Published online Jul 21, 2013. doi: 10.3748/wjg.v19.i27.4445
Revised: June 4, 2013
Accepted: June 8, 2013
Published online: July 21, 2013
Processing time: 148 Days and 23.5 Hours
The therapeutic potential of long-term ketotifen in irritable bowel syndrome and postoperative ileus is currently under investigation. Ambiguous results of prolonged postoperative ketotifen use on gastrointestinal passage have been found. The current data point at a hampered gastrointestinal transit after prolonged postoperative ketotifen use in a rodent ileus induction model. Therefore, caution should be taken when administering ketotifen in the perioperative phase.
Core tip: Prolonged postoperative ketotifen impairs gastrointestinal transit in a rodent ileus induction model.
- Citation: Reisinger KW, de Haan JJ, Schreinemacher MH. Word of caution before implementing ketotifen for gastrointestinal transit improvement. World J Gastroenterol 2013; 19(27): 4445-4446
- URL: https://www.wjgnet.com/1007-9327/full/v19/i27/4445.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i27.4445
Postoperative ileus has a major impact on length of hospital stay after bowel resection[1]. Currently, the therapeutic potential of long-term ketotifen in postoperative ileus and irritable bowel syndrome is under investigation[2,3]. The underlying mechanism, however, remains unclear as ketotifen exerts both mast cell stabilizing and H1 receptor blocking effects. Furthermore, the long-term effects on gastrointestinal (GI) transit deserve definition. The and colleagues showed that ketotifen improved gastric emptying at 24 h follow-up; however continuing treatment up to 48 h postoperatively produced ambiguous results on GI passage[2]. Therefore, we performed a rat study to assess the influence of prolonged use of ketotifen on GI transit time at 5 d follow-up after ileus induction.
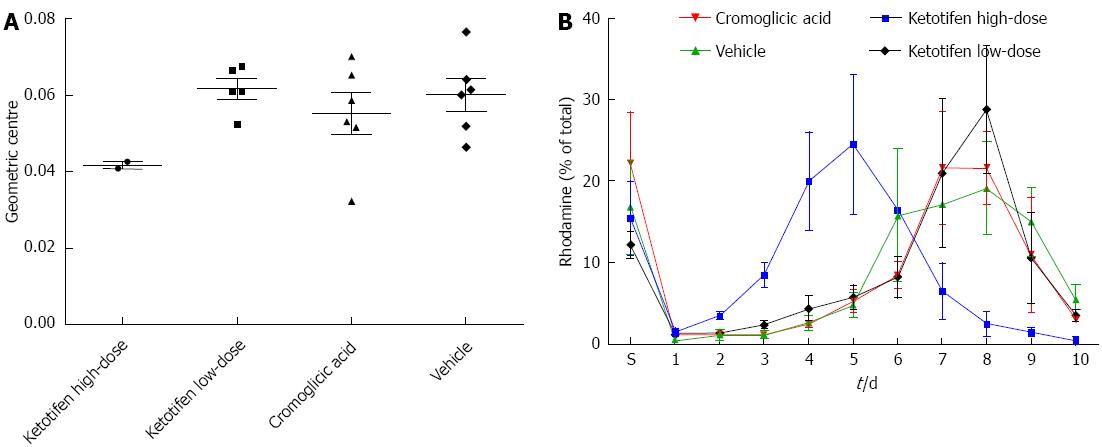
The same postoperative ileus induction model was used as previously described[4]. Male Wistar rats (250-300 g) were anesthetized using buprenorphine 0.1 mg/kg sc and anesthesia was maintained using 2.5% isoflurane. Subsequently, rats underwent a laparotomy via a midline abdominal incision under aseptic conditions. The small intestine was placed on moist gauze pads outside the abdomen, manipulated with moist cotton swab sticks for 5 min and kept moist at all times. After manipulation, the small intestine was placed back in the abdomen and the abdomen closed in 2 layers with continuous sutures. Rats (6 in each group) received either ketotifen in a high-dose (1 mg/kg) or low-dose (0.1 mg/kg), mast cell stabilizer cromoglicic acid (50 mg/kg) or vehicle (saline). The high dose of ketotifen is comparable to doses prescribed for humans. Cromoglicic acid prevents the release of mediators from mast cells through a non-H1/2-receptor pathway. Doses were administered twice daily in a volume of 1.5 mL via oral gavage starting at 2 d preoperatively until sacrifice. GI transit time was measured at the time of sacrifice (5 d postoperatively, by cervical dislocation after anesthesia with 4% isoflurane) by evaluating the GI distribution of rhodamine-B-labeled dextran (Sigma-Aldrich, St. Louis, MO, United States). Rhodamine [200 μL of 6.25 mg/mL in phosphate buffered saline (PBS)] was administered via oral gavage. One hour after administration the animals were sacrificed, the small bowel divided in 10 equal parts (part 1: beginning at jejunum, part 10: ending at the transition of ileum to coecum) and resected together with the stomach. A fluorescence reader was used to quantify the rhodamine-containing gut content in the supernatant after vigorous mixing and centrifuging of the gastric and bowel contents in 2 mL PBS. A histogram of fluorescence distribution per segment (% of total recovered rhodamine) was plotted for transit analysis and expressed as geometric center for statistical analysis. Geometric centers were calculated for each animal as (Σ% fluorescence per segment × segment number)/100.
In the high-dose ketotifen group, 4 out of 6 rats died before reaching 5 d follow-up with an extremely distended stomach at necropsy. The geometric centers of the surviving animals were also markedly lower than the other groups, but numbers (n = 2) were too low to allow for statistical analysis (Figure 1). However, GI transit times in the low-dose group were comparable with the control group (P = 0.66, Mann-Whitney U test) implying that the beneficial ketotifen effects after postoperative ileus are dose-dependent and probably restricted to the very early postoperative period, i.e., less than 5 d. GI transit in the cromoglicic acid group was comparable to control (P = 0.70, Mann-Whitney U test). These results suggest that the effects of ketotifen on GI transit may indeed not, or not fully, depend on mast cell stabilization but rather a H1 receptor pathway.
As stated earlier by The et al[2], caution should be taken when administering ketotifen in the perioperative phase as prolonged postoperative treatment may have an inhibitory effect on enteric smooth muscle contraction. Indeed, the current data point at a hampered GI transit after prolonged postoperative ketotifen use. A careful treatment regimen as proposed by de Jonge et al[5], i.e., preoperative treatment only, is therefore mandatory.
P- Reviewer Brogna A S- Editor Wen LL L- Editor A E- Editor Ma S
| 1. | Augestad KM, Delaney CP. Postoperative ileus: impact of pharmacological treatment, laparoscopic surgery and enhanced recovery pathways. World J Gastroenterol. 2010;16:2067-2074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 87] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (2)] |
| 2. | The FO, Buist MR, Lei A, Bennink RJ, Hofland J, van den Wijngaard RM, de Jonge WJ, Boeckxstaens GE. The role of mast cell stabilization in treatment of postoperative ileus: a pilot study. Am J Gastroenterol. 2009;104:2257-2266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Klooker TK, Braak B, Koopman KE, Welting O, Wouters MM, van der Heide S, Schemann M, Bischoff SC, van den Wijngaard RM, Boeckxstaens GE. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut. 2010;59:1213-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 304] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 4. | Lubbers T, Luyer MD, de Haan JJ, Hadfoune M, Buurman WA, Greve JW. Lipid-rich enteral nutrition reduces postoperative ileus in rats via activation of cholecystokinin-receptors. Ann Surg. 2009;249:481-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | de Jonge WJ, The FO, van der Coelen D, Bennink RJ, Reitsma PH, van Deventer SJ, van den Wijngaard RM, Boeckxstaens GE. Mast cell degranulation during abdominal surgery initiates postoperative ileus in mice. Gastroenterology. 2004;127:535-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |