Published online Jul 14, 2013. doi: 10.3748/wjg.v19.i26.4155
Revised: May 6, 2013
Accepted: June 4, 2013
Published online: July 14, 2013
AIM: To explore the role of CDX2 in the multi-drug resistance (MDR) process of gastric cancer in vitro and in vivo.
METHODS: A cisplatin-resistant gastric cancer cell line with stable downregulation of CDX2 was established. mRNA and protein expression levels of CDX2, survivin, cyclin D1, and c-Myc were detected by western blotting and semi-quantitative reverse-transcriptase polymerase chain reaction (RT-PCR). The influence of downregulation of CDX2 on MDR was assessed by measuring IC50 of SGC7901/DDP cells to cisplatin, doxorubicin, and 5-fluorouracil, rate of doxorubicin efflux, apoptosis, and cell cycle progression detected by flow cytometry. In addition, we determined the in vivo effects of CDX2 small interfering RNA (siRNA) on tumor size, and apoptotic cells in tumor tissues were detected by deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling and hematoxylin and eosin staining.
RESULTS: CDX2 siRNA led to downregulation of endogenous CDX2 mRNA (0.31 ± 0.05 vs 1.10 ± 0.51, 0.31 ± 0.05 vs 1.05 ± 0.21, P = 0.003) and protein (0.12 ± 0.08 vs 0.51 ± 0.07, 0.12 ± 0.08 vs 0.55 ± 0.16, P = 2.57 × 10-4) expression. It significantly promoted the sensitivity of SGC7901/DDP cells to cisplatin (0.12 ± 0.05 vs 0.33 ± 0.08, 0.12 ± 0.05 vs 0.39 ± 0.15, P = 0.001), doxorubicin (0.52 ± 0.13 vs 4.11 ± 1.25, 0.52 ± 0.13 vs 4.05 ± 1.44, P = 2.81 × 10-4), and 5-fluorouracil (0.82 ± 0.13 vs 2.81 ± 0.51, 0.82 ± 0.13 vs 3.28 ± 1.03, P = 1.71 × 10-4). Flow cytometry confirmed that the percentage of apoptotic cells increased after CDX2 downregulation (32.15% ± 2.15% vs 17.63% ± 3.16%, 32.15% ± 2.15% vs 19.3% ± 2.25%, P = 1.73 × 10-6). This notion was further supported by the observation that downregulation of CDX2 blocked entry into the S-phase of the cell cycle (31.53% ± 3.78% vs 65.05% ± 7.25%, 31.53% ± 3.78% vs 62.27% ± 5.02%, P = 7.55 × 10-7). Furthermore, downregulation of CDX2 significantly increased intracellular accumulation of doxorubicin (0.21 ± 0.06 vs 0.41 ± 0.11, 0.21 ± 0.06 vs 0.40 ± 0.08, P = 0.003). In molecular studies, semiquantitative RT-PCR and western blotting revealed that CDX2 downregulation could inhibit expression of c-Myc, survivin and cyclin D1.
CONCLUSION: CDX2 may be involved in regulating multiple signaling pathways in reversing MDR, suggesting that CDX2 may represent a novel target for gastric cancer therapy.
Core tip: Modulator of multidrug resistance (MDR) gene is a direct transcriptional target of CDX2. However, we still speculate whether CDX2 affects MDR through other ways. Our results showed that downregulation of CDX2 significantly promoted sensitivity of SGC7901/DDP cells to anticancer drugs, and increased the percentage of apoptotic cells. Downregulation of CDX2 potentiated G1 phase arrest of the cell cycle. Furthermore, it significantly increased intracellular accumulation of doxorubicin. We conclude that downregulation of CDX2 can efficiently reverse MDR via inhibition of apoptosis/cell-cycle-related gene expression (c-Myc, survivin and cyclin D1).
- Citation: Yan LH, Wang XT, Yang J, Lian C, Kong FB, Wei WY, Luo W, Xiao Q, Xie YB. Reversal of multidrug resistance in gastric cancer cells by CDX2 downregulation. World J Gastroenterol 2013; 19(26): 4155-4165
- URL: https://www.wjgnet.com/1007-9327/full/v19/i26/4155.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i26.4155
The transcription factor, CDX2, is a member of the caudal-related homeobox gene family. It is expressed exclusively in the small and large intestine, playing important roles in proliferation and differentiation of intestinal epithelial cells[1]. Several investigators have reported that low levels of CDX2 are a characteristic feature of human colon and squamous esophageal cancer[2,3], but others have shown that strong and robust expression of CDX2 is found in > 80% of colorectal cancer and non-small cell lung cancer[4,5]. In addition, CDX2 enhances proliferation and has tumorigenic potential in human colon cancer cell lines LoVo and SW48[6]. These studies have suggested that CDX2 also has oncogenic activity. Together, these conflicting findings point to a complex role for CDX2 in cell regulation. In adult humans, CDX2 is associated with intestinal metaplasia in the stomach in which ectopic expression of CDX2 is speculated to cause the gastric epithelial cells to trans-differentiate and assume the intestinal phenotype[7]. In addition, CDX2 transgenic mice have been shown to have intestinal metaplasia and a high incidence of gastric carcinoma[8,9].
In a previous study[10], it has been reported that RNA interference (RNAi)-mediated inhibition of CDX2 decreases endogenous MDR1 expression. MDR1 was originally identified as an overexpressed and amplified gene in multidrug-resistant cells. Its product, P-glycoprotein (P-gp), appears to play a critical role in drug resistance, which suggests that CDX2 is associated with multidrug resistance (MDR) of gastric cancer. Previously, we have reported that CDX2 affects the cell cycle and apoptosis of gastric cancer[11], Furthermore, apoptosis is just one of the important mechanisms of reversal MDR[12]. CDX2 may play a crucial role in the control of reversal MDR.
In the present study, we constructed small interfering RNA (siRNA) sequences that targeted CDX2, transfected them into a cisplatin-resistant gastric cancer cell line SGC7901/DDP, selected stable transfectants, and explored changes in IC50, rate of doxorubicin efflux, cell cycle, and apoptosis. We also observed the effect of CDX2 siRNA on the expression of genes associated with apoptosis, including c-Myc and survivin. Moreover, we investigated the effects of CDX2 downregulation on the growth and apoptosis of SGC7901/DDP cells in nude mice.
5-fluorouracil, cisplatin and doxorubicin were purchased from Sigma-Aldrich (St Louis, MO, United States). Cell culture medium RPMI-1640 was purchased from Invitrogen-Gibco (Carlsbad, CA, United States). Fetal bovine serum (FBS) was from Invitrogen-Gibco. Trypsin, streptomycin and penicillin were obtained from Sunshine Biotechnology (Nanjing, China). CDX2, c-Myc, survivin, cyclin D1, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and β-actin antibody were from Santa Cruz Biotechnology (Santa Cruz, CA, United States). All other chemicals were of the highest commercial grade available.
The cells were cultured in RPMI 1640 supplemented with 10% FBS (Sijiqing Biotec, Co. Ltd., Hangzhou, China), antibiotics (100 U/mL penicillin and 100 mg/mL streptomycin) in a humidified 5% CO2 atmosphere at 37.8 °C. For SGC7901/DDP cells, 0.6 μg/mL cisplatin was supplemented in the medium to maintain the drug-resistance phenotype.
Recombinant lentiviral vector for CDX2 gene (siRNA-CDX2) and null vector (siRNA-NC) were stored in our laboratory[13]. SGC7901/DDP cells were seeded in six-well plates with antibiotic-free medium. After 24 h incubation, cells were infected with viral supernatant at a multiplicity of infection of 150 PFU per cell (MOI = 150), and the stable-transfected cell lines were obtained by culturing transfected cells in the presence of 700 mg/mL G418 (Invitrogen, Carlsbad, CA, United States) for 3-4 wk. The cells were divided into three groups: SGC7901/DDP + siRNA-CDX2, SGC7901/DDP + siRNA-NC, and SGC7901/DDP.
The IC50 was determined by MTT [3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazoliumbromide] assay. Cells were plated in 96-well plates (5000 cells/well), and after adherence, the cells were exposed to cisplatin, doxorubicin, and 5-fluorouracil. After incubation for 48 h, the cells were incubated with 20 μL MTT (at a final concentration of 0.5 mg/mL) at 37 °C for 4 h. The medium was removed and the precipitated formazan was dissolved in 100 μL DMSO. The absorbance at 490 nm was detected using a microplate reader (Bio-Rad, Hercules, CA, United States). The IC50 was estimated by the relative survival curve. Each assay was performed in triplicate.
The cells were inoculated into six-well plates and 4 mg/mL doxorubicin was added, and all wells were placed at 37 °C for 30 min. Flow cytometry was used to measure the fluorescent intensity of doxorubicin in cells with an excitation wavelength of 488 nm and emission wavelength of 575 nm. The cells were then washed twice with fresh culture medium and incubated with the new medium at 37 °C for 1 h to detect the retained doxorubicin. Subtraction of the fluorescence retained from the total fluorescence was the fluorescent index of doxorubicin. The procedure was repeated three times and an average value was obtained to calculate the pump rate of doxorubicin. The pump rate of the drug from the cells = (accumulated quantity of doxorubicin-retained quantity of doxorubicin)/accumulated quantity of doxorubicin.
SGC7901/DDP cells (1 × 106) were washed twice with ice-cold PBS, treated with trypsin, and fixed in cold 70% ethanol at 4 °C for 30 min. The cell pellet was incubated in a solution containing 50 ng/mL propidium iodide, 0.2 mg/mL RNase, and 0.1% Triton X-100 at room temperature for 30 min. The cells were analyzed by flow cytometry using an EPICS XL-MCL FACScan (Becton-Dickinson, Mountain View, CA, United States). The data was analyzed with the MultiCycle Software for Windows (Phoenix Flow Systems, San Diego, CA, United States).
Total RNA was extracted from SGC7901/DDP + siRNAi-CDX2 cells, SGC7901/DDP + siRNAi-NC cells, and SGC7901/DDP cells using TRIzol Reagent (Invitrogen). All gene segments were amplified and verified by semiquantitative reverse-transcriptase polymerase chain reaction (RT-PCR). cDNAs were reverse-transcribed from 2 μg total RNA. The PCR primer sequences (CDX2 primers were sense: 5’-CGG CAG CCA AGT GAA AAC-3’ and antisense: 5’-GAT GGT GAT GTA GCG ACT GTA-3’. Survivin primers were sense: 5’-AAA TGC ACT CCA GCC TCT GT-3’ and antisense: 5’-TGT CGA GGA AGC TTT CAGGT-3’. Cyclin D1 primers were sense: 5’-CCC TCG GTG TCC TAC TTC AA-3’ and antisense: 5’-GGG GAT GGT CTC CTT CAT CT-3. c-Myc primers were sense: 5’-TTC TCT CCG TCC TCG GAT TC-3’ and antisense: 5’-GTA GTT GTG CTG ATG TGT GG-3’. GAPDH primers were sense: 5’-ACC ACA GTC CAT GCC ATC AC-3’ and antisense: 5’-TCA CCA CCC TGT TGC TGT A-3’). The products of PCR were checked by agarose gel electrophoresis, and the abundance of each mRNA was detected and normalized to that of GAPDH mRNA.
Cell lysates were prepared in a buffer containing 100 mmol/L NaCl, 10 mmol/L Tris-HCl (pH 7.6), 1 mmol/L EDTA (pH 8.0), 1 μg/mL aprotinin, 100 μg/mL phenylmethylsulfonyl fluoride, and 1% (v/v) NP-40. After protein quantitation using the Lowery protein assay, equal amounts of proteins were separated by SDS-PAGE and blotted onto nitrocellulose membranes by the semi-dry blotting method using a three-buffer system. The membranes were incubated with a dilution of primary antibody (anti-CDX2: 1:500, anti-c-Myc: 1:1000, anti-survivin: 1:1500, anti-cyclin D1:1:3000), overnight at 4 °C. The membrane was washed with TBST and incubated with a peroxidase-conjugated secondary antibody (1:1000) (Santa Cruz Biotechnology) for 1 h. Specific antibody binding was detected using a chemiluminescence detection system (Pierce, Rockford, IL, United States), according to the manufacturer’s recommendations. Western blot film was scanned, and the net intensities of the bands were quantified using Image-QuanT software (Molecular Dynamics, Sunnyvale, CA, United States). After development, the membrane was stripped and reprobed with antibody against GAPDH (1:1000) or β-actin (1:1500) to confirm equal sample loading.
BALB/c 5-wk-old male nude mice (Guangxi Animal Center, Nanning, China) were kept under specific pathogen-free conditions and tended to in accordance with institutional guidelines. All experimental studies were approved by the Guangxi Medical University Animal Care and Use Committee. SGC7901/DDP cells were used for tumor implantation. Approximately 2 × 106 tumor cells were harvested, resuspended in 100 μL PBS, implanted subcutaneously into the flanks of the BALB/c nude mice, and resulting tumor was named as SGC7901/DDP tumor. After 7 d, when the SGC7901/DDP tumor measured 3-5 mm in diameter, these nude mice were randomly divided into the following three groups (6 mice/per group): SGC7901/DDP + siRNA-CDX2, SGC7901/DDP + siRNA-NC, and SGC7901/DDP. The animals were administered an intratumoral injection of LV-siRNA- CDX2 or LV-siRNA-NC at a titer of 5 × 106 TU in 100 μL PBS, and injection of an equal volume of PBS was used as a blank control. After the first injection, the animals were administered a similar injection every 2 d. DDP was administered by intraperitoneal injection at a dose of 25 mg/kg. After the first injection, the animals were administered a similar injection every 2 d. The tumors were monitored every day and measured every 2 d with a caliper, and the diameters were recorded. The tumor volume (TV) was calculated by the formula: TV = W2×L/2, where L is the length and W is the width of the tumor. The relative tumor volume (RTV) was calculated by the formula: RTV = Vt/V0 (V0 is the TV at the day when the chemicals were given, and Vt is the TV of subsequent measurement). The animals were sacrificed at 12 d after tumor injection and the tumors were analyzed.
For hematoxylin and eosin (HE) staining tumor tissues were fixed in 4% formaldehyde, dehydrated with gradient ethanol, and embedded in paraffin wax. Tissue sections were dewaxed and rehydrated according to a standard protocol. Sections were stained with HE. For the deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) assay, apoptotic cells in sections of mouse tumor tissue were detected using an in situ apoptosis detection kit (KEYGEN, Nanjing, China) as instructed by the manufacturer. Cells were visualized with a light microscope (Olympus IX70, Tokyo, Japan). The apoptotic index was calculated as follows: the apoptotic index = number of apoptotic cells/total number of cells. The in vivo experiments strictly obeyed the ethical principles and guidelines for scientific experiments on animals.
Data are expressed as mean ± SE. Statistical significance was determined using χ2 test, Student’s t test, or one-way analysis of variance (ANOVA). Statistical analysis were carried out using SPSS version 13.0 (Chicago, IL, United States) or Origin 7.5 software programs (OriginLab, Northampton, MA, United States). A value of P < 0.05 was considered as statistically significant.
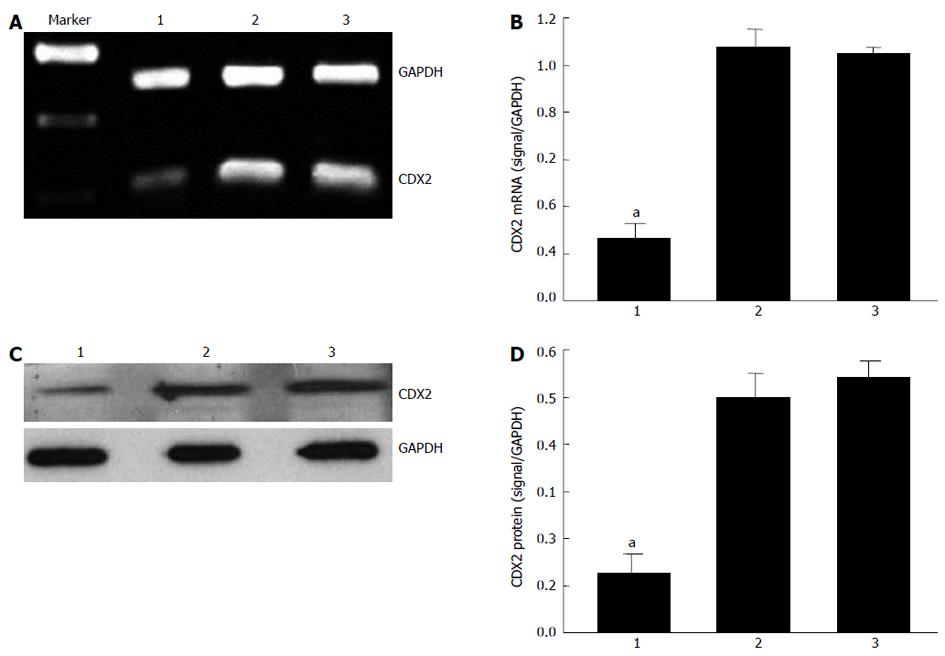
Our previous study suggested that recombinant lentiviral vector for CDX2 gene (siRNA-CDX2) successfully inhibited CDX2 mRNA and protein expression in MGC-803 cells[13]. In the present study, we further tested the hypothesis that CDX2 siRNA downregulates CDX2 mRNA and protein expression in SGC7901/DDP cells. We treated SGC7901/DDP cells with siRNA-CDX2 and siRNA-NC (negative control). Transfection of siRNA-CDX2 into SGC7901/DDP cells led to marked inhibition of CDX2 mRNA (Figure 1A) and protein expression (Figure 1C). Densitometry analysis showed that CDX2 mRNA (Figure 1B) and protein (Figure 1D) in SGC7901/DDP + siRNA-CDX2 cells were about 3.5- and 4-fold lower, respectively, than those in SGC7901/DDP + siRNA-NC cells and SGC7901/DDP cells (P < 0.05). There were no differences between SGC7901/DDP + siRNA-NC cells and SGC7901/DDP cells. These results suggested that CDX2 siRNA could downregulate CDX2 mRNA and protein expression in SGC7901/DDP.
Although SGC7901/DDP cells were selected with the single anticancer drug cisplatin, they also displayed multiple resistances to other anticancer drugs. We studied the regulatory effects of CDX2 siRNA on the drug sensitivity of gastric cancer cells. MTT assay was used to detect the sensitivity of cells to one P-gp-related drug (doxorubicin) and two P-gp-non-related drugs (5-fluorouracil and cisplatin). As showed in Table 1, compared with SGC7901/DDP + siRNA-NC cells and SGC7901/DDP cells, SGC7901/DDP + siRNA-CDX2 exhibited significantly decreased IC50 values for cisplatin, doxorubicin and 5-fluorouracil (P < 0.05).
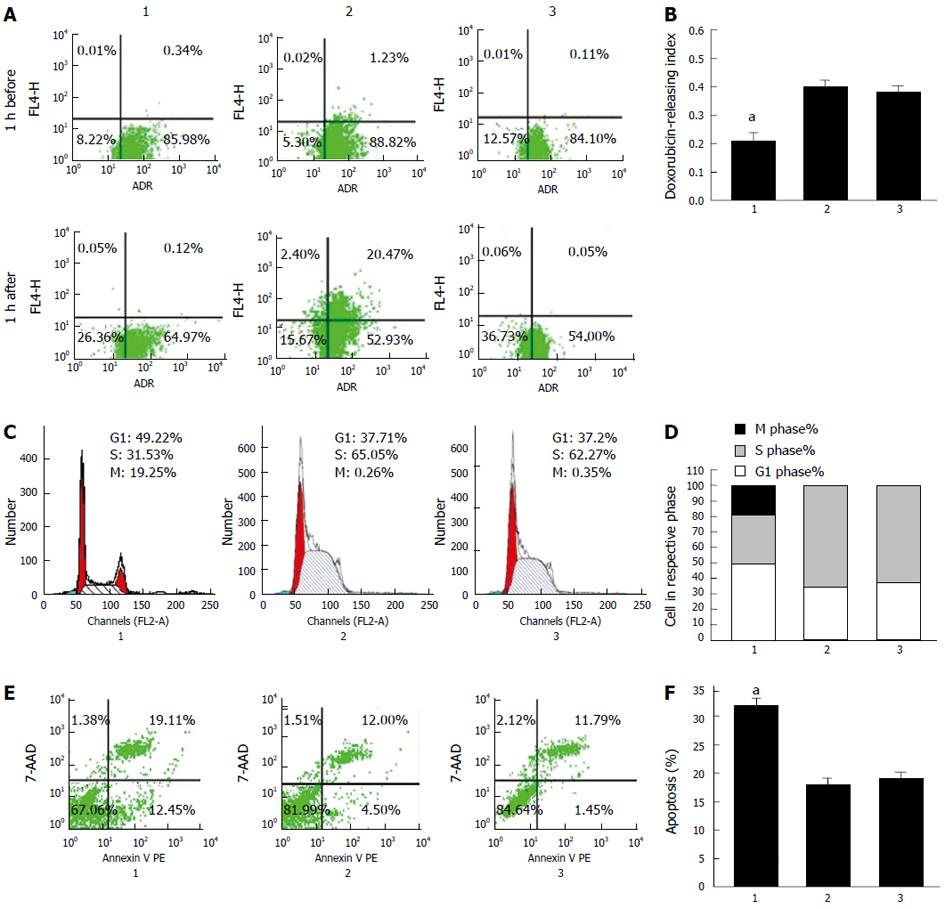
Pumping out chemotherapeutic agents is the key process in MDR[14]. We proposed that downregulation of CDX2 inhibited drug efflux in gastric cancer cells. To test this hypothesis, intracellular drug accumulation and retention were evaluated using doxorubicin as a probe. As shown in Figure 2A, compared with SGC7901/DDP + siRNA-NC cells and SGC7901/DDP cells, SGC7901/DDP + siRNA-CDX2 cells exhibited significantly increased accumulation and retention as well as a lower releasing index of doxorubicin (Figure 2B) (P < 0.05).
We used flow cytometry to determine whether reversal of MDR by CDX2 siRNA in SGC7901/DDP cells was mediated, at least in part, through an effect on cell cycle progression. We found that the number of cells in G1 phase markedly increased, while those in S phase decreased in SGC7901/DDP + siRNA-CDX2 cells, compared with SGC7901/DDP + siRNA-NC cells and SGC7901/DDP cells (Figure 2C) (P < 0.05).
Anti-apoptosis is an important mechanism of MDR, therefore, we investigated the effect of siRNA-CDX2 on cisplatin-induced gastric cancer cell apoptosis by calculating apoptosis index. Cells were stained with annexin V PE and 7-AAD, and then subsequently analyzed by flow cytometry. The dual parameter fluorescent dot plots showed that the viable cells were in the lower left quadrant, and the apoptotic cells were in the right quadrant. As shown in Figure 2E, compared with SGC7901/DDP + siRNA-NC cells and SGC7901/DDP cells, SGC7901/DDP + siRNA-CDX2 cells exhibited significantly increased apoptosis index (Figure 2F) (P < 0.05).
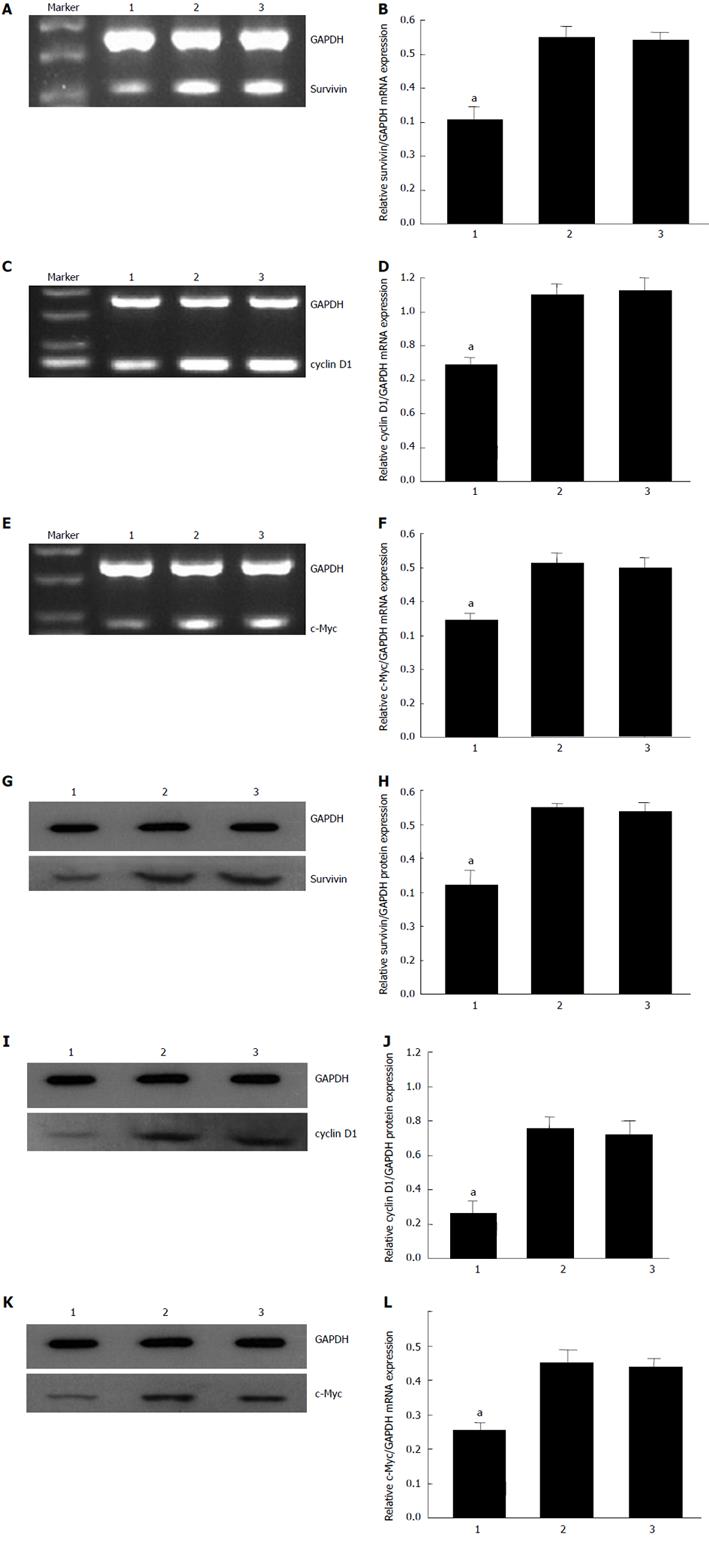
To investigate the mechanism by which CDX2 siRNA induces reversal of MDR in SGC7901/DDP cells, we detected expression levels of some well-known regulators of apoptosis (caspase-9, caspase-3, p53, bax, bcl-2, Survivin, and c-Myc), and an important cell cycle molecule (cyclin D1) by semiquantitative RT-PCR and Western blotting (Figure 3). The mRNA and protein expression level of c-Myc, survivin and cyclin D1 in SGC7901/DDP + siRNA-CDX2 cells was lower than that in SGC7901/DDP + siRNA-NC cells and SGC7901/DDP cells (P < 0.05). However, no significant difference in the expression level of caspase-9, caspase-3, p53, bax and bcl-2 was found in the cell models (data not shown).
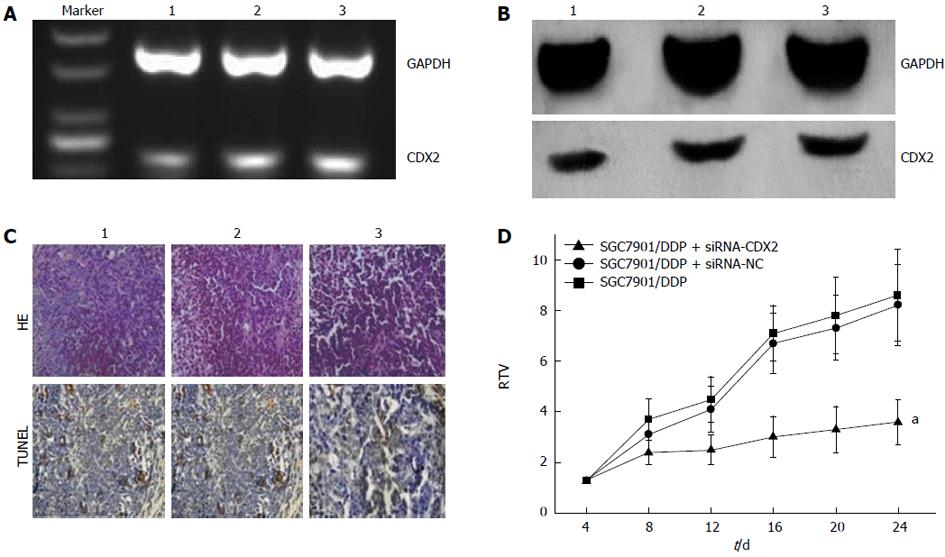
We examined the effect of CDX2 siRNA on growth of SGC7901/DDP cells in vivo, by implanting LV-siRNA-CDX2 and LV-siRNA-NC subcutaneously into the flanks of BALB/c nude mice. We detected expression levels of CDX2 in vivo by semi-quantitative RT-PCR and Western blotting. The mRNA (Figure 4A) and protein (Figure 4B) expression level of CDX2 in SGC7901/DDP + siRNA-CDX2 group was lower than that in SGC7901/DDP + siRNA-NC group and SGC7901/DDP group. Three weeks after implantation, TV in the SGC7901/DDP + siRNA-CDX2 group was significantly less than in the SGC7901/DDP and SGC7901/DDP + siRNA-NC groups (P < 0.05) (Figure 4D). The percentage of apoptotic tumor cells in SGC7901/DDP + siRNA-CDX2 cells was 7.2% ± 1.3%, which was more than the 3.1% ± 1.2% in SGC7901/DDP + siRNA-NC cells and 3.1% ± 1.4% in SGC7901/DDP cells, as determined by the HE staining and TUNEL assay (Figure 4C).
The development of MDR to cancer chemotherapy is a major obstacle to the effective treatment of gastric cancer[14]. However, the mechanism of MDR remains obscure. P-gp was the first molecule identified as a modulator of MDR. After that, various other molecules were shown to be involved, including transporters that eject anticancer drugs from cells, such as MDR-associated protein (MRP)[15], genes regulating apoptosis, such as p53[16], PKC[17], and Bcl-2 family[18]. Recently, the distribution of drugs in cancer cells was also considered to play a part in MDR[19]. According to our previous report, some classic molecules are involved in MDR, including caspase-3 (apoptosis-related cysteine peptidase) and caspase-9 (an initiator caspase, has been linked to the mitochondrial death pathway)[20,21], but there may be other mechanisms that control MDR of gastric cancer cells[12].
The CDX2 homeobox gene, which is homologous to the Drosophila gene caudal, has an essential role during early development[2], an important study by Ma et al[22] demonstrated that short interfering RNA-mediated knockdown of CDX2 resulted in reduced apical sodium-dependent bile acid transporter (ASBT) mRNA expression in intestinal cells. Overexpression of CDX2 in human colon cancer cells induces a less malignant phenotype, inhibiting proliferation, invasion, and migration[23]. Furthermore, CDX2 has a crucial role in the regulation of MDR1 gene expression in drug resistance[10]. However, the precise molecular mechanism of CDX2 in reversing MDR in gastric cancer cells is still poorly characterized. The present study is believed to be the first to correlate CDX2 with MDR of gastric cancer cells, and we found that expression of CDX2 regulated drug efflux pumping, the cell cycle, and apoptosis. The multiple changes conferred by CDX2 on gastric cancer cells are not surprising, given the involvement of CDX2 in a wide range of biochemical reactions, and CDX2 is a homeobox transcription factor that contributes to reversing MDR.
Our study indicated that CDX2 siRNA led to marked downregulation of CDX2 mRNA and protein expression in SGC7901/DDP cells, caused cell cycle arrest in the G0/G1 phase, and induced apoptosis. Furthermore, downregulation of CDX2 in SGC7901/DDP cells enhanced sensitivity to cisplatin, 5-fluorouracil (P-gp-non-related drug), and doxorubicin (P-gp-related drug). The ability to pump doxorubicin was reduced significantly, moreover, a strong antitumor effect of CDX2 siRNA in vivo was observed, as tumor growth was suppressed and tumor apoptosis was increased in nude mice when CDX2 mRNA and protein were downregulated. These findings suggest that CDX2 siRNA reversed MDR of human gastric cancer cells.
Doxorubicin is a common substrate for P-gp, but SGC7901/DDP + siRNA-CDX2 cells also exhibited significantly decreased IC50 values for cisplatin and 5-fluorouracil. It should be noted that P-gp-mediated drug efflux was not the only mechanism involved in drug resistance. Previous studies have shown that the effect of P-gp on drug resistance is closely related to cell cycle distribution and apoptosis[24-26]. Cyclin D1 is a regulatory kinase of cell cycle distribution. Previously, overexpression of cyclin D1 in a human fibrosarcoma cell line has been shown to confer resistance to methotrexate[27], which suggests that cyclin D1 overexpression can contribute to the resistance of cancer cells to chemotherapeutic agents. Conversely, suppression of cyclin D1 levels has been shown to potentiate the response of human pancreatic cancer cells to cisplatinum, transfection and multidrug selection experiments have demonstrated that resistance to mitoxantrone can be associated with MDR1 and/or multidrug resistance-associated protein (MRP) overexpression[28]. Indeed, subsequent analysis of MDR1 and MRP expression has revealed that cyclin D1 suppression decreases MDR1 and MRP mRNA levels[24]. Besides regulation of cell cycle distribution, apoptosis is a common pathway that finally mediates the killing effects of anticancer drugs, which is an important cause of MDR. Mitochondria are known to play an active role in the apoptotic process by various mechanisms, including release of caspase activators, disruption of electron transport and energy metabolism, and production of reactive oxygen species[29]. Survivin induces mitochondrial fragmentation and reduces mitochondrial respiration[30]. These data indicate that survivin is closely related to apoptosis. Therefore, in the present study, inhibition of CDX2 expression may have decreased cyclin D1 and survivin expression directly or indirectly, which was responsible for reversal of MDR in human gastric cancer cells in vitro and in vivo. Further studies are needed to confirm our results.
The term MDR was originally coined to define a condition enabling a disease-causing organism or cancer cells to resist distinct drugs or chemicals with a wide variety of structure and function, targeted at eradicating the organism/cancer cell. Much routine chemotherapy cannot achieve good therapeutic effects because of MDR. It is important to find a new way to reverse MDR. In this study, we showed that CDX2 plays a critical role in reversing MDR. Downregulation of CDX2 using RNAi reversed the progression of MDR in gastric cancer SGC7901/DDP cells in vitro and in vivo. In conclusion, this study lays the foundation for treatment of MDR in gastric cancer through manipulation of CDX2 expression.
The term multidrug resistance (MDR) was originally coined to define a condition enabling a disease-causing organism or cancer cells to resist distinct drugs or chemicals with a wide variety of structure and function, targeted at eradicating the organism/cancer cell. Much routine chemotherapy cannot achieve good therapeutic effects because of MDR. It is believed that MDR is the key factor in the failure of gastric cancer chemotherapy. It is important to find a new way to reverse MDR. The caudal-type homeobox gene, CDX2, plays an important role in intestinal metaplasia, and is a precursor of intestinal-type gastric carcinoma. However, the effect of CDX2 in reversing MDR is still not clear.
CDX2 has a crucial role in the regulation of MDR1 gene expression in drug resistance, but P-glycoprotein (P-gp)-mediated drug efflux is not the only mechanism involved in drug resistance. The CDX2 research hotspot is how it affects the reversal of MDR by other pathways.
This study is believed to be the first to demonstrate that downregulation of CDX2 causes cell cycle arrest in the G0/G1 phase, and induces apoptosis. Furthermore, downregulation of CDX2 in SGC7901/DDP cells enhances the sensitivity of SGC7901/DDP cells to cisplatin, 5-fluorouracil (P-gp-non-related drug), and doxorubicin (P-gp-related drug). The ability to pump doxorubicin was reduced significantly, moreover, a strong antitumor effect of CDX2 siRNA in vivo was observed, as tumor growth was suppressed and tumor apoptosis was increased in nude mice when CDX2 mRNA and protein were downregulated. CDX2 siRNA also decreased c-Myc, survivin and cyclin D1 expression as determined by semiquantitative reverse-transcriptase polymerase chain reaction and Western blotting.
This study lays the foundation for treatment of MDR in gastric cancer through manipulation of CDX2 expression.
The transcription factor, CDX2, is a member of the caudal-related homeobox gene family, and is mainly expressed in the intestine. It is also known to be a key factor in reversing MDR by manipulation of MDR1 expression.
This is a well-written manuscript and most of the experiments were properly controlled and clearly presented.
P- Reviewers Aoyagi K, Baba H, Chen JL, Yun S, Zhu BS S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Zhao J, Gregersen H. Relationships of CDXs and apical sodium-dependent bile acid transporter in Barrett’s esophagus. World J Gastroenterol. 2013;19:2736-2739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 3] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Guo RJ, Suh ER, Lynch JP. The role of Cdx proteins in intestinal development and cancer. Cancer Biol Ther. 2004;3:593-601. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 3. | Guo M, House MG, Suzuki H, Ye Y, Brock MV, Lu F, Liu Z, Rustgi AK, Herman JG. Epigenetic silencing of CDX2 is a feature of squamous esophageal cancer. Int J Cancer. 2007;121:1219-1226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Witek ME, Nielsen K, Walters R, Hyslop T, Palazzo J, Schulz S, Waldman SA. The putative tumor suppressor Cdx2 is overexpressed by human colorectal adenocarcinomas. Clin Cancer Res. 2005;11:8549-8556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Grimminger P, Ling FC, Neiss S, Vallböhmer D, Lurje G, Schneider PM, Hölscher AH, Metzger R, Brabender J. The role of the homeobox genes BFT and CDX2 in the pathogenesis of non-small cell lung cancer. Anticancer Res. 2009;29:1281-1286. [PubMed] [Cited in This Article: ] |
| 6. | Dang LH, Chen F, Ying C, Chun SY, Knock SA, Appelman HD, Dang DT. CDX2 has tumorigenic potential in the human colon cancer cell lines LOVO and SW48. Oncogene. 2006;25:2264-2272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Barros R, da Costa LT, Pinto-de-Sousa J, Duluc I, Freund JN, David L, Almeida R. CDX2 autoregulation in human intestinal metaplasia of the stomach: impact on the stability of the phenotype. Gut. 2011;60:290-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Almeida R, Silva E, Santos-Silva F, Silberg DG, Wang J, De Bolós C, David L. Expression of intestine-specific transcription factors, CDX1 and CDX2, in intestinal metaplasia and gastric carcinomas. J Pathol. 2003;199:36-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 212] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 9. | Mutoh H, Sakurai S, Satoh K, Tamada K, Kita H, Osawa H, Tomiyama T, Sato Y, Yamamoto H, Isoda N. Development of gastric carcinoma from intestinal metaplasia in Cdx2-transgenic mice. Cancer Res. 2004;64:7740-7747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 135] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 10. | Takakura Y, Hinoi T, Oue N, Sasada T, Kawaguchi Y, Okajima M, Akyol A, Fearon ER, Yasui W, Ohdan H. CDX2 regulates multidrug resistance 1 gene expression in malignant intestinal epithelium. Cancer Res. 2010;70:6767-6778. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Xie Y, Li L, Wang X, Qin Y, Qian Q, Yuan X, Xiao Q. Overexpression of Cdx2 inhibits progression of gastric cancer in vitro. Int J Oncol. 2010;36:509-516. [PubMed] [Cited in This Article: ] |
| 12. | Fan K, Fan D, Cheng LF, Li C. Expression of multidrug resistance-related markers in gastric cancer. Anticancer Res. 2000;20:4809-4814. [PubMed] [Cited in This Article: ] |
| 13. | Wang XT, Xie YB, Xiao Q. siRNA targeting of Cdx2 inhibits growth of human gastric cancer MGC-803 cells. World J Gastroenterol. 2012;18:1903-1914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Fan D, Liu X. New progresses in researches on multidrug resistance in gastric cancer. Chin J Digest. 2000;20:77-78. [Cited in This Article: ] |
| 15. | Chuman Y, Sumizawa T, Takebayashi Y, Niwa K, Yamada K, Haraguchi M, Furukawa T, Akiyama S, Aikou T. Expression of the multidrug-resistance-associated protein (MRP) gene in human colorectal, gastric and non-small-cell lung carcinomas. Int J Cancer. 1996;66:274-279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 16. | Matsuhashi N, Saio M, Matsuo A, Sugiyama Y, Saji S. The evaluation of gastric cancer sensitivity to 5-FU/CDDP in terms of induction of apoptosis: time- and p53 expression-dependency of anti-cancer drugs. Oncol Rep. 2005;14:609-615. [PubMed] [Cited in This Article: ] |
| 17. | Han Y, Han ZY, Zhou XM, Shi R, Zheng Y, Shi YQ, Miao JY, Pan BR, Fan DM. Expression and function of classical protein kinase C isoenzymes in gastric cancer cell line and its drug-resistant sublines. World J Gastroenterol. 2002;8:441-445. [PubMed] [Cited in This Article: ] |
| 18. | Xiao B, Shi YQ, Zhao YQ, You H, Wang ZY, Liu XL, Yin F, Qiao TD, Fan DM. Transduction of Fas gene or Bcl-2 antisense RNA sensitizes cultured drug resistant gastric cancer cells to chemotherapeutic drugs. World J Gastroenterol. 1998;4:421-425. [PubMed] [Cited in This Article: ] |
| 19. | Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583-592. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2014] [Cited by in F6Publishing: 1964] [Article Influence: 109.1] [Reference Citation Analysis (0)] |
| 20. | Cappellini A, Chiarini F, Ognibene A, McCubrey JA, Martelli AM. The cyclin-dependent kinase inhibitor roscovitine and the nucleoside analog sangivamycin induce apoptosis in caspase-3 deficient breast cancer cells independent of caspase mediated P-glycoprotein cleavage: implications for therapy of drug resistant breast cancers. Cell Cycle. 2009;8:1421-1425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Sherbakova EA, Stromskaia TP, Rybalkina EIu, Kalita OV, Stavrovskaia AA. [Role of PTEN protein in multidrug resistance of prostate cancer cells]. Mol Biol (Mosk). 2008;42:487-493. [PubMed] [Cited in This Article: ] |
| 22. | Ma L, Jüttner M, Kullak-Ublick GA, Eloranta JJ. Regulation of the gene encoding the intestinal bile acid transporter ASBT by the caudal-type homeobox proteins CDX1 and CDX2. Am J Physiol Gastrointest Liver Physiol. 2012;302:G123-G133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Brabletz T, Spaderna S, Kolb J, Hlubek F, Faller G, Bruns CJ, Jung A, Nentwich J, Duluc I, Domon-Dell C. Down-regulation of the homeodomain factor Cdx2 in colorectal cancer by collagen type I: an active role for the tumor environment in malignant tumor progression. Cancer Res. 2004;64:6973-6977. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 111] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 24. | Kornmann M, Danenberg KD, Arber N, Beger HG, Danenberg PV, Korc M. Inhibition of cyclin D1 expression in human pancreatic cancer cells is associated with increased chemosensitivity and decreased expression of multiple chemoresistance genes. Cancer Res. 1999;59:3505-3511. [PubMed] [Cited in This Article: ] |
| 25. | Sicari BM, Troxell R, Salim F, Tanwir M, Takane KK, Fiaschi-Taesch N. c-myc and skp2 coordinate p27 degradation, vascular smooth muscle proliferation, and neointima formation induced by the parathyroid hormone-related protein. Endocrinology. 2012;153:861-872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Tsubaki M, Satou T, Itoh T, Imano M, Komai M, Nishinobo M, Yamashita M, Yanae M, Yamazoe Y, Nishida S. Overexpression of MDR1 and survivin, and decreased Bim expression mediate multidrug-resistance in multiple myeloma cells. Leuk Res. 2012;36:1315-1322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 27. | Hochhauser D, Schnieders B, Ercikan-Abali E, Gorlick R, Muise-Helmericks R, Li WW, Fan J, Banerjee D, Bertino JR. Effect of cyclin D1 overexpression on drug sensitivity in a human fibrosarcoma cell line. J Natl Cancer Inst. 1996;88:1269-1275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 53] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Kornmann M, Arber N, Korc M. Inhibition of basal and mitogen-stimulated pancreatic cancer cell growth by cyclin D1 antisense is associated with loss of tumorigenicity and potentiation of cytotoxicity to cisplatinum. J Clin Invest. 1998;101:344-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 103] [Article Influence: 4.0] [Reference Citation Analysis (0)] |