Published online Aug 21, 2013. doi: 10.3748/wjg.v19.i31.5138
Revised: June 28, 2013
Accepted: July 17, 2013
Published online: August 21, 2013
Processing time: 110 Days and 6.4 Hours
AIM: To investigate the therapeutic efficacy and mechanisms of action of oncolytic-herpes-simplex-virus encoding granulocyte-macrophage colony-stimulating factor (HSVGM-CSF) in pancreatic carcinoma.
METHODS: Tumor blocks were homogenized in a sterile grinder in saline. The homogenate was injected into the right armpit of each mouse. After vaccination, the mice were randomly assigned into four groups: a control group, a high dose HSVGM-CSF group [1 × 107 plaque forming units (pfu)/tumor], a medium dose HSVGM-CSF group (5 × 106 pfu/tumor) and a low dose HSVGM-CSF group (5 × 105 pfu/tumor). After initiation of drug administration, body weights and tumor diameters were measured every 3 d. Fifteen days later, after decapitation of the animal by cervical dislocation, each tumor was isolated, weighed and stored in 10% formaldehyde solution. The drug effectiveness was evaluated according to the weight, volume and relative volume change of each tumor. Furthermore, GM-CSF protein levels in serum were assayed by enzyme-linked immunosorbent assays at 1, 2, 3 and 4 d after injection of HSVGM-CSF.
RESULTS: Injection of the recombinant mouse HSV encoding GM-CSF resulted in a significant reduction in tumor growth compared to the control group, and dose-dependent effects were observed: the relative tumor proliferation rates of the low dose, medium dose and high dose groups on 15 d after injection were 45.5%, 55.2% and 65.5%, respectively. The inhibition rates of the tumor weights of the low, middle, and high dose groups were 41.4%, 46.7% and 50.5%, respectively. Furthermore, the production of GM-CSF was significantly increased in the mice infected with HSVGM-CSF. The increase in the GM-CSF level was more pronounced in the high dose group compared to the other two dose groups.
CONCLUSION: Our study provides the first evidence that HSVGM-CSF could inhibit the growth of pancreatic cancer. The enhanced GM-CSF expression might be responsible for the phenomenon.
Core tip: Herpes-simplex-virus encoding granulocyte-macrophage colony-stimulating factor (HSVGM-CSF) is an engineered oncolytic virus. The key features of HSVGM-CSF include the deletion of both copies of γ134.5 and the ICP47 gene as well as interruption of the ICP6 gene and insertion of the therapeutic gene GM-CSF. Our study provides the first evidence that HSVGM-CSF could inhibit the growth of pancreatic cancer in a dose-dependent manner. Enhanced GM-CSF expression might be responsible for the phenomenon.
- Citation: Liu H, Yuan SJ, Chen YT, Xie YB, Cui L, Yang WZ, Yang DX, Tian YT. Preclinical evaluation of herpes simplex virus armed with granulocyte-macrophage colony-stimulating factor in pancreatic carcinoma. World J Gastroenterol 2013; 19(31): 5138-5143
- URL: https://www.wjgnet.com/1007-9327/full/v19/i31/5138.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i31.5138
Pancreatic cancer is a rapidly fatal malignancy with one-year relative survival rates less than 30% and nearly all patients die from their disease within 7 years of surgery[1,2]. More than 80% patients are unsuitable for radical resection. Further more, it is insensitive to current chemotherapy, radiotherapy and immunotherapy.
Gene therapy of pancreatic carcinoma is considered a novel model, and has become an emerging research area in recent years. Successful drugs for gene therapy may result in prolonged survival. Oncolytic herpes simplex virus encoding granulocyte-macrophage colony-stimulating factor (HSVGM-CSF) is an attenuated, replication-competent oncolytic virus. It can activate the host’s own immune system against infected tumor cells. Some clinical trials of HSV for the treatment of various cancers have been completed, providing preliminary data about its safety and effectiveness[3-7]. However, there is little data for pancreatic cancer.
Therefore, we conducted a preclinical evaluation of effects of HSVGM-CSF on pancreatic cancer and explored the mechanisms that may be involved in any antitumor response.
The OrienGene Biotechnology Ltd. (Beijing, China) provided the mouse recombinant GM-CSF herpes simplex virus (HSVGM-CSF) (OrienX010).
Panc-2 cells: All cells used in this study represent mouse pancreatic carcinoma cell lines. Panc-2 cells were grown in Dulbecco’s modification of Eagle’s medium.
Animals: Female C-57B mice (4-6 wk, 16-18 g) were provided by the Experimental Animal Center, Peking Union Medical College. The Committee of Animal Care and Use of the university approved the experimental protocol, which met the regulatory requirements of Tumor Hospital, Chinese Academy of Medical Science for the use of experimental animals. All mice were bred in a standard environment and were provided with free access to food and water.
Injection of transplanted tumors and drug administration in mice followed standard methods used internationally. The Discussion Draft of Guidance Principles of Pharmacodynamics of Antitumor Drugs[8] and Anticancer Drug Development Guide: Preclinical Screening, Clinical Trials and Approval[9] were used for guidance. Panc-2 cells were first recovered and amplified for collection of tumor cells, of which a total of 1 × 107-1 × 108 plaque forming units (pfu) virus were subcutaneously injected into each mouse. When the resulting tumor had grown to 2-3 cm in diameter, the tumor tissue was dissected under sterile conditions and cut into blocks of 2 mm3 with sterile scissors. The tumor blocks were homogenized in a sterile grinder with normal saline. The homogenate was injected into the right armpit of each mouse. After vaccination, the mice were randomly grouped for intratumoral administration of drugs. After initiation of drug administration, body weights and tumor diameters were measured every 3 d. Fifteen days later, after decapitation of the animals by cervical dislocation, each tumor was isolated, weighed and stored in 10% formaldehyde solution (Figure 1). The drug effectiveness was evaluated according to the weight, volume and relative volume change of each tumor.
In vivo blood collected by tail vein bleed was centrifuged, and serum was collected and stored at -20 °C. Mouse GM-CSF concentration was determined by an enzyme-linked immunosorbent assay (ELISA) (Abcam Inc, MA, United States), according to manufacturer’s protocol, for cells infected with 1 × 107, 5 × 106 and 5 × 105 pfu/mL.
Three days after vaccination of tumors, the mice were randomly divided into groups with the weights of the animals being similar in each group: control group, high dose group (1 × 107 pfu/tumor), middle dose group (5 × 106 pfu/tumor) and low dose group (5 × 105 pfu/tumor). The drug was administered via intratumoral injections of 0.1 mL/tumor on the first day.
Data were expressed as mean ± SD. The inhibition rate of tumor proliferation = (tumor weight of control group - tumor weight of drug group)/tumor weight of control group × 100%. Tumor volume (V) = 1/2ab2 (a = tumor major diameter; b = tumor minor diameter). The inhibition rate of tumor volume proliferation = (tumor volume of control group - tumor volume of drug group)/tumor volume of control group × 100%. Relative tumor volume (RTV) = Vt/Vo (Vo = tumor volume pre-drug, Vt = tumor volume measured each time after drug administration). The relative tumor proliferation rates (T/C) = RTV of drug group/RTV of control group × 100%. SPSS13 was used for statistical analysis of inter-group difference using t tests and for plotting of the tumor volume, relative growth curve of volume-time, tumor weight and related tables and figures.
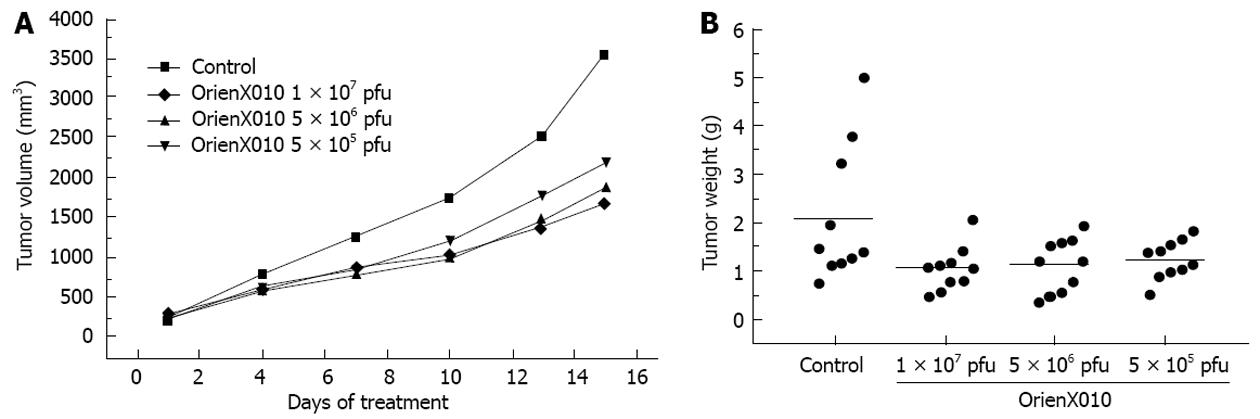
The tumor volume on day 15 post-treatment in the control group was 3555.8 ± 1849.8 mm3. The tumor volume of the group treated with a single intratumoral injection of low dose virus was 2200.3 ± 826.5 mm3 (P < 0.05 vs control). For the middle dose virus group, the tumor volume on day 15 post-treatment was 1869.8 ± 846.6 mm3 (P < 0.05 vs control). The tumor volume on day 15 post-treatment of the high dose virus group was 1668.3 ± 661.9 mm3 (P < 0.01 vs control) (Figure 2A). The inhibition rates of the tumor volumes of dose of the low, middle, and high dose groups were 38.1%, 47.4% and 54.3%, respectively. Thus, HSVGM-CSF could inhibit pancreatic cancer in a dose-dependent manner. The relative tumor proliferation rates of the low, middle, and high dose groups were 45.5%, 55.2% and 65.5%, respectively (Table 1).
| Administration | Host (mice) reaction | Tumor reaction | ||||||
| Animal number | Body weight, g (mean ± SD) | Tumor weight, g | Z | Tumor volume | J | RTV | T/C (%) | |
| beginning/end | beginning/end | (mean ± SD) | (mm3) | |||||
| 10/10 | 16.5 ± 1.2/19.8 ± 1.9 | 2.10 ± 1.41 | 3555.8 ± 1849.8 | 15.4 ± 5.7 | ||||
| Tumor injection × 4 | 10/10 | 16.4 ± 0.9/18.2 ± 2.4 | 1.04 ± 0.45 | 50.50% | 1668.3 ± 661.9 | 53.10% | 7.0 ± 2.5b | 45.5 |
| Tumor injection × 4 | 10/10 | 16.0 ± 0.6/18.6 ± 1.3 | 1.12 ± 0.55 | 46.70% | 1869.8 ± 846.6 | 47.40% | 8.5 ± 4.4b | 55.2 |
| Tumor injection × 4 | 10/10 | 16.2 ± 0.8/18.5 ± 0.8 | 1.23 ± 0.39 | 41.40% | 2200.3 ± 826.5 | 38.10% | 10.1 ± 4.2a | 65.6 |
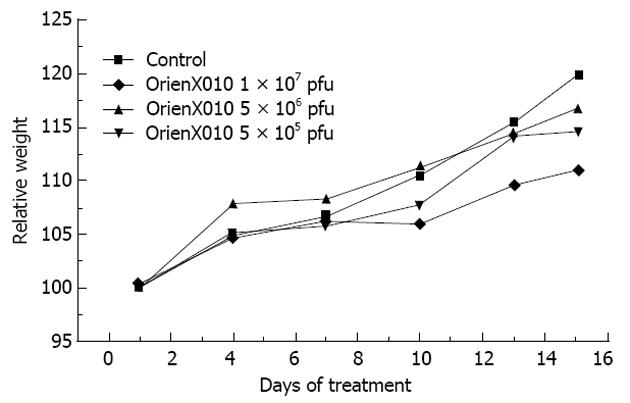
The present study showed that the tumor weight of the control group was 2.10 ± 1.41 g, 1.23 ± 0.39 g in the low dose group (P > 0.05 vs control), 1.12 ± 0.55 g in the middle dose group (P > 0.05 vs control), and 1.04 ± 0.45 g in the high dose group (P < 0.05 vs control). The inhibition rates of the tumor weights of the low, middle, and high dose groups were 41.4%, 46.7% and 50.5%, respectively (Figure 2B). Only the high dose group showed a significant difference compared with the control group (P < 0.05). There was no significant difference in mouse body weight among these four groups (P > 0.05) (Figure 3). Also, none of the mice died or showed skin ulceration/necrosis at the tumor location during the experiment.
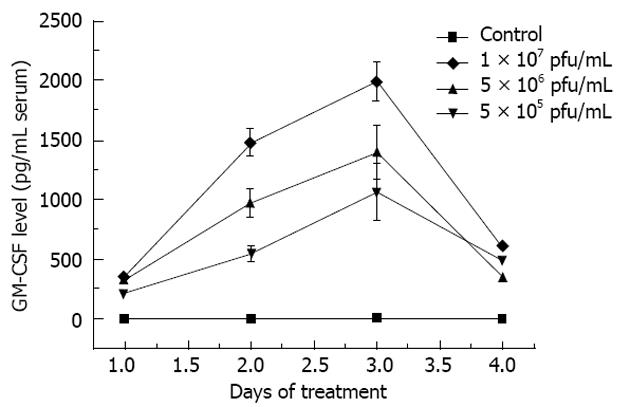
The results of serum GM-CSF protein level showed that HSVGM-CSF significantly increased GM-CSF production, peaking at day 3 after treatment (Figure 4). There may be a correlation between the dose of HSVGM-CSF and the GM-CSF protein level.
Dissection of the mice at 15th day after administration of the drug showed no adhesions around the tumors, and there were no ascites or metastasis of the tumors in the peritoneal cavity; the tumors appeared as gray in color, had uniform textures and showed no necrosis.
Compared to the traditional therapeutic methods, gene therapy is a recent and active research field. Since 1999, Germany, the United Kingdom and the United States have approved gene therapy projects for pancreatic carcinoma to enter clinical stage I/II trials, some of which are complete, providing preliminary data about its safety and effectiveness. The data suggested that the gene drugs were well tolerated in cancer patients and could suppress tumor growth[3-7]. The aim of the present study was to evaluate the efficacy of HSVGM-CSF, an attenuated, replication-competent oncolytic virus, for treating mouse pancreatic carcinoma.
HSVGM-CSF is an engineered oncolytic virus. It belongs to a conditional replication HSV-1 mutant that uses the differences in cellular structure and metabolic pathways between tumors and normal tissues and retains the genes related to virus replication. The key features of HSVGM-CSF include the deletion of both copies of γ134.5 and ICP47 genes, as well as interruption of the ICP6 gene and insertion of the therapeutic gene GM-CSF. GM-CSF is a pleiotropic cytokine secreted by many kinds of cells, including activated lymphocytes, macrophages and endothelial cells. Several previous studies demonstrated that GM-CSF was one of the most potent cytokines[5] that could influence the immune response in several ways, including recruitment and stimulation of antigen-presenting cells, such as dendritic cells, and induction of myeloid precursor cells to proliferate and differentiate into monocytes, macrophages, neutrophils and eosinophils[10]. Viral lysis and the mechanism mediated by the transgene protein, represent two parallel mechanisms of tumor destruction that can be achieved using HSVGM-CSF.
The encouraging results of the present study suggested that the proliferation speed of tumors in the mouse experimental groups was reduced after 15 d of administration of HSVGM-CSF compared with the control group (P < 0.05). The reduction of tumor growth was dose-dependent. However, there was no obvious difference in the host response between the different dosages, which may be related to the small differences in drug dosages and could be resolved with the promotion of pharmacological techniques for high-concentration drugs.
The sera of cells infected with the three doses of the virus showed high expression of GM-CSF. There was no GM-CSF secretion in the control group. The results suggested that the HSVGM-CSF enhanced GM-CSF gene expression. Additionally, the increase was more pronounced in the group injected by the high dose virus than in the middle and low dose groups. There may be a correlation between the dose of HSVGM-CSF and the GM-CSF protein level. These results indicated that HSVGM-CSF could regulate immunity in cancer-bearing mice. The increased GM-CSF levels might be responsible for the dose-dependent relationship between the drug and the tumor response.
Currently, HSV vectors alone, and HSV vectors armed with GM-CSF or other recombinant genes have been successfully tested for safety in humans and have exhibited efficacy in preclinical animal models against various human cancers. And HSV mutant has been shown to be an effective strategy for lysing tumor cells in vitro and in multiple experimental animal models[10-16]. Geevarghese et al[17] evaluated the anti-tumor effects of NV1020 (another HSV-1 mutant), which showed that the NV1020 stabilized liver metastases in patients, and extended survival by resensitizing the cancer cells to chemotherapy. Both Yang et al[18] and Malhotra et al[19] suggested that the HSV vectors armed with GM-CSF had a significantly better antitumor effect compared to treatment with HSV vectors alone in mouse colon cancer. Furthermore, Derubertis et al[20] declared that mouse colorectal cancer hepatic metastases could be suppressed by HSV vectors armed with GM-CSF. HSVGM-CSF combined with cisplatin-based chemoradiotherapy was well tolerated in patients with stage III/IV head and neck cancer. The present study showed that HSVGM-CSF enhanced the inhibition rate of mouse pancreatic cancer by regulating the expression of GM-CSF. The results were similar to those provided in previous studies[19,20].
Although the agent was highly attenuated and replication restricted, the use of a virus still raises concerns about viral proliferation and dissemination. During the experimental period, the body weights of the mice in the experimental groups were similar to the control group at the beginning, and gradually and stably increased. At the later stages, the body weights slowly increased, particularly in the high-dosage group, compared to the control group, but there was no statistical difference. In addition, there was no occurrence of treatment-related death or ulceration/necrosis of the skin, suggesting that the drug is safe and effective, with low toxicity and side effects, and is tolerated by mice. The existence of antiviral drugs, such as ganciclovir, provides us with a further margin of safety.
In the present study, the transplanted tumor cell was injected into the armpits of mice and the drug was administrated by intratumoral injection. If applied in a clinic, the drug could be administered through a fine needle puncture technique with the guidance of CT/endoscopic ultrasonography or through vascular intervention, which several research centers have proved to be effective. Mulvihill et al[21] performed a clinical trial of intratumoral injection of the ONYX-015 gene with the guidance of CT, while Löhr et al[22] reported their experimental results of clinical stage I and II trials of drug administration via vascular intervention.
Previous studies indicated that the HSV vector had a significant effect on multiple solid tumors[23-26], and could enhance the effect of other combined common therapies, such as radiotherapy and chemotherapy. Most studies that combined viral gene therapy with other therapies observed a synergistic effect in preclinical models[27-29]. Recently a stageI/II clinical trial of combined HSV with radiotherapy in head and neck tumors ended and showed no obvious side effects[7]. We are performing experimental research using an injection solution of recombined mouse HSVGM-CSF combined with radiotherapy to find a new approach in treating pancreatic carcinoma.
During the last two decades, gene therapy has made great progress. Simultaneous use of basic research and clinical experiments may become one of the fastest-developing areas in the field of medicine in the next 10 years. The development of gene therapy has proved difficult, and application in the clinic is still a long way off. The immunity, safety, transduction rate and tissue specificity of current vectors require further study and improvement, which is a common problem in gene therapy. The vectors used in the clinic in the future should have the advantage of combining non-viral vectors and alternative viral vectors that can be customized according to different requirements to express the target gene in specific tissues, and effectively modulate their expression level and duration.
Pancreatic cancer is a rapidly fatal malignancy with one-year relative survival rates less than 30%; nearly all patients die from their disease within 7 years of surgery. Gene therapy of pancreatic carcinoma is considered a novel model, and has become an emerging research area in recent years.
The gene therapy model for pancreatic carcinoma has emerged recently. Successful drugs for gene therapy may result in prolonged survival. Oncolytic herpes simplex virus encoding granulocyte-macrophage colony-stimulating factor (HSVGM-CSF) is an attenuated, replication-competent oncolytic virus. It can activate the host’s own immune system against infected tumor cells. Some clinical trials of HSV for the treatment of various cancers had been completed, providing preliminary data about its safety and effectiveness. However, there is little data for pancreatic cancer.
HSVGM-CSF is an engineered oncolytic virus. It is a conditional replication HSV-1 mutant that utilizes differences in cellular structure and metabolic pathways between tumor and normal tissues, and retains the genes related with virus replication. The key features of HSVGM-CSF include the deletion of both copies of γ134.5 and ICP47 gene as well as interruption of the ICP6 gene and insertion of the therapeutic gene GM-CSF.
The study is very interesting. Liu et al investigated the therapeutic efficacy of oncolytic HSVGM-CSF in a mouse model of pancreatic carcinoma and explored mechanisms that may be involved in the antitumor response. The authors provide evidence that HSVGM-CSF could inhibit the growth of pancreatic cancer.
P- Reviewers Liauw SL, Linnebacher M S- Editor Song XX L- Editor Stewart GJ E- Editor Li JY
| 1. | Garcea G, Dennison AR, Pattenden CJ, Neal CP, Sutton CD, Berry DP. Survival following curative resection for pancreatic ductal adenocarcinoma. A systematic review of the literature. JOP. 2008;9:99-132. [PubMed] |
| 2. | Ferrone CR, Brennan MF, Gonen M, Coit DG, Fong Y, Chung S, Tang L, Klimstra D, Allen PJ. Pancreatic adenocarcinoma: the actual 5-year survivors. J Gastrointest Surg. 2008;12:701-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 193] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 3. | Rampling R, Cruickshank G, Papanastassiou V, Nicoll J, Hadley D, Brennan D, Petty R, MacLean A, Harland J, McKie E. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000;7:859-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 426] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 4. | Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, Hunter WD, Palmer CA, Feigenbaum F, Tornatore C, Tufaro F. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7:867-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 734] [Cited by in RCA: 716] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 5. | MacKie RM, Stewart B, Brown SM. Intralesional injection of herpes simplex virus 1716 in metastatic melanoma. Lancet. 2001;357:525-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 119] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Hu JC, Coffin RS, Davis CJ, Graham NJ, Groves N, Guest PJ, Harrington KJ, James ND, Love CA, McNeish I. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 2006;12:6737-6747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 435] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 7. | Harrington KJ, Hingorani M, Tanay MA, Hickey J, Bhide SA, Clarke PM, Renouf LC, Thway K, Sibtain A, McNeish IA. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin Cancer Res. 2010;16:4005-4015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 204] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 8. | Han R, Sun Y. Cancer chemoprevention and drug therapy or the new millennium. : People’s Military Medical Press 2005; . |
| 9. | Beverly AT, Paul AA, Michel P, Michael DB, David AE, Kety H, Axel RH, Susan GH, Daniel VH, William RW. Anticancer Drug Development Guide: Preclinical Screening, Clinical Trials, and Approval. : Humana Press 2004; 153-183. |
| 10. | Bennett JJ, Kooby DA, Delman K, McAuliffe P, Halterman MW, Federoff H, Fong Y. Antitumor efficacy of regional oncolytic viral therapy for peritoneally disseminated cancer. J Mol Med (Berl). 2000;78:166-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Carroll NM, Chiocca EA, Takahashi K, Tanabe KK. Enhancement of gene therapy specificity for diffuse colon carcinoma liver metastases with recombinant herpes simplex virus. Ann Surg. 1996;224:323-329; discussion 329-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Andreansky SS, He B, Gillespie GY, Soroceanu L, Markert J, Chou J, Roizman B, Whitley RJ. The application of genetically engineered herpes simplex viruses to the treatment of experimental brain tumors. Proc Natl Acad Sci USA. 1996;93:11313-11318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 117] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Yoon SS, Nakamura H, Carroll NM, Bode BP, Chiocca EA, Tanabe KK. An oncolytic herpes simplex virus type 1 selectively destroys diffuse liver metastases from colon carcinoma. FASEB J. 2000;14:301-311. [PubMed] |
| 14. | Advani SJ, Chung SM, Yan SY, Gillespie GY, Markert JM, Whitley RJ, Roizman B, Weichselbaum RR. Replication-competent, nonneuroinvasive genetically engineered herpes virus is highly effective in the treatment of therapy-resistant experimental human tumors. Cancer Res. 1999;59:2055-2058. [PubMed] |
| 15. | Kooby DA, Carew JF, Halterman MW, Mack JE, Bertino JR, Blumgart LH, Federoff HJ, Fong Y. Oncolytic viral therapy for human colorectal cancer and liver metastases using a multi-mutated herpes simplex virus type-1 (G207). FASEB J. 1999;13:1325-1334. [PubMed] |
| 16. | Cozzi PJ, Malhotra S, McAuliffe P, Kooby DA, Federoff HJ, Huryk B, Johnson P, Scardino PT, Heston WD, Fong Y. Intravesical oncolytic viral therapy using attenuated, replication-competent herpes simplex viruses G207 and Nv1020 is effective in the treatment of bladder cancer in an orthotopic syngeneic model. FASEB J. 2001;15:1306-1308. [PubMed] |
| 17. | Geevarghese SK, Geller DA, de Haan HA, Hörer M, Knoll AE, Mescheder A, Nemunaitis J, Reid TR, Sze DY, Tanabe KK. Phase I/II study of oncolytic herpes simplex virus NV1020 in patients with extensively pretreated refractory colorectal cancer metastatic to the liver. Hum Gene Ther. 2010;21:1119-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 18. | Yang SH, Oh TK, Kim ST. Increased anti-tumor effect by a combination of HSV thymidine kinase suicide gene therapy and interferon-gamma/GM-CSF cytokine gene therapy in CT26 tumor model. J Korean Med Sci. 2005;20:932-937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Malhotra S, Kim T, Zager J, Bennett J, Ebright M, D’Angelica M, Fong Y. Use of an oncolytic virus secreting GM-CSF as combined oncolytic and immunotherapy for treatment of colorectal and hepatic adenocarcinomas. Surgery. 2007;141:520-529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Derubertis BG, Stiles BM, Bhargava A, Gusani NJ, Hezel M, D’Angelica M, Fong Y. Cytokine-secreting herpes viral mutants effectively treat tumor in a murine metastatic colorectal liver model by oncolytic and T-cell-dependent mechanisms. Cancer Gene Ther. 2007;14:590-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Mulvihill S, Warren R, Venook A, Adler A, Randlev B, Heise C, Kirn D. Safety and feasibility of injection with an E1B-55 kDa gene-deleted, replication-selective adenovirus (ONYX-015) into primary carcinomas of the pancreas: a phase I trial. Gene Ther. 2001;8:308-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 199] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 22. | Löhr M, Hoffmeyer A, Kröger J, Freund M, Hain J, Holle A, Karle P, Knöfel WT, Liebe S, Müller P. Microencapsulated cell-mediated treatment of inoperable pancreatic carcinoma. Lancet. 2001;357:1591-1592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 138] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Fujiwara S, Nawa A, Luo C, Kamakura M, Goshima F, Kondo C, Kiyono T, Kikkawa F, Nishiyama Y. Carrier cell-based delivery of replication-competent HSV-1 mutants enhances antitumor effect for ovarian cancer. Cancer Gene Ther. 2011;18:77-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Watanabe D, Goshima F, Mori I, Tamada Y, Matsumoto Y, Nishiyama Y. Oncolytic virotherapy for malignant melanoma with herpes simplex virus type 1 mutant HF10. J Dermatol Sci. 2008;50:185-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 25. | Kimata H, Imai T, Kikumori T, Teshigahara O, Nagasaka T, Goshima F, Nishiyama Y, Nakao A. Pilot study of oncolytic viral therapy using mutant herpes simplex virus (HF10) against recurrent metastatic breast cancer. Ann Surg Oncol. 2006;13:1078-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Nakao A, Kasuya H, Sahin TT, Nomura N, Kanzaki A, Misawa M, Shirota T, Yamada S, Fujii T, Sugimoto H. A phase I dose-escalation clinical trial of intraoperative direct intratumoral injection of HF10 oncolytic virus in non-resectable patients with advanced pancreatic cancer. Cancer Gene Ther. 2011;18:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 27. | Post DE, Fulci G, Chiocca EA, Van Meir EG. Replicative oncolytic herpes simplex viruses in combination cancer therapies. Curr Gene Ther. 2004;4:41-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Blank SV, Rubin SC, Coukos G, Amin KM, Albelda SM, Molnar-Kimber KL. Replication-selective herpes simplex virus type 1 mutant therapy of cervical cancer is enhanced by low-dose radiation. Hum Gene Ther. 2002;13:627-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Passer BJ, Castelo-Branco P, Buhrman JS, Varghese S, Rabkin SD, Martuza RL. Oncolytic herpes simplex virus vectors and taxanes synergize to promote killing of prostate cancer cells. Cancer Gene Ther. 2009;16:551-560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |