INTRODUCTION
The fox-tapeworm Echinococcus multilocularis (E. multilocularis) is the causative agent of alveolar echinococcosis (AE), a potentially deadly parasitic disease. AE is prevalent in the northern hemisphere and central Europe is an endemic focus[1-3]. In the era prior to anthelmintic treatment the cumulative lethality for AE was about 90% ten years after a diagnosis has been established[4]. Imaging techniques of hepatic involvement by AE commonly reveal an ill-defined lesion of the liver parenchyma and contrast computer tomography (CT) and magnetic resonance imaging (MRI) are considered to clearly demonstrate infiltrative structure and extension of the parasitic tumour to adjacent structures[5-8]. In macroscopic sections of the human liver the larval parasite usually exhibits an alveolar (spongy) structure composed of numerous irregular vesicles with diameters between less than 1 and up to 20 mm[9]. Biologically, the lesions behave like a slow-growing liver cancer, without sharp boundaries between the parasitic tissue and the liver parenchyma.
Publications on surgical procedures and results are rare but essential, and prospective studies are not available because the incidence of the disease is low. According to current treatment guidelines, surgery should be the first choice if the parasitic mass is resectable in toto[10]. Complete resections of the parasitic lesion can cure the patient while available drugs are only parasitostatic[10-12].
Emerging clinical data indicate that the parasite’s geographic range has widened in recent years[13,14]. Growing fox populations in Europe, especially in urban zones, have drawn attention to a potentially increased infection risk for humans with a phase lag of 10-20 years[15-17]. In addition, since AE is not adequately considered as a differential diagnosis, the disease remains thus underdiagnosed in Europe[18].
We report on a 76-year old patient with AE of the liver, who underwent a curative resection. Prior to resection the patient had an eventful course due to the development of a postinterventional pseudoaneurysm (aneurysm spurium) of the hepatic artery following a biopsy of the liver lesion. Interestingly, during surgery the parasitic mass appeared typical for cystic echinococcosis (CE) and revealed no infiltrations or extensive adhesions to adjacent structures as contrastingly expected from the preoperative imaging results of the abdomen. Nevertheless, the definite histology as well as referral evaluations [serology and polymerase chain reaction (PCR)] confirmed the diagnosis of AE. The patient has not been abroad for the last 20 years and at his farm he has been in constant contact to various animal species, including dogs and wild foxes. To our knowledge this is the first case of autochthonous AE infection in the federal state of Saxony, Germany.
CASE REPORT
In April 2010 a 76-year old farmer from Saxony was presented in the emergency department of a general district hospital with a severe abdominal pain under the suspicion of a biliary colic and pancreatitis, respectively. In the performed imaging of the abdomen a large cystic tumour in the left sided liver was detected. In the further course, suspecting a highly malignant atypical primary liver cancer, a biopsy for the presumed confirmation of the diagnosis was performed. After the intervention the patient was discharged in good clinical condition. Surprisingly, the histological findings from the tumour biopsy were consistent with the larval stage of E. multilocularis. Subsequent serological investigation by a referral laboratory for echinococcosis confirmed specific antibodies for AE in the patient’s serum. Moreover, a pan-cestode 12S rRNA gene-PCR from the paraffin block was positive and sequencing of the amplicon revealed 100% identity with E. multilocularis. Therefore, an anti-infective drug treatment with albendazole was initiated.
Several days after liver biopsy the patient presented again with severe abdominal pain and jaundice. In the emergency CT scan of the abdomen the gross tumour revealed no progression. Though, a postjunctional pseudoaneurysm of a branch of the left hepatic artery in segment 4a in direct proximity to the tumour tissue was newly diagnosed. A subsequent coil-embolization of the aneurysm was successfully performed.
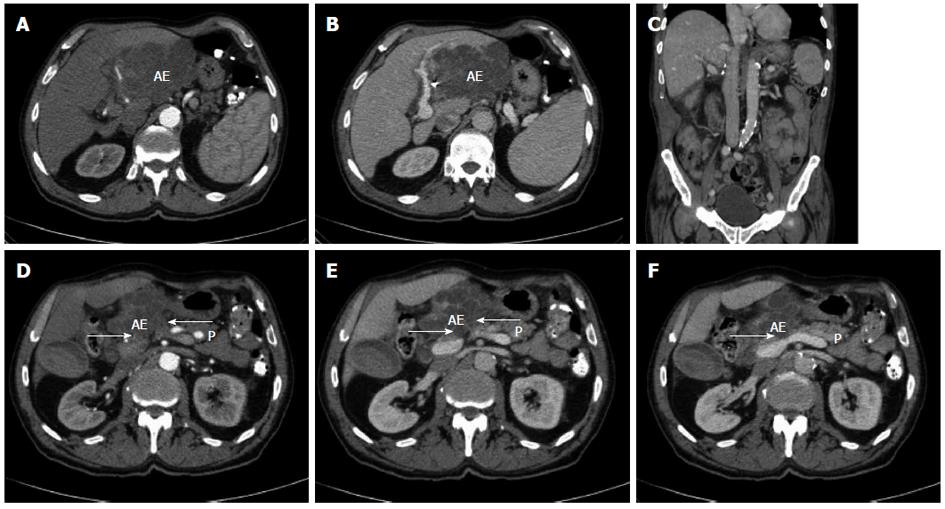
One month later, in May 2010, the patient was referred to our centre as a potential candidate for abdominal surgery. A thorough examination of patient data and history files revealed that the farmer has not been abroad in the last few decades. In his farm he has been in a constant contact and exposure to numerous domestic and wild animals, including dogs and foxes. The patient had neither B-symptoms nor further major ailments and was in good general condition. The blood tests revealed normal findings for alpha-1-fetoprotein, carbohydrate-antigen 19-9 and carcinoembryonic antigen. Preoperatively, due to pronounced cholestasis and hyperbilirubinaemia, an endoscopic retrograde cholangiography (ERC) with stenting of the main bile duct was performed. Clinical imaging prior to surgery revealed that the echinococcal lesion infiltrated liver segments 2 and 3 and showed a maximum diameter of 13.5 cm (Figure 1A and B). In addition, CT suggested the presence of adhesions in the area of the diaphragm and cystic infiltration of the pancreatic head and corpus (Figure 1 D-F). The diagnostic evaluation revealed no further extrahepatic manifestations. Hence, additionally to extended left hemihepatectomy, we considered a concomitant resection of the pencreatic head and corpus.
Figure 1 Radiological findings prior and after curative resection.
A, B: Computed tomography of the abdomen displaying an extended tumour manifestation prior to resection; C: Computed tomography of the abdomen following extended left hemihepatectomy; D-F: Computed tomography of the abdomen prior to resection. Arrows: possible extensive adhesions to adjacent pancreatic head and corpus. AE: Alveolar echinococcus tumour; P: Pancreas.
During surgery the tumorous manifestation in the left lobe of the liver was confirmed but, surprisingly, no infiltrative growth to neighboring structures was detectable. Basically, we observed a gross tumor with multiple large cystic structures that varied in size, i.e., a growth pattern that is rather typical for CE. Further exploration revealed no cystic adhesions to the diaphragm. After exploration of the bursa omentalis, no infiltration of the pancreas was notable either. No evidence for further extrahepatic tumorous dissemination or lymph node metastases was found. As the restriction of the tumour to the left liver lobe was confirmed, we affirmed the indication for extended left hemihepatectomy with curative intent. The situs was then suffused with cloths imbued in hypertonic (10%) Sodium chloride (NaCl) solution. Subsequently the isolation of the proper hepatic artery and the selective division of the left hepatic artery followed. After isolation of the main trunk of the portal vein and the left portal vein branch, the latter was divided. Then cholecystectomy was performed. After the left hepatic vein was divided, lobus caudatus (segment 1) was mobilized. Then, a liver resection was completed without any intraoperative complications. Parenchymal transection was performed using an ultrasonic dissection device (cavitron ultrasonic surgical aspirator, CUSA®). The caudate lobe did not appear to be infiltrated but to ensure an additional safety distance it was resected as well. The parenchymal resection was performed along the level of the middle hepatic vein in direction to the gall bladder bed and then caudally to the hepatic hilum. At the hilum the liver dissection diverged to the left and then ended in the parenchymal bridge leading to the caudate lobe. In the region of the hilum the left hepatic duct was isolated and then selectively divided. After removing the left liver lobe and the caudate lobe, the situs was rinsed with hypertonic NaCl solution. A T-Drain was inserted in the main bile duct for decompression and easy access cholangiography. During the postoperative stay in the intensive care unit the patient did not develop any significant complications.
Based on the finding of multiple large cystic formations of the tumorous lesion an additional histological evaluation was performed in a referral centre for pathology. There, the original diagnosis of hepatic AE was confirmed (Figure 2). In the postoperative course no signs of insufficient liver function were notable. The conclusive pathological result showed the parasitic tumour was entirely resected (Figures 1C and 3).
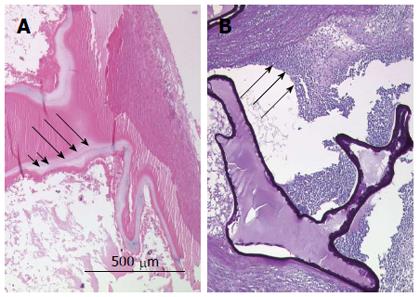
Figure 2 Referral evaluations for diagnosis of alveolar echinococcosis.
A: The hematoxylin and eosin stain of paraffin sections displays the laminated layer as a narrow band (long arrows). The germinal layer is marked by short arrows; B: Periodic acid-Schiff (PAS) stain shows a strongly PAS-positive basophilic laminated layer displaying a bizarre narrow structural pattern. The long arrows indicate the typical severe inflammatory process associated with the characteristic tubular growth pattern of the parasite.
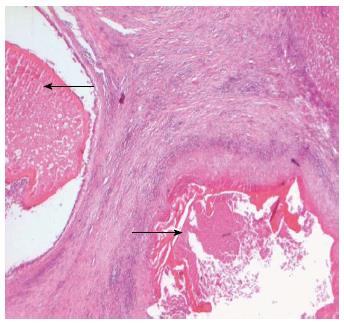
Figure 3 Histological findings after curative resection.
Hematoxylin and eosin stain of paraffin sections displaying two daughter cysts containing no vital protoscoleces embedded in a larger lesion. Black arrows indicate avital protoscoleces.
After a total postoperative hospitalization of 30 d the patient was dismissed. The anti-infective drug treatment with albendazole was maintained as long-term therapy. The follow up visit 6 and 12 mo after resection revealed normal liver function and no evidence for recurrent disease.
DISCUSSION
AE, caused by the larval (metacestode) stage of E. multilocularis, is found in the northern hemisphere and is a potentially fatal disease. The parasite is transmitted to humans by eggs of the helminth shed into the environment by feces of foxes. Almost exclusively, the liver is affected[19]. Recent studies have shown that the endemic area of E. multilocularis is larger than previously known and has regionally expanded from rural to urban areas[20-22]. In addition, increasing fox populations are associated with higher infection risk in humans with a phase lag up to 20 years[15].
The macroscopic appearance of an echinococcal lesion is distinct in regard of its species and developmental stage. While parasitic masses of CE ordinarily display a typical structure of a single or multiple fluid-filled large unilocular cysts that can reach monstrous dimensions, AE preferentially exhibits metastasis and an infiltrative growth to adjacent host tissues with a spongy structure composed of numerous irregular small vesicles of several millimeters. Thus, the surgical therapy for hepatic AE conforms the operative principles established for malignant liver tumours, i.e., in toto removal of the tumour with additional safety distance and tumour free resection margins[4].
Chemotherapy with benzimidazoles is the backbone of the comprehensive treatment of AE and long-term anti-infective drug treatment has been established in many centres in Europe as well as in China[9]. In spite of remarkable improvement of long term patient survival after the introduction of anti-helmintic drug treatment, this therapeutic modality proved to be mainly parasitostatic. Therefore, surgical resection represents the therapy of choice for patients with operable lesions of AE.
In the present report we have described the first case of autochthonous infection with E. multilocularis in our federal state of Saxony, Germany. The tumour masses affected liver segments 2 and 3 and the patient received a curative extended left hemihepatectomy. Due to a suspected liver malignancy an interventional biopsy of the lesion was preoperatively performed. Usually, such a diagnostic step is considered a contraindication in cases of AE because of the risk of abdominal seeding and anaphylaxis. Fortunately, the postinterventional iatrogenic pseudoaneurysm of the left hepatic artery could be treated with success. Interestingly, the parasitic mass showed a macroscopic pattern that appeared typical for CE which is caused by the larval stage of the dog tapeworm, Echinococcus granulosus (E. granulosus). The cause of this phenomenon remains for the most part unknown but has been reported occasionally in historic reports. Dual infection with E. granulosus and E. multilocularis could have also been possible. Indeed, concomitant infections with both echinococcal species have been reported in the literature but in the present case the definite histology, PCR results, serology, and immunohistology for specific structural proteins were all clearly positive for E. multilocularis only[23]. Additionally, a major distinguishing factor between E. granulosus cysts and E. multilocularis is the presence of an adventitial layer around the E. granulosus metacestode. The histological evaluations did not detect such a structure in the present case. Furthermore, despite the expected extensive adhesions to the diaphragm and pancreas seen by preoperative imaging, no such condition or infiltration to neighboring structures could be confirmed, showing current limitations of modern imaging techniques. All together this data indicate that in the current era diagnosis as well as assessment of extent of local disease still remain a challenge for clinicians.
Recent investigations suggest that AE remains underreported and human infection can also occur in regions with low overall parasite prevalence. Case reports from regions remote from the areas of high prevalence may be strong hints of new areas at risk[18,24]. In addition to the high prevalence rates for AE in the southern geographic regions of Germany, recent data suggest growing numbers of AE cases in neighboring European countries, such as the Czech Republic. Some of these cases indicate an autochthonous character of the infection[25,26]. Thus, a possible enlargement of fox populations in the last decades as well as migration of infected animals might have been a possible source for infection in the current case.
In conclusion, we have here described the first autochthonous infection with E. multilocularis in Saxony, Germany, providing the first evidence for a new geographical area at risk for the acquisition of AE. Albeit, curatively treated with an extended left hemihepatectomy the disease presented with uncommon findings and had an eventful course, constituting a challenge for clinicians.
P- Reviewer Hemphill A S- Editor Zhai HH L- Editor A E- Editor Zhang DN