teague BD, Metcalfe MS, Wemyss-Holden SA, Dennison AR, Maddern GJ. Subtotal hepatectomy: a porcine model for the study of liver regeneration. J Surg Res. 2004;116:181-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Filipponi F, Fabbri LP, Marsili M, Falcini F, Benassai C, Nucera M, Romagnoli P. A new surgical model of acute liver failure in the pig: experimental procedure and analysis of liver injury. Eur Surg Res. 1991;23:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Itasaka H, Suehiro T, Wakiyama S, Yanaga K, Shimada M, Sugimachi K. Significance of hyaluronic acid for evaluation of hepatic endothelial cell damage after cold preservation/reperfusion. J Surg Res. 1995;59:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Eriksson S, Fraser JR, Laurent TC, Pertoft H, Smedsrød B. Endothelial cells are a site of uptake and degradation of hyaluronic acid in the liver. Exp Cell Res. 1983;144:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 214] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Kahn D, Stadler J, Terblanche J, van Hoorn-Hickman R. Thymidine kinase: an inexpensive index of liver regeneration in a large animal model. Gastroenterology. 1980;79:907-911. [PubMed] |
| 13. | McGowan JA, Fausto N. Ornithine decarboxylase activity and the onset of deoxyribonucleic acid synthesis in regenerating liver. Biochem J. 1978;170:123-127. [PubMed] |
| 14. | Couvelard A, Scoazec JY, Feldmann G. Expression of cell-cell and cell-matrix adhesion proteins by sinusoidal endothelial cells in the normal and cirrhotic human liver. Am J Pathol. 1993;143:738-752. [PubMed] |
| 15. | Fondevila C, Hessheimer AJ, Taurá P, Sánchez O, Calatayud D, de Riva N, Muñoz J, Fuster J, Rimola A, García-Valdecasas JC. Portal hyperperfusion: mechanism of injury and stimulus for regeneration in porcine small-for-size transplantation. Liver Transpl. 2010;16:364-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Iwanaga S, Morita T, Harada T, Nakamura S, Niwa M, Takada K, Kimura T, Sakakibara S. Chromogenic substrates for horseshoe crab clotting enzyme. Its application for the assay of bacterial endotoxins. Haemostasis. 1978;7:183-188. [PubMed] |
| 17. | Lane DJ. 16S/23S rRNA sequencing. Nucleic acid techniques in bacterial systematics. New York: John Wiley and Sons 1991; 115–175. |
| 18. | Such J, Francés R, Muñoz C, Zapater P, Casellas JA, Cifuentes A, Rodríguez-Valera F, Pascual S, Sola-Vera J, Carnicer F. Detection and identification of bacterial DNA in patients with cirrhosis and culture-negative, nonneutrocytic ascites. Hepatology. 2002;36:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 201] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 19. | Singer AL, Olthoff KM, Kim H, Rand E, Zamir G, Shaked A. Role of plasmapheresis in the management of acute hepatic failure in children. Ann Surg. 2001;234:418-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 86] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Rittler P, Ketscher C, Inthorn D, Jauch KW, Hartl WH. Use of the molecular adsorbent recycling system in the treatment of postoperative hepatic failure and septic multiple organ dysfunction--preliminary results. Liver Int. 2004;24:136-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Matsubara S. Combination of plasma exchange and continuous hemofiltration as temporary metabolic support for patients with acute liver failure. Artif Organs. 1994;18:363-366. [PubMed] |
| 22. | Hassanein TI, Tofteng F, Brown RS, McGuire B, Lynch P, Mehta R, Larsen FS, Gornbein J, Stange J, Blei AT. Randomized controlled study of extracorporeal albumin dialysis for hepatic encephalopathy in advanced cirrhosis. Hepatology. 2007;46:1853-1862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 259] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 23. | Wei AC, Tung-Ping Poon R, Fan ST, Wong J. Risk factors for perioperative morbidity and mortality after extended hepatectomy for hepatocellular carcinoma. Br J Surg. 2003;90:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 234] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 24. | Dimick JB, Pronovost PJ, Cowan JA, Lipsett PA. Postoperative complication rates after hepatic resection in Maryland hospitals. Arch Surg. 2003;138:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Kellersmann R, Gassel HJ, Bühler C, Thiede A, Timmermann W. Application of Molecular Adsorbent Recirculating System in patients with severe liver failure after hepatic resection or transplantation: initial single-centre experiences. Liver. 2002;22 Suppl 2:56-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Eisenhuber E, Madl C, Kramer L, Steininger R, Yeganehfar W, Ratheiser K, Gangl A. [Prognostic factors in acute liver failure]. Wien Klin Wochenschr. 1998;110:564-569. [PubMed] |
| 27. | Garcia-Tsao G, Albillos A, Barden GE, West AB. Bacterial translocation in acute and chronic portal hypertension. Hepatology. 1993;17:1081-1085. [PubMed] |
| 28. | De Silvestro G, Marson P, Brandolese R, Pittoni G, Ongaro G. A single institution’s experience (1982-1999) with plasma-exchange therapy in patients with fulminant hepatic failure. Int J Artif Organs. 2000;23:454-461. [PubMed] |
| 29. | Topuz Ö, Ilhan YS, Doğru O, Aygen E, Sözen S. Effect of melatonin and misoprostol on bacterial translocation in portal hypertensive rats. J Gastroenterol Hepatol. 2012;27:562-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Ishizaki Y, Kawasaki S, Sugo H, Yoshimoto J, Fujiwara N, Imamura H. Left lobe adult-to-adult living donor liver transplantation: Should portal inflow modulation be added. Liver Transpl. 2012;18:305-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 31. | Li J, Liang L, Ma T, Yu X, Chen W, Xu G, Liang T. Sinusoidal microcirculatory changes after small-for-size liver transplantation in rats. Transpl Int. 2010;23:924-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |