Published online Jul 14, 2013. doi: 10.3748/wjg.v19.i26.4119
Revised: April 23, 2013
Accepted: April 28, 2013
Published online: July 14, 2013
Processing time: 175 Days and 16.5 Hours
Toll-like receptors (TLRs) recognize specific motifs which are frequently present in bacteria, fungi, prokaryotes and viruses. Amongst TLRs, TLR9 can be activated by such bacterial or viral DNA fragments, immunoglobulin-DNA complexes or synthetic oligonucleotides, which all contain unmethylated cytosine-guanine nucleotide sequences (CpGs). Emerging data indicate that TLR9 signaling has a role in, and may influence, colorectal carcinogenesis and colonic inflammation. CpGs are classified into three groups according to their influence on both the antigen-specific humoral- and cellular immunity, and the production of type 1 interferons and proinflammatory cytokines. TLR9 activation via CpGs may serve as a new therapeutic target for several cancerous and various inflammatory conditions. Due to its probable anti-cancer effects, the application possibilities of TLR9-signaling modulation may be extremely diverse even in colorectal tumors. In this review we aimed to summarize the current knowledge about TLR-signaling in the pathogenesis and therapy of inflammatory bowel diseases and colorectal cancer. Due to the species-specific differences in TLR9 expression, however, one must be careful in translating the animal model data into the human system, because of the differences between CpG-oligodeoxynucleotide-responsive cells. TLR9 agonist DNA-based immunomodulatory sequences could also represent a promising therapeutic alternative in systemic inflammatory conditions and chronic colonic inflammations as their side effects are not significant.
Core tip: Toll-like receptor 9 mediated signaling influences and regulates the severity of mucosal inflammation, and seems to have a protective role against malignant transformation. The modulation of toll-like receptor 9 signaling by synthetic oligodeoxynucleotide agonists or antagonists seems to have beneficial therapeutic effect in inflammatory and cancerous colonic disorders.
- Citation: Fűri I, Sipos F, Germann TM, Kalmár A, Tulassay Z, Molnár B, Műzes G. Epithelial toll-like receptor 9 signaling in colorectal inflammation and cancer: Clinico-pathogenic aspects. World J Gastroenterol 2013; 19(26): 4119-4126
- URL: https://www.wjgnet.com/1007-9327/full/v19/i26/4119.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i26.4119
The immunostimulatory effect of DNA was discovered by William Coley, a surgeon from New York[1]. He used living and heat-treated bacteria as a therapeutic option for different kinds of tumors. It has long been known that microbes contain many immunostimulatory ingredients. In 1980, Tokunaga et al[2] identified the bacterial DNA as a main ingredient of the Coley-lysate. Later, they also showed that the same immunostimulatory effect could be achieved by using short synthetic oligodeoxynucleotide sequences (ODNs)[3,4]. In 2000, it was proven that DNA sequences are mainly recognized by the members of the Toll-like receptor (TLR) superfamily[5]. It has also been proven that in TLR9 knock out mice, microbial DNA fragments cannot result in an immune response[5]. It was also shown that the immunomodulating effect of natural and synthetic ODNs is mainly transmitted by TLR9[6].
Peyer’s patches (PPs) and isolated lymphoid follicles (ILFs) are immunologic and regenerative organizers of the gut mucosa, and they also represent a unique switch point between colonic inflammation and cancer[7]. Microfold (M) cells are located in the follicle associated epithelium (FAE) of PPs and ILFs, where they mediate the uptake and transcytosis of luminal antigens to the underlying lymphoid tissue. TLR9 was found to be preferentially expressed in M cells[8]. Some TLR polymorphisms are known to be associated with the susceptibility of inflammatory bowel diseases (IBD)[9-11] and sporadic colorectal cancer (CRC) development[12,13], but the current and concrete pathogenic role of TLRs, including TLR9, remains uncertain in these conditions.
In this review we aimed to summarize the current knowledge about TLR-signaling in the pathogenesis of IBD and CRC, focusing especially on TLR9. Recent data indicate that targeting TLR9-signaling may yield new and promising therapeutic approaches to these conditions.
TLRs belong to the type 1 transmembrane glycoproteins, which contain extracellular leucin-reach repeated sequences and Toll/interleukin-1 receptor signaling domains. TLR4 was the first to be identified, and currently 10 TLRs have been identified in humans, while 13 have been identified in mice[14]. TLRs are mainly expressed in the cells of the innate and adaptive immunity (i.e., monocytes, macrophages, lymphocytes, mast cells, dendritic cells), however, some (TLR4, -5 and -9) may be expressed by modified epithelial cells as well[15]. Apical epithelial TLR9 activation by bacterial DNA fragments has been reported to maintain colonic homeostasis[16].
TLRs usually recognize microbial wall components, DNA and ribonucleic acid (RNA) fragments. TLR1, -2, -4, -5, and -6 are localized to the cell surface, while TLR3, -7, -8, and -9 are present in the intracellular compartment[17-20]. TLRs bind specific motifs, which frequently appear in bacteria, fungi, protozoa, and viruses[21,22]. These motifs can be lipids and lipopeptides (TLR1, -2, -4, -6), bacterial flagelline (TLR5), and fragments of nucleic acids (TLR3, - 7, -8, -9). TLR3 binds double stranded RNA from viruses, while TLR7 and -8 can recognize single stranded RNAs. Moreover, TLR7 recognizes immunoglobuline-self-RNA complexes in autoimmune disease conditions. Imiquimod is a specific ligand for TLR7. TLR9 could be activated by bacterial and viral DNA, immunoglobuline-DNA complexes, and synthetic ODNs, which contain unmethylated CpG sequences[21,22].
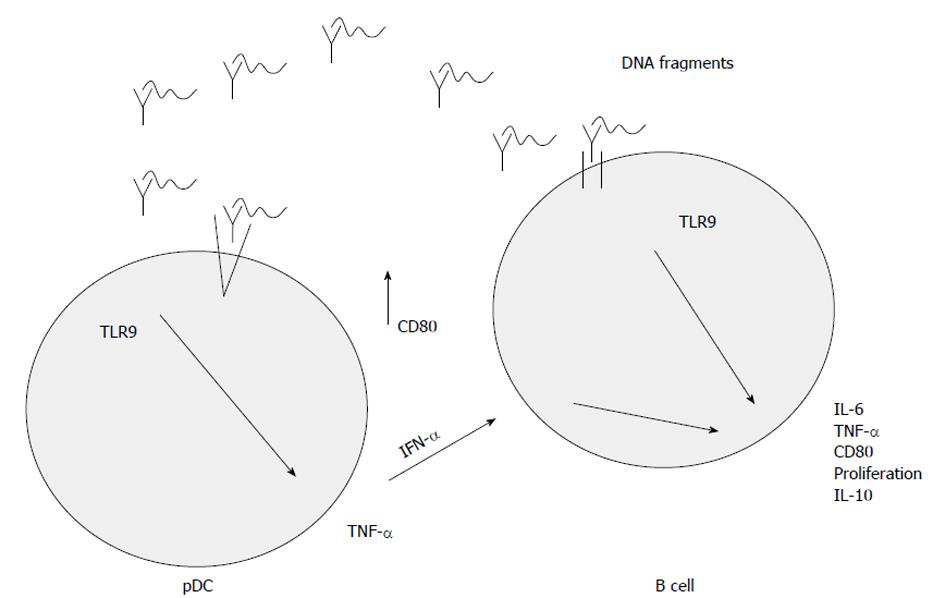
The signals transmitted by TLRs activate both innate and adaptive immunity. Due to the immune evasion nature of tumor cells, the dysregulated activation of adaptive and innate immune systems could result in cytotoxic effects. This could in turn eradicate the diseased cells or even control the tumorous progression. TLRs recognize pathogen-associated molecular patterns (PAMPs) originating from microbiota and could also bind endogenous ligands, such as danger-associated molecular patterns (DAMPs)[23]. Both bacterial DNA and synthetic ODNs activate the innate and adaptive immune system via plasmacytoid dendritic cells (pDCs) and macrophages[24].
Due to TLR9-associated activation, pDCs produce interferon-α which influences the cytokine production of B cells[24] resulting in pro- (e.g., interleukin 6, tumor-necrosis factor-α) and anti-inflammatory (e.g., interleukin 10) cytokine release and co-expression of MHC II type surface antigens.
The activation of TLR9 is a complex pathway. The uptake of DNA sequences is the most unclear process, which is influenced by the structure of the DNA fragments. Many cell types can easily take up single stranded DNA, but the uptake of double stranded DNA may be more effective if a cationic lipid is used for packing it in, because TLR9 is located in the intracellular compartment of endosomes[24,25]. It was shown that fluorescein isothiocyanate labelled CpG DNA is transferred to the intracellular compartment by non-specific endocytosis[25]. This transport is non-specific, because DNA sequences lacking CpG dinucleotides may also be recognized by TLR9, and this way of immune activation can be competitively inhibited by non-CpG sequences[25]. After transportation to the intracellular compartment, endosomal acidic maturation occurs. This process may be inhibited by pH raising agents (e.g., chloroquine, bafilomycin A)[25]. Finally pro- and anti-inflammatory cytokines may be released and B cell proliferation may be enhanced[25]. The main steps of TLR9-signaling are summarized in Figure 1.
The signal molecules of this pathway [e.g., myeloid differentiation primary response gene 88 (Myd88), tumor necrosis factor receptor-associated factor 6 (TRAF6), interleukin receptor associated kinase (IRAK)-1, -4; p50/p65 heterodimer of nuclear factor (NF)-κB] are non- specific and are also involved in the signaling of other TLRs. Interferons may also be released by a mitogen activated protein kinase (MAPK)-associated pathway, which is also intensively being investigated[26].
The immunostimulatory effect of unmethylated CpG sequences has been proven in mice and in other species, as well as in in vitro human cell line experiments[27,28]. The CpG DNA sequences can be classified into three classes based on the different immune cell-mediated immune responses and their chemical structure. It has already been documented how these differences in the chemical structure may determine the immunostimulatory effect of these sequences on immune cells[29]. Liu et al[29] demonstrated in mice that three CpG-ODN classes can differentially affect antigen-specific humoral and cellular immune responses. Specifically, the B- and C-class CpG-ODNs induced a potent Th1-mediated immunity with comparable antibody levels as well as CD4+ and CD8+ T cell responses. In contrast, although the A-class CpG-ODNs weakly enhanced antibody titers and CD8+ T cell response regarding cytotoxic activity, they were not able to change the IgG1/IgG2a ratio or elicit antigen-specific, interferon γ-secreting CD4+ and CD8+ T cells. Consistent with this, three CpG-ODN classes provided differential antigen-specific protection against an intracellular bacterial infection (i.e., Listeria monocytogenes). These three classes of CpG-ODNs did not show significant differences regarding the interleukin 12 producing effect[30,31]. These results may provide not only better understanding of the adjuvant activities of three CpG-ODN classes, but also of implications for the rational design of CpG-ODN adjuvants.
Components of Gram-negative bacterial cell walls alert the host to invading bacteria and activate innate immunity. These responses are usually effective in combating infection and restoring normal host function. However, in individuals susceptible to IBD, they may become excessive and lead to mucosal damage.
In genes for all the contributing proteins, single nucleotide polymorphisms (SNPs) have been identified that may increase IBD susceptibility[32]. There are several lines of sensing bacterial components (described earlier), all of which result in activation of NF-κB, and thereby stimulate the innate immune response. In genes of TLR9 signaling, SNPs have been found that may increase IBD susceptibility[9,33]. Török et al[9] reported that a SNP in the promoter region of the TLR9 gene was associated with increased risk of Crohn’s disease in a German cohort. These genetic findings confirm an important role for innate immunity, pro- and anti-inflammatory immune responses for both gut homeostasis and the development of chronic inflammation in IBD.
Regarding the connection between polymorphisms in TLR9 genes and the risk of colorectal cancer no data are currently available.
The commensal microbiota of the intestinal tract confer multiple health benefits to the host, including amelioration of IBD. It was recently identified that TLR9-induced type 1 interferons mediate the anti-inflammatory effects in experimental colitis[34]. The addition of neutralization antibodies to type 1 interferons abolished the anti-inflammatory effects, whereas the administration of recombinant interferon-β mimicked the anti-inflammatory effects induced by TLR9 agonists.
The relapse of IBD may occur following an infection with Campylobacter jejuni (C. jejuni). In a murine model of dextran sulfate sodium (DSS) induced colitis, the infection of the animals by C. jejuni disrupted TLR9-induced reinforcement of the intestinal epithelial barrier and colonization by C. jejuni increased the severity of DSS-induced colitis[35].
In humans, the gene expression and protein expression level of not just TLR2, -4, and -8, but also TLR9 increased in the biopsy samples of active ulcerative colitis patients. Furthermore, the levels of these TLRs positively correlated with the severity of intestinal inflammation as well as with inflammatory cytokine production[36]. Based on these results, it is plausible that TLR9 mediated signaling influences and regulates the severity of the mucosal inflammation.
In colonic carcinogenesis the role of TLR9 signaling is not well studied. It was recently published that ODNs targeting TLR9 oppositely modulate DNA repair genes in tumor versus immune cells and enhance the biologic effects of chemotherapy[37]. The first publication about the relation of TLR9 expression to colonic carcinogenesis was also published nowadays[38]. Eiró et al[38] found TLR9 expression to be higher in hyperplastic or adenomatous polyps compared to other polyp types. TLR9 expression was decreased in hyperplastic and villous polyps from patients who developed colorectal cancer. Their findings suggest a possible protective role of TLR9 expression against malignant transformation in the colorectal mucosa.
The therapeutic targeting of TLRs may be useful in diseases such as tumors, allergies or viral infections. In these disorders, TLR agonists and antagonists result in a different immune response. In allergic diseases, like asthma or inflammatory conditions, such as IBD, these agents have an important effect on T cells. For a wider spectrum of anti-tumoral immune response TLR agonists in tumorous diseases require the involvement of the innate immunity, pDCs, monocytes and macrophages, as well as the activation of Th1-dependent immunity and induction of apoptosis[28].
In 2011, Rosa et al[39] demonstarted that an immunomodulatory oligonucleotide sequence (IMO) in combination with cetuximab has an antitumorous effect on a K-ras mutated colorectal carcinoma model. This is probably based on the alteration of MAPK phosphorylation and results in structural and functional changes in the relationship between epidermal growth factor receptor (EGFR) and TLR9[39]. They used a synthetic IMO having free 5’ end. The CpG DNA sequence had dimer structure, where the 3-3’ ends were connected by glycerin or 2’-deoxy-7-deazaguanosine modification. Mutation of the K-ras gene has a critical role in colon, lung and pancreatic cancers, and may cause a resistance to anti-EGFR therapy[40,41]. This is the reason why panitumumab and cetuximab therapy do not show a positive effect on the control of proliferation and metastasis of K-ras mutated colon cancer. This kind of biologic therapy could be only useful in the case of patients carrying the wild type K-ras gene[40].
It was shown in an in vivo murine xenograft model and in vitro human cancer cell lines (GEO, SW48 and LS174T) that IMOs can restore the therapy sensitivity for K-ras mutant colon and pancreatic cancers[40]. These cell lines, except GEO, were resistant for EGFR inhibition therapy, if they had a K-ras mutation. A small number of GEO cells carrying K-ras mutations showed sensitivity to anti-EGFR antibodies. This demonstrates that cells could carry a different K-ras mutation and could respond to EGFR inhibition therapy in a different way based on their K-ras status[39-41].
TLR9 agonists were also tested on a breast cancer cell line which was estrogen receptor positive[42]. After estrogen-TLR9 agonist combination the test showed significant reduction of transactivation via the estrogen receptor. Estrogen receptors may also take part in colorectal carcinogenesis[43,44], therefore, this interaction may have further therapeutic importance in colorectal cancer as well.
Currently, TLR9 agonist therapy has been tested clinically on colon, pancreatic and breast cancers[45-48], and experiments are running on oesophageal squamous cell cancer[49], melanomas[50], lymphomas[51,52], non-small cell lung carcinomas[53], renal tumors[47] and androgen resistant prostate cancers[54].
It has long been known that in IBD patients, antibodies against own or microbial antigens can be detected. Antibodies against Saccharomyces cerevisiae, outer membrane porin, Pseudomonas fluorescens, pancreas, bacterial flagelline as well as anti-chitobioside-, anti-laminaribioside-, and anti-mannobioside antibodies[55] have all been identified. These antibodies are recognized by PAMP and DAMP receptors. The most important members of these receptor families are the nucleotide oligomerization domain (NOD) - caspase recruitment domain (CARD) system (mainly NOD2 receptor in Crohn‘s disease) and TLRs. These receptors are localized in the intestinal mucosa, and by increased activation and genetic polymorphisms these receptors create an excessive immune response. At the end of the pathway pro-inflammatory cytokines are released, regulatory T cells are thought to loose their control function and the Th1/Th17 cell subpopulation becomes over-expressed[55,56-58].
Rachmilewitz et al[56] used IMOs in a DSS-induced colitis mouse model and found decreased IL-6, IL-12 and interferon mRNA levels. The levels of matrix metalloproteinases were found to be proportionally decreased. The immunological, clinical, biochemical and histological results showed decreased activity index of the inflammation. From these data one could suggest that the continuous presence of bacteria and bacterial DNA, which densely contain non-methylated CpG sequences, may act as a physiological factor. Furthermore, they could influence the release of inflammatory cytokines in IBD and thus may serve as a therapeutic tool[57].
A newly developed therapeutic agent is a synthetic DNA-based immunomodulatory sequence (DIMS0150), which acts through TLR9 signaling[57]. Based on the results of clinical trials, DIMS0150 seems to restore the steroid sensitivity of the mucosa in steroid-resistant ulcerative colitis patients. In the third phase of clinical trials, 71% of patients achieved remission after 12 weeks of administration of this drug[57]. Although it has no notable side effects, the mode of its administration, namely it has to be spread over the inflamed mucosa with the help of a spray catheter during colonoscopy, makes its use widely intolerable for patients. New ways of drug administration (i.e., colon solvent capsules) must be developed in the near future.
Based on the results of clinical trials[45-54], TLR9 agonists are therapeutically safe in vivo. Only some minor side effects, mainly a dose-dependent local inflammation of the connective tissue were observed.
Due to complex signaling of oligodeoxynucleotide binding TLRs (including TLR9) a dynamic regulation of pro- and anti-inflammatory cytokines is present[59]. Therefore, TLR9 antagonists and inhibitory oligodeoxynucleotides (inh-ODNs) also may represent new therapeutic options[60] in the treatment of autoimmune diseases. The mechanism of their action is by controlling and blocking the dangerous immune response activated by the self-antigen recognizing receptors. Interestingly, inh-ODNs have TLR9 (and TLR7) antagonist activity, but this effect is sequence dependent. These inhibitory oligonucleotides competitively inhibit TLR9 activation in a manner that competitively antagonizes the binding of ligands to the active, proteolytically cleaved TLR9 sequence. Their therapeutic use shows promise in systemic autoimmune diseases, DNA-mediated sepsis, and chronic inflammatory conditions (e.g., IBD) in which TLR9 plays an important role[60].
In the majority of in vivo studies mice were used as animal models for showing that CpG-ODNs are effective both as adjuvants and for therapeutic intervention in infectious and tumour model systems. However, one must be careful in translating the murine data into the human system, because of the differences between CpG-ODN-responsive cells in mice and humans. One major and important difference between mice and humans refers to the expression pattern of TLR9. In humans, only pDC and B cells express TLR9 and respond directly to TLR9 activation. All other effects of TLR9 ligands on human immune cells seem to be indirect and depend on factors produced by pDCs and B cells[61].
The situation in mice is different because not only pDCs and B cells, but other dendritic cell subsets and macrophages express TLR9 and thus respond directly to TLR9 activation[61]. Given this important species-specific difference in TLR9 expression, mice are not ideal animal models for establishing TLR9-based therapeutic strategies. The natural ligands for TLR9 can be mimicked by special CpG-ODNs[62].
Besides rodent models, a few studies have analysed the immune response of CpG-ODNs in other animals[62]. Guzylack-Piriou et al[63] demonstrated that pig pDCs are the main producers of interferon-α in response to certain CpG-ODNs. Importantly, they additionally showed that myeloid DCs and monocytes/macrophages are refractory to CpG-ODNs. Thus, the CpG-ODN responsiveness in pigs seems to mimic the situation in humans, and therefore recommends the pig as an animal model for preclinical studies with CpG-ODN.
Since the immunomodulatory effects of TLRs are known, they are the center of biological, immunological and therapeutic research. Most of the research teams are dealing with the potential therapeutic use of TLR9 agonists and antagonists because their use is not restricted to a specific group of patients. They can be widely applied in almost all diseases where dysregulated immunity plays a central role via antibody production or phagocytosis by macrophages.
TLR9 plays a central role in both innate and adaptive immunity. The signalling cascade mediated by CpG ODNs is a complicated pathway and contains many steps, including the synthesis of proinflamatory cytokines and the production of interferons, and thus significant activation of pDCs and T-lymphocytes. The activation of TLR9 acts as a new therapeutic modality in bacterial, viral, inflammatory and neoplastic diseases. In inflammatory circumstances, TLR9 agonists act by both decreasing the enormous immune activation, especially in IBD, and setting the balance of the Th1/Th2 immune response. They may have an effect on the suppression of Th1/Th17 overexpression as well. In tumorous conditions, especially in colorectal cancer, these agents were able to restore anti-EGFR therapy sensitivity caused by a K-ras mutation. They also seem to be effective therapeutic agents in estrogen receptor positive breast cancers, androgen-resistant prostate tumors, melanomas, lymphomas, large cell lung cancers and renal tumors. The side effects of TLR9 agonists are not significant. Further investigations of these new therapeutic modalities may have promising results in the near future.
P- Reviewer Spisni E S- Editor Wen LL L- Editor A E- Editor Zhang DN
| 1. | Wiemann B, Starnes CO. Coley’s toxins, tumor necrosis factor and cancer research: a historical perspective. Pharmacol Ther. 1994;64:529-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 306] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 2. | Tokunaga T, Yamamoto H, Shimada S, Abe H, Fukuda T, Fujisawa Y, Furutani Y, Yano O, Kataoka T, Sudo T. Antitumor activity of deoxyribonucleic acid fraction from Mycobacterium bovis BCG. I. Isolation, physicochemical characterization, and antitumor activity. J Natl Cancer Inst. 1984;72:955-962. [PubMed] |
| 3. | Yamamoto S, Yamamoto T, Shimada S, Kuramoto E, Yano O, Kataoka T, Tokunaga T. DNA from bacteria, but not from vertebrates, induces interferons, activates natural killer cells and inhibits tumor growth. Microbiol Immunol. 1992;36:983-997. [PubMed] |
| 4. | Yamamoto T, Yamamoto S, Kataoka T, Komuro K, Kohase M, Tokunaga T. Synthetic oligonucleotides with certain palindromes stimulate interferon production of human peripheral blood lymphocytes in vitro. Jpn J Cancer Res. 1994;85:775-779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 49] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4803] [Cited by in RCA: 4821] [Article Influence: 192.8] [Reference Citation Analysis (0)] |
| 6. | Jurk M, Vollmer J. Therapeutic applications of synthetic CpG oligodeoxynucleotides as TLR9 agonists for immune modulation. BioDrugs. 2007;21:387-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Sipos F, Muzes G. Isolated lymphoid follicles in colon: switch points between inflammation and colorectal cancer? World J Gastroenterol. 2011;17:1666-1673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Cashman SB, Morgan JG. Transcriptional analysis of Toll-like receptors expression in M cells. Mol Immunol. 2009;47:365-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Török HP, Glas J, Endres I, Tonenchi L, Teshome MY, Wetzke M, Klein W, Lohse P, Ochsenkühn T, Folwaczny M. Epistasis between Toll-like receptor-9 polymorphisms and variants in NOD2 and IL23R modulates susceptibility to Crohn’s disease. Am J Gastroenterol. 2009;104:1723-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Baumgart DC, Buning C, Geerdts L, Schmidt HH, Genschel J, Fiedler T, Gentz E, Molnar T, Nagy F, Lonovics J. The c.1-260C& gt; T promoter variant of CD14 but not the c.896A& gt; G (p.D299G) variant of toll-like receptor 4 (TLR4) genes is associated with inflammatory bowel disease. Digestion. 2007;76:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | De Jager PL, Franchimont D, Waliszewska A, Bitton A, Cohen A, Langelier D, Belaiche J, Vermeire S, Farwell L, Goris A. The role of the Toll receptor pathway in susceptibility to inflammatory bowel diseases. Genes Immun. 2007;8:387-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Davoodi H, Seow HF. Variant Toll-like receptor4 (Asp299Gly and Thr399Ile alleles) and Toll-like receptor2 (Arg753Gln and Arg677Trp alleles) in colorectal cancer. Iran J Allergy Asthma Immunol. 2011;10:91-99. [PubMed] |
| 13. | Slattery ML, Herrick JS, Bondurant KL, Wolff RK. Toll-like receptor genes and their association with colon and rectal cancer development and prognosis. Int J Cancer. 2012;130:2974-2980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 14. | O’Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: 10 years of progress. Immunol Rev. 2008;226:10-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 467] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 15. | Fischer M, Ehlers M. Toll-like receptors in autoimmunity. Ann N Y Acad Sci. 2008;1143:21-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 16. | Lee J, Mo JH, Katakura K, Alkalay I, Rucker AN, Liu YT, Lee HK, Shen C, Cojocaru G, Shenouda S. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat Cell Biol. 2006;8:1327-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 457] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 17. | Latz E, Schoenemeyer A, Visintin A, Fitzgerald KA, Monks BG, Knetter CF, Lien E, Nilsen NJ, Espevik T, Golenbock DT. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004;5:190-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1011] [Cited by in RCA: 1061] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 18. | Leifer CA, Kennedy MN, Mazzoni A, Lee C, Kruhlak MJ, Segal DM. TLR9 is localized in the endoplasmic reticulum prior to stimulation. J Immunol. 2004;173:1179-1183. [PubMed] |
| 19. | Latz E, Visintin A, Espevik T, Golenbock DT. Mechanisms of TLR9 activation. J Endotoxin Res. 2004;10:406-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Espevik T, Latz E, Lien E, Monks B, Golenbock DT. Cell distributions and functions of Toll-like receptor 4 studied by fluorescent gene constructs. Scand J Infect Dis. 2003;35:660-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003;85:85-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 803] [Cited by in RCA: 828] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 22. | Kanzler H, Barrat FJ, Hessel EM, Coffman RL. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat Med. 2007;13:552-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 648] [Cited by in RCA: 678] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 23. | Pinto A, Morello S, Sorrentino R. Lung cancer and Toll-like receptors. Cancer Immunol Immunother. 2011;60:1211-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Lamphier MS, Sirois CM, Verma A, Golenbock DT, Latz E. TLR9 and the recognition of self and non-self nucleic acids. Ann N Y Acad Sci. 2006;1082:31-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Häcker H, Mischak H, Miethke T, Liptay S, Schmid R, Sparwasser T, Heeg K, Lipford GB, Wagner H. CpG-DNA-specific activation of antigen-presenting cells requires stress kinase activity and is preceded by non-specific endocytosis and endosomal maturation. EMBO J. 1998;17:6230-6240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 493] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 26. | Zhu J, Mohan C. Toll-like receptor signaling pathways--therapeutic opportunities. Mediators Inflamm. 2010;2010:781235. [PubMed] |
| 27. | Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5:471-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 935] [Cited by in RCA: 986] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 28. | Klinman DM. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat Rev Immunol. 2004;4:249-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 781] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 29. | Liu Y, Luo X, Yang C, Yu S, Xu H. Three CpG oligodeoxynucleotide classes differentially enhance antigen-specific humoral and cellular immune responses in mice. Vaccine. 2011;29:5778-5784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Vollmer J, Weeratna R, Payette P, Jurk M, Schetter C, Laucht M, Wader T, Tluk S, Liu M, Davis HL. Characterization of three CpG oligodeoxynucleotide classes with distinct immunostimulatory activities. Eur J Immunol. 2004;34:251-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 460] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 31. | Krug A, Rothenfusser S, Hornung V, Jahrsdörfer B, Blackwell S, Ballas ZK, Endres S, Krieg AM, Hartmann G. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur J Immunol. 2001;31:2154-2163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 32. | Petermann I, Huebner C, Browning BL, Gearry RB, Barclay ML, Kennedy M, Roberts R, Shelling AN, Philpott M, Han DY. Interactions among genes influencing bacterial recognition increase IBD risk in a population-based New Zealand cohort. Hum Immunol. 2009;70:440-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Lammers KM, Ouburg S, Morré SA, Crusius JB, Gionchett P, Rizzello F, Morselli C, Caramelli E, Conte R, Poggioli G. Combined carriership of TLR9-1237C and CD14-260T alleles enhances the risk of developing chronic relapsing pouchitis. World J Gastroenterol. 2005;11:7323-7329. [PubMed] |
| 34. | Lee J, Rachmilewitz D, Raz E. Homeostatic effects of TLR9 signaling in experimental colitis. Ann N Y Acad Sci. 2006;1072:351-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | O’Hara JR, Feener TD, Fischer CD, Buret AG. Campylobacter jejuni disrupts protective Toll-like receptor 9 signaling in colonic epithelial cells and increases the severity of dextran sulfate sodium-induced colitis in mice. Infect Immun. 2012;80:1563-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Sánchez-Muñoz F, Fonseca-Camarillo G, Villeda-Ramírez MA, Miranda-Pérez E, Mendivil EJ, Barreto-Zúñiga R, Uribe M, Bojalil R, Domínguez-López A, Yamamoto-Furusho JK. Transcript levels of Toll-Like Receptors 5, 8 and 9 correlate with inflammatory activity in Ulcerative Colitis. BMC Gastroenterol. 2011;11:138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 37. | Sommariva M, De Cecco L, De Cesare M, Sfondrini L, Ménard S, Melani C, Delia D, Zaffaroni N, Pratesi G, Uva V. TLR9 agonists oppositely modulate DNA repair genes in tumor versus immune cells and enhance chemotherapy effects. Cancer Res. 2011;71:6382-6390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Eiró N, González L, González LO, Andicoechea A, Fernández-Díaz M, Altadill A, Vizoso FJ. Study of the expression of toll-like receptors in different histological types of colorectal polyps and their relationship with colorectal cancer. J Clin Immunol. 2012;32:848-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 39. | Rosa R, Melisi D, Damiano V, Bianco R, Garofalo S, Gelardi T, Agrawal S, Di Nicolantonio F, Scarpa A, Bardelli A. Toll-like receptor 9 agonist IMO cooperates with cetuximab in K-ras mutant colorectal and pancreatic cancers. Clin Cancer Res. 2011;17:6531-6541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 40. | Yu D, Kandimalla ER, Bhagat L, Tang JY, Cong Y, Tang J, Agrawal S. ‘Immunomers’--novel 3’-3’-linked CpG oligodeoxyribonucleotides as potent immunomodulatory agents. Nucleic Acids Res. 2002;30:4460-4469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 41. | Bardelli A, Siena S. Molecular mechanisms of resistance to cetuximab and panitumumab in colorectal cancer. J Clin Oncol. 2010;28:1254-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 533] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 42. | Qiu J, Wang X, Guo X, Zhao C, Wu X, Zhang Y. Toll-like receptor 9 agonist inhibits ERalpha-mediated transactivation by activating NF-kappaB in breast cancer cell lines. Oncol Rep. 2009;22:935-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | Rath-Wolfson L, Purim O, Ram E, Morgenstern S, Koren R, Brenner B. Expression of estrogen receptor β1 in colorectal cancer: correlation with clinicopathological variables. Oncol Rep. 2012;27:2017-2022. [PubMed] |
| 44. | Saleiro D, Murillo G, Benya RV, Bissonnette M, Hart J, Mehta RG. Estrogen receptor-β protects against colitis-associated neoplasia in mice. Int J Cancer. 2012;131:2553-2561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 45. | Brody JD, Ai WZ, Czerwinski DK, Torchia JA, Levy M, Advani RH, Kim YH, Hoppe RT, Knox SJ, Shin LK. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. J Clin Oncol. 2010;28:4324-4332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 408] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 46. | Thompson JA, Kuzel T, Drucker BJ, Urba WJ, Bukowski RM. Safety and efficacy of PF-3512676 for the treatment of stage IV renal cell carcinoma: an open-label, multicenter phase I/II study. Clin Genitourin Cancer. 2009;7:E58-E65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 47. | Hofmann MA, Kors C, Audring H, Walden P, Sterry W, Trefzer U. Phase 1 evaluation of intralesionally injected TLR9-agonist PF-3512676 in patients with basal cell carcinoma or metastatic melanoma. J Immunother. 2008;31:520-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 48. | Yamada K, Nakao M, Fukuyama C, Nokihara H, Yamamoto N, Sekine I, Kunitoh H, Ohe Y, Ohki E, Hashimoto J. Phase I study of TLR9 agonist PF-3512676 in combination with carboplatin and paclitaxel in patients with advanced non-small-cell lung cancer. Cancer Sci. 2010;101:188-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 49. | Katsuda M, Iwahashi M, Matsuda K, Miyazawa M, Nakamori M, Nakamura M, Naka T, Ojima T, Iida T, Yamaue H. [Peptide vaccine therapy with TLR-9 agonist for patients with esophageal squamous cell carcinoma]. Gan To Kagaku Ryoho. 2011;38:1942-1944. [PubMed] |
| 50. | Tarhini AA, Leng S, Moschos SJ, Yin Y, Sander C, Lin Y, Gooding WE, Kirkwood JM. Safety and immunogenicity of vaccination with MART-1 (26-35, 27L), gp100 (209-217, 210M), and tyrosinase (368-376, 370D) in adjuvant with PF-3512676 and GM-CSF in metastatic melanoma. J Immunother. 2012;35:359-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 51. | Kim YH, Gratzinger D, Harrison C, Brody JD, Czerwinski DK, Ai WZ, Morales A, Abdulla F, Xing L, Navi D. In situ vaccination against mycosis fungoides by intratumoral injection of a TLR9 agonist combined with radiation: a phase 1/2 study. Blood. 2012;119:355-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 163] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 52. | Zent CS, Smith BJ, Ballas ZK, Wooldridge JE, Link BK, Call TG, Shanafelt TD, Bowen DA, Kay NE, Witzig TE. Phase I clinical trial of CpG oligonucleotide 7909 (PF-03512676) in patients with previously treated chronic lymphocytic leukemia. Leuk Lymphoma. 2012;53:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 53. | Manegold C, van Zandwijk N, Szczesna A, Zatloukal P, Au JS, Blasinska-Morawiec M, Serwatowski P, Krzakowski M, Jassem J, Tan EH. A phase III randomized study of gemcitabine and cisplatin with or without PF-3512676 (TLR9 agonist) as first-line treatment of advanced non-small-cell lung cancer. Ann Oncol. 2012;23:72-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 54. | Rayburn ER, Wang W, Zhang Z, Li M, Zhang R, Wang H. Experimental therapy of prostate cancer with an immunomodulatory oligonucleotide: effects on tumor growth, apoptosis, proliferation, and potentiation of chemotherapy. Prostate. 2006;66:1653-1663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 55. | Henckaerts L, Pierik M, Joossens M, Ferrante M, Rutgeerts P, Vermeire S. Mutations in pattern recognition receptor genes modulate seroreactivity to microbial antigens in patients with inflammatory bowel disease. Gut. 2007;56:1536-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 56. | Rachmilewitz D, Karmeli F, Takabayashi K, Hayashi T, Leider-Trejo L, Lee J, Leoni LM, Raz E. Immunostimulatory DNA ameliorates experimental and spontaneous murine colitis. Gastroenterology. 2002;122:1428-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 267] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 57. | Musch E, Lutfi T, von Stein P, Zargari A, Admyre C, Malek M, Löfberg R, von Stein OD. Topical treatment with the toll-like receptor agonist DIMS0150 has potential for lasting relief of symptoms in patients with chronic active ulcerative colitis by restoring glucocorticoid sensitivity. Inflamm Bowel Dis. 2013;19:283-292. [RCA] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 58. | Liu L, Shen L, Liu X, Yu Y, Li Y, Wang L, He C, Sun J, Li B. A safety study of a B-class CpG ODN in Sprague-Dawley rats. J Appl Toxicol. 2012;32:60-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 59. | Chi H, Barry SP, Roth RJ, Wu JJ, Jones EA, Bennett AM, Flavell RA. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc Natl Acad Sci USA. 2006;103:2274-2279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 474] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 60. | Matesic D, Lenert A, Lenert P. Modulating toll-like receptor 7 and 9 responses as therapy for allergy and autoimmunity. Curr Allergy Asthma Rep. 2012;12:8-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 61. | Hochrein H, Wagner H. Of men, mice and pigs: looking at their plasmacytoid dendritic cells [corrected]. Immunology. 2004;112:26-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 62. | Krieg AM. CpG motifs: the active ingredient in bacterial extracts? Nat Med. 2003;9:831-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 204] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 63. | Guzylack-Piriou L, Balmelli C, McCullough KC, Summerfield A. Type-A CpG oligonucleotides activate exclusively porcine natural interferon-producing cells to secrete interferon-alpha, tumour necrosis factor-alpha and interleukin-12. Immunology. 2004;112:28-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 130] [Article Influence: 6.2] [Reference Citation Analysis (0)] |