Published online Jun 21, 2013. doi: 10.3748/wjg.v19.i23.3602
Revised: April 1, 2013
Accepted: April 18, 2013
Published online: June 21, 2013
Processing time: 234 Days and 0.1 Hours
AIM: To study an assessment of the number of enterochromaffin cells and expression of hydroxyindole-O-methyltransferase in colonic mucosa and urine excretion of 6-sulfatoxymelatonin in patients with ulcerative colitis.
METHODS: The study included 30 healthy subjects (group I-C), 30 patients with ulcerative proctitis [group II-ulcerative proctitis (UP)] and 30 patients with ulcerative colitis [group III-ulcerative colitis (UC)] in acute phases of these diseases. The number of enterochromaffin cells (EC) was estimated in rectal and colonic mucosa. Bioptates were assembled from many different parts of the large intestine. Immunorective cells collected from various parts of the colon were counted according to the Eurovision DAKO (Dako A/S, Copenhagen, Denmark) System in the range of 10 fields in each bioptate at × 200 magnification. The level of mRNA expression of hydroxyindole-O-methyltransferase (HIOMT) in colonic mucosa was estimated with RT-PCR. Urine 6-sulfatoxymelatonin (6-HMS) excretion was determined immunoenzymatically using an IBL (IBL International GmbH, Hamburg, Germany) kit (RE 54031).
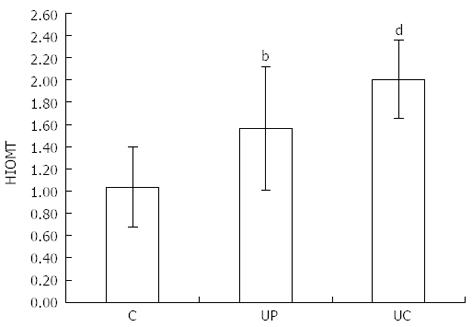
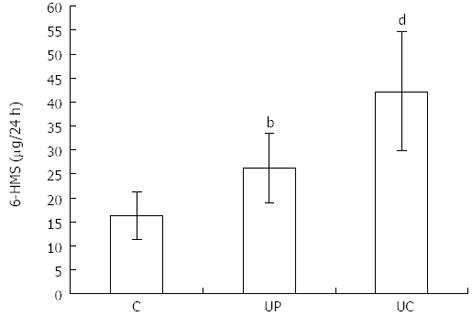
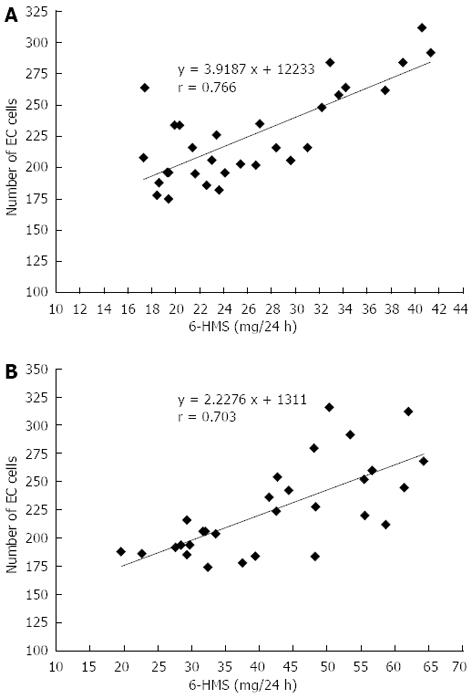
RESULTS: The number of EC cells in healthy subjects (C) was 132.40 ± 31.26. In patients of group II (UP) and group III (UC) the number of these cells was higher - 225.40 ± 37.35 (P < 0.001) and - 225.24 ± 40.50 (P < 0.001) respectively. Similar differences were related to HIOMT expression, which was 1.04 ± 0.36 in group C, 1.56 ± 0.56 (P < 0.01) in group UP and 2.00 ± 0.35 (P < 0.001) in group UC. Twenty-four hour 6-HMS urinary excretion was as follows: C - 16.32 ± 4.95 μg/24 h, UP - 26.30 ± 7.29 μg/24 h (P < 0.01), UC - 42.30 ± 12.56 μg/24h (P < 0.001). A correlation between number of EC cells and 6-HMS excretion was noted in all groups: r = 0.766 in patients with UP, r = 0.703 with UC and r = 0.8551 in the control group; the correlation between the results is statistically significant.
CONCLUSION: In the acute phases of both UP and UC, proliferation of EC cells and high expression of HIOMT and urine excretion of 6-HMS is noted. These changes may represent a beneficial response in the anti-inflammatory and defense mechanism.
Core tip: In the gastrointestinal tract melatonin is secreted mainly by enterochromaffin cells (EC). It appears that hydroxyindole-O-methyltransferase (HIOMT) is enzyme determining melatonin synthesis. This indoleamine exert, antioxidant, and anti-inflammatory effects. Its synthesis can be disturbed by pro-inflammatory cytokines. The obtained results noted the proliferation of EC cells and the increase of HIOMT expression in ulcerative colitis ulcerative colitis (UC). Positive correlation between the amount of EC cells and urinary 6-sulfatoxymelatonin excretion points to the increase of melatonin secretion in the colon, but its beneficial anti-inflammatory effect was insufficient. The results indicate that the supplementation of melatonin may be useful in complex treatment of UC.
- Citation: Chojnacki C, Wiśniewska-Jarosińska M, Kulig G, Majsterek I, Reiter RJ, Chojnacki J. Evaluation of enterochromaffin cells and melatonin secretion exponents in ulcerative colitis. World J Gastroenterol 2013; 19(23): 3602-3607
- URL: https://www.wjgnet.com/1007-9327/full/v19/i23/3602.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i23.3602
Ulcerative colitis (UC) is a chronic disease with periods of exacerbation and remission. Its pathogenesis is complex and inflammatory and immune factors, as well as toxic forms of oxygen produced in this condition, play a major role in the destruction of colonic mucosa[1].
The phase of exacerbation is characterized by, inter alia, the increased production of pro-inflammatory cytokines, cell adhesion molecules and acute-phase proteins. In addition, various defense mechanisms are activated, including increased production of specific antibodies and antioxidants. Melatonin is also a potent agent in antioxidative defense through its antioxidant and anti-inflammatory properties[2,3].
Melatonin (N-acetyl-5-methoxytryptamine) is a hormone produced by the cells of the neuroendocrine system located throughout the body amine precursor uptake and decarboxylation (APUD). It is produced from L-tryptophan, an exogenous amino acid, which is first converted in the body into serotonin with the involvement of tryptophan hydroxylase and 5-hydroxytryptophan decarboxylase. The serotonin is then converted to melatonin by arylalkylamine N-acetyltransferase (AANAT) and 5-hydroxyindole-O-methyltransferase hydroxyindole-O-methyltransferase (HIOMT)[4].
This indoleamine is secreted by pineal cells according to circadian rhythms, which are regulated mainly by light stimuli[5] and by enterochromaffin cells (EC) which are distributed throughout the whole gastrointestinal tract[6]. Melatonin from the gut is transported via the portal vein to the liver, where it is metabolized to 6-sulfatoxymelatonin (6-HMS)[7]. The amounts of urinary 6-HMS excretion are known to be indices of the synthesis and metabolism of this hormone[8].
Melatonin is secreted from the gastrointestinal tract under the influence of a range of stimuli, including nutritional factors, but the precise mechanisms regulating its release have not been recognized thoroughly. This hormone, released from enterochromaffin cells, fulfils important enteroprotective functions via the paraendocrine mechanism[9].
In inflammatory processes, melatonin inhibits nitric oxide and cyclooxygenase-2 synthesis and decreases the concentration of endogenous oxidants[10]. Furthermore, it induces other systems which in turn reduce oxygen free radicals: Superoxide dismutase and glutathione peroxidase. It also exerts a beneficial effect on microcirculation and tissue blood supply by inhibiting COX-2[11].
In experimental colitis in animals, the administration of exogenous melatonin decreased lipid, protein and nucleic acid oxidation, and also ameliorated epithelial damage and inflammatory infiltration[12,13]. In our studies, carried out on patients with ulcerative colitis, melatonin was demonstrated to reduce DNA damage and to stimulate the repair of hydrogen-peroxide-induced oxidative DNA damage in enterocytes[14]. Melatonin also has an influence on beneficial immune processes. Melatonin receptors have been identified on immunologically active cells. It also stimulates Th lymphocytes to produce IL-2 and IFN-γ, monocytes to produce IL-1, IL-6 and IL-12 and decreases TNF-α concentration and the expression of adhesion molecules (ICAM-1, P-selectin and MAdCAM-1)[15,16].
All these properties of melatonin can have a beneficial effect on the course of ulcerative colitis, as the mentioned pro-inflammatory factors play a significant role in the pathogenesis of this disease. Melatonin deficiency has been suggested to be one of the causes of upper digestive tract mucosal defects[17], whereas the results related to the colon are not consistent. The amount of melatonin secreted in an organism depends on the number of EC cells and their activity. Spiller et al[18] found an increased number of EC cells in rectal mucosa in patients with diarrhea predominant IBS. Similar observations were made by Osadchuk et al[19]. However, El-Salhy et al[20] demonstrated that patients with constipation-predominant IBS have a decreased number of EC cells in the colonic mucosa.
Some authors have tried to relate the changes observed in IBS patients to inflammatory bowel diseases but no reliable results have been obtained, either. Most of the researchers demonstrated that the number of EC cells increases in colonic mucosa in patients with ulcerative colitis[21-23]. In turn, others have detected a decreased number of EC cells in this group of patients[24,25]. These differences may result from a heterogeneous clinical and morphological evaluation of colonic mucosa.
The aim of the present study was to evaluate the number of EC cells, HIOMT expression in colonic mucosa and urinary 6-HMS excretion in patients with acute phase of ulcerative proctitis (UP) and ulcerative colitis (UC).
The study included 30 healthy subjects (control group I (C)-aged 32.4 ± 9.8 years), 26 patients with an acute phase of ulcerative proctitis [group II (UP)-aged 31.9 ± 11. 6 years] and 30 patients with ulcerative colitis (group III (UC)-aged 33.0 ± 16.9 years). The severity of the disease was classified according to modified Mayo Clinic Score[26]. Bioptates were collected from many different parts (10-16) of the rectum and colon close to erosions and ulcerations. Histopathological activity was expressed according to the criteria of truelove and richards[27].
To determine the number of enterochromaffin cells in the bioptates, an immunohistochemical method was used with rabbit polyclonal antibodies (PSE) at a dilution of 1: 200 (Eurodiagnostica). Immunoreactive cells were counted with a computer according to the Eurovision DAKO system, in the range of 10 fields in each bioptate at × 200 magnification.
The level of mRNA expression of HIOMT was estimated with RT-PCR, and for this purpose, 50 mg of colonic tissue was used. Total RNA was isolated with trizol (Gibco) reagents, and then purified with DNase using gigagen RNeasy mini kit. The quantity and quality of RNA was estimated by using spectrophotometry. The obtained extract was used as a matrix in analyses of gene expression. cDNA synthesis was performed with Oligo (dT) 12-18 in an MJ Research PTG-1000 thermocycler. cDNA was obtained in reverse transcription and applied as the matrix for a PCR reaction incorporating selected fragments of the analyzed gene. The hypoxanthine phosphoribosyltransferase gene was the quantitative marker for the evaluation of the activity of the selected genes.
The reaction products were first separated on 6%- 10% polyacrylamide gel stained with ethidium bromide and then subjected to densitometry to determine the reaction efficacy and the level of mRNA of the investigated genes. The expression of the investigated genes was compared to HPRT gene product to normalize the expression.
The urine concentration of 6-HMS was determined immunoenzymatically using an IBL kit (RE54031).
On the day of testing excretion of 6-HMS through the urine, the patients remained in a room in which white light was off at night, and they received condensed liquid meals (Nutridrink-400 mL there times a day) with a total energy value of 1800 kcal and drank 1500 mL non-carbonated isotonic mineral water. After completion of 24-hour urine collection, the urine was centrifuged and the samples were stored at -70 °C. The 6-HMS concentration of the urine was determined immunoenzymatically using a Immuno-Biological Laboratories kit (No. RE54031). The measurements were performed by photometry at a wavelength of 450 nm using an Expert 96 reader (Biogent).
The study was conducted in accordance with the Declaration of Helsinki and the principles of Good Clinical Practice. Written consent was obtained from each patient enrolled in the study and the study protocol was approved by Bioethics Committee of the Medical University of Lodz (RNN/242/06/KB).
The non-parametric Krushal-Wallis test was used to evaluate the number of enterochromaffin cells, as well as the expression of HIOMT and urinary 6-HMS excretion in the three groups: C, UP and UC. The Mann-Whitney Test was used for comparison of median values. The correlation between the number of EC cells and urinary 6-HMS extraction was estimated by the determination of Pearson’s correlation coefficient and linear regression equation. The differences between the results was regarded as significant at a P value 0.05-0.001. Statistica 9.0 (StatSoft, Inc., United States) and MS Excel 2007 (Microsoft Co, United States) were used for statistical analysis.
The mean number of colonic EC cells in 10 fields of view in healthy subjects (group I) was 132.40 ± 31.26. However, in patients with ulcerative colitis, the number was twice as high: 225.40 ± 37.35 (P < 0.001) in the rectal mucosa (group II) and - 225.24 ± 40.50 (P < 0.001) in patients with pancolitis (group III) (Figure 1).
Similar differences were related to HIOMT expression: 1.04 ± 0.36 in healthy subjects, 1.56 ± 0.56 (P < 0.01) in proctitis and 2.00 ± 0.35 (P < 0.001) in pancolitis (Figure 2).
Urinary 6-HMS excretion in healthy subjects was 16.32 ± 4.95 and it was lower than in patients with proctitis - 26.30 ± 7.29 (P < 0.01) and with pancolitis - 42.30 ± 12.56 (P < 0.001; Figure 3).
Urinary 6-HMS excretion was found to be dependent on the number of EC cells in all groups: In healthy subjects, r = 0.855, and in patients with proctitis, r = 0.766 (Figure 4A). The number of EC cells was found to have a particularly strong positive correlation with urinary 6-HMS excretion in patients with pancolitis r = 0.703 (Figure 4B).
The obtained results confirm earlier observations, which indicate that EC cell proliferation is present in the active phase of ulcerative colitis, regardless of the location of inflammatory changes in colonic mucosa[21-23]. Additional observations found in this group of patients are firstly, an increase of HIOMT expression in the colon and secondly, a high level of urinary 6-HMS excretion. All these observations indicate increased activity of the melatonergic system in patients with ulcerative colitis. The increase of urinary 6-HMS excretion, in particular, is recognized to be the correct indicator of total melatonin production and content in the whole organism.
This pool of melatonin, which includes both the intestinal and pineal components, which representnearly the whole amount, is metabolized in the liver. Nevertheless, the positive correlation between the number of EC cells and level of urinary 6-HMS excretion indicates an increase of melatonin production mainly in the colon, as confirmed by the high HIOMT expression, compared to healthy subjects. However, it should be emphasized that melatonin catabolism both in the liver and in the colon can be modified by other factors; an excessive increase of pro-inflammatory cytokines in the colonic wall can disturb metabolism of this hormone.
Furthermore, due to the “consumption” of melatonin in the reaction with oxygen free radicals at the infection site, and its metabolism in the cells of the immune system, other metabolites such as 2-hydroxymelatonin, 3-hydroxymelatonin can be formed[28,29]. In turn, amino salicylates and immunosuppressive drugs applied in the treatment of ulcerative colitis exert a hepatotoxic effect and can also impair the melatonin metabolism in hepatocytes.
Due to these reasons, and probably also to the profound destruction of the colonic mucosa, decreased urinary 6-HMS excretion was observed in patients with severe ulcerative colitis. In less severe forms of the disease, these values were higher[30]. Therefore, it should be acknowledged that inflammatory processes in the colon are accompanied by increased secretion of enteral melatonin. This hormone plays a crucial enteroprotective role and its increase in ulcerative colitis is an important defense mechanism.
However, these beneficial changes in melatonin homeostasis are not sufficient to inhibit the inflammatory process and to obtain spontaneous remission of the disease. Remission requires the administration of many medications, including anti-inflammatory ones and immunosuppressants, because the pathogenesis of ulcerative colitis is complex and conditioned by numerous pro-inflammatory factors.
Serotonin is one such factor. It is secreted by the same EC cells and it is the precursor of melatonin. EC cell proliferation can, at the same time, lead to increase of serotonin secretion. The balance between serotonin and melatonin depends on the expression of enzymes regulating their synthesis and catabolism. Previous studies have not given unequivocal results. Carpuso et al[31] and Magro et al[32] found decreased serotonin concentration in colonic mucosa in patients with ulcerative colitis. However, in experimental animals with ulcerative colitis, a significant increase was observed both of EC cells and serotonin concentration in the colonic mucosa[33,34].
Regardless of the factors which contribute to the maintenance of the inflammatory process, the increase of melatonin secretion is a beneficial reaction. In our earlier studies, exogenous melatonin was also found to exert a positive effect on maintaining the remission of ulcerative colitis[35], which is the main goal of pharmacotherapy in this disease.
In conclusion, EC proliferation, HIOMT expression and increased urine excretion of 6-HMS is seen in the acute phases of ulcerative proctitis and ulcerative colitis. Consequently, the increase of enteral melatonin secretion is a beneficial response in antiinflammatory and defense mechanisms.
Melatonin is synthesized mainly by pinealocytes and by enterochromaffin cells in the gastrointestinal tract. This indoleamine displays endocrine and paracrine properties which account for enteroprotective action. Particular, melatonin and its metabolites are powerful antioxidants.
It is known that melatonin deficiency plays an important role in the pathogenesis of certain gastrointestinal diseases, such as gastroesophageal diseases, duodenal ulcer and functional disorders, dyspepsia, irritable bowel syndrome and others. Melatonin homeostasis in inflammatory bowel diseases is not determined and the results of some researches are not unequivocal.
It is believed to be the first study of report an association between the number of enterochromaffin cells, expression of hydroxyindole-O-methyltransferase (HIOMT) and urine excretion of 6-sulfatoxymelatonin (6-HMS) in patients with ulcerative colitis.
Evaluation of 24-h urinary excretion of 6-sulfatoxymelatonin may be a useful method for the estimation of melatonin secretion and intensity of inflammatory process in colon mucosa in patients with acute phase of ulcerative colitis. During non active phase of this disease secretion of melatonin is probably decreased and the supplementation of melatonin may by useful in complex treatment to maintain the remission.
Melatonin is secreted by enterochromaffin cells (EC) in the gastrointestinal tract under the effect of HIOMT but the mechanism regulating its release have not been recognized thoroughly. In the gastrointestinal tract melatonin fulfils important enteroprotective role via paraendocrine activity. Melatonin from the gut is transported via portal vain to the liver where is metabolized mainly to 6-HMS. The amounts of the urinary 6-HMS excretion are recognized indices of the synthesis and metabolism of this hormone.
The authors evaluated the melatonin synthetic pathway in EC in the colonic mucosa in patients with ulcerative proctitis and ulcerative colitis. This is novel paper reporting an important role of melatonin in the injured gastrointestinal tract. The role showed that during acute phase of this disease there was a proliferation of EC cells accompanied with elevated expression of HIOMT and urinary excretion of 6-HMS. The authors concluded that the response of melatonin synthesis may account for a beneficial response against the inflammatory process.
P- Reviewers Acuna-Castroviejo D, Shirazi A S- Editor Huang XZ L- Editor A E- Editor Zhang DN
| 1. | Kruidenier L, Kuiper I, Lamers CB, Verspaget HW. Intestinal oxidative damage in inflammatory bowel disease: semi-quantification, localization, and association with mucosal antioxidants. J Pathol. 2003;201:28-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 281] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 2. | Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, Martín V, Reiter RJ. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res. 2004;36:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1419] [Cited by in RCA: 1454] [Article Influence: 69.2] [Reference Citation Analysis (0)] |
| 3. | Radogna F, Diederich M, Ghibelli L. Melatonin: a pleiotropic molecule regulating inflammation. Biochem Pharmacol. 2010;80:1844-1852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 257] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 4. | Brzezinski A. Melatonin in humans. N Engl J Med. 1997;336:186-195. [PubMed] |
| 5. | Stehle JH, Saade A, Rawashdeh O, Ackermann K, Jilg A, Sebestény T, Maronde E. A survey of molecular details in the human pineal gland in the light of phylogeny, structure, function and chronobiological diseases. J Pineal Res. 2011;51:17-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 316] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 6. | Bubenik GA. Gastrointestinal melatonin: localization, function, and clinical relevance. Dig Dis Sci. 2002;47:2336-2348. [PubMed] |
| 7. | Bubenik GA, Pang SF, Cockshut JR, Smith PS, Grovum LW, Friendship RM, Hacker RR. Circadian variation of portal, arterial and venous blood levels of melatonin in pigs and its relationship to food intake and sleep. J Pineal Res. 2000;28:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Ma X, Idle JR, Krausz KW, Gonzalez FJ. Metabolism of melatonin by human cytochromes p450. Drug Metab Dispos. 2005;33:489-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 233] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 9. | Reiter RJ, Tan DX, Mayo JC, Sainz RM, Leon J, Czarnocki Z. Melatonin as an antioxidant: biochemical mechanisms and pathophysiological implications in humans. Acta Biochim Pol. 2003;50:1129-1146. [PubMed] |
| 10. | Deng WG, Tang ST, Tseng HP, Wu KK. Melatonin suppresses macrophage cyclooxygenase-2 and inducible nitric oxide synthase expression by inhibiting p52 acetylation and binding. Blood. 2006;108:518-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 196] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 11. | Dong WG, Mei Q, Yu JP, Xu JM, Xiang L, Xu Y. Effects of melatonin on the expression of iNOS and COX-2 in rat models of colitis. World J Gastroenterol. 2003;9:1307-1311. [PubMed] |
| 12. | Cuzzocrea S, Mazzon E, Serraino I, Lepore V, Terranova ML, Ciccolo A, Caputi AP. Melatonin reduces dinitrobenzene sulfonic acid-induced colitis. J Pineal Res. 2001;30:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Necefli A, Tulumoğlu B, Giriş M, Barbaros U, Gündüz M, Olgaç V, Güloğlu R, Toker G. The effect of melatonin on TNBS-induced colitis. Dig Dis Sci. 2006;51:1538-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Chojnacki J, Wiśniewska-Jarosińska M, Śliwiński . Błasiak J, Chojnacki C. Melatonin modulates DNA damage and repair in colonocytes of subjects with ulcerative colitis. Pol Gastroenterol. 2011;18:67-71. |
| 15. | Mei Q, Yu JP, Xu JM, Wei W, Xiang L, Yue L. Melatonin reduces colon immunological injury in rats by regulating activity of macrophages. Acta Pharmacol Sin. 2002;23:882-886. [PubMed] |
| 16. | Mazzon E, Esposito E, Crisafulli C, Riccardi L, Muià C, Di Bella P, Meli R, Cuzzocrea S. Melatonin modulates signal transduction pathways and apoptosis in experimental colitis. J Pineal Res. 2006;41:363-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Klupińska G, Wiśniewska-Jarosińska M, Harasiuk A, Chojnacki C, Stec-Michalska K, Błasiak J, Reiter RJ, Chojnacki J. Nocturnal secretion of melatonin in patients with upper digestive tract disorders. J Physiol Pharmacol. 2006;57 Suppl 5:41-50. [PubMed] |
| 18. | Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, Neal KR. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 805] [Cited by in RCA: 817] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 19. | Osadchuk AM, Osadchuk MA, Balashov AV, Kvetnoĭ IM. [The role of diffuse endocrine system and colonocytes cellular renovation in formation of clinical variants of irritable colon syndrome in young persons]. Klin Med (Mosk). 2008;86:33-37. [PubMed] |
| 20. | El-Salhy M, Norrgård O, Spinnell S. Abnormal colonic endocrine cells in patients with chronic idiopathic slow-transit constipation. Scand J Gastroenterol. 1999;34:1007-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Shen B, Liu W, Remzi FH, Shao Z, Lu H, DeLaMotte C, Hammel J, Queener E, Bambrick ML, Fazio VW. Enterochromaffin cell hyperplasia in irritable pouch syndrome. Am J Gastroenterol. 2008;103:2293-2300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | El-Salhy M, Danielsson A, Stenling R, Grimelius L. Colonic endocrine cells in inflammatory bowel disease. J Intern Med. 1997;242:413-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 161] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Rybakova MG, Botina AV, Solov’eva OI. [Immunomorphological characteristics of mucosal and endocrine cells of the colon in patients with chronic ulcerative colitis]. Arkh Patol. 2005;67:30-33. [PubMed] |
| 24. | Ahonen A, Kyösola K, Penttilä O. Enterochromaffin cells in macrophages in ulcerative colitis and irritable colon. Ann Clin Res. 1976;8:1-7. [PubMed] |
| 25. | Kyösola K, Penttilä O, Salaspuro M. Rectal mucosal adrenergic innervation and enterochromaffin cells in ulcerative colitis and irritable colon. Scand J Gastroenterol. 1977;12:363-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 78] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Osada T, Ohkusa T, Yokoyama T, Shibuya T, Sakamoto N, Beppu K, Nagahara A, Otaka M, Ogihara T, Watanabe S. Comparison of several activity indices for the evaluation of endoscopic activity in UC: inter- and intraobserver consistency. Inflamm Bowel Dis. 2010;16:192-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Truelove SC, Richards WC. Biopsy studies in ulcerative colitis. Br Med J. 1956;1:1315-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 271] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J Pineal Res. 2007;42:28-42. [PubMed] |
| 29. | Konturek SJ, Konturek PC, Brzozowska I, Pawlik M, Sliwowski Z, Cześnikiewicz-Guzik M, Kwiecień S, Brzozowski T, Bubenik GA, Pawlik WW. Localization and biological activities of melatonin in intact and diseased gastrointestinal tract (GIT). J Physiol Pharmacol. 2007;58:381-405. [PubMed] |
| 30. | Boznańska P, Wiśniewska-Jarosińska M, Chojnacki J. 6-hydroxymelatonin sulfate urine concentration in patients with ulcerative colitis. Gut. 2006;55:A109. |
| 31. | Capurso L, Friedmann CA. Distribution of 5-OH tryptamine (serotonin) in ulcerative colitis. Proc R Soc Med. 1970;63 Suppl:20-21. [PubMed] |
| 32. | Magro F, Vieira-Coelho MA, Fraga S, Serrão MP, Veloso FT, Ribeiro T, Soares-da-Silva P. Impaired synthesis or cellular storage of norepinephrine, dopamine, and 5-hydroxytryptamine in human inflammatory bowel disease. Dig Dis Sci. 2002;47:216-224. [PubMed] |
| 33. | Oshima S, Fujimura M, Fukimiya M. Changes in number of serotonin-containing cells and serotonin levels in the intestinal mucosa of rats with colitis induced by dextran sodium sulfate. Histochem Cell Biol. 1999;112:257-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 88] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 34. | Linden DR, Chen JX, Gershon MD, Sharkey KA, Mawe GM. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2003;285:G207-G216. [PubMed] |
| 35. | O’Hara JR, Ho W, Linden DR, Mawe GM, Sharkey KA. Enteroendocrine cells and 5-HT availability are altered in mucosa of guinea pigs with TNBS ileitis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G998-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 94] [Article Influence: 4.5] [Reference Citation Analysis (0)] |