Published online Jun 7, 2013. doi: 10.3748/wjg.v19.i21.3358
Revised: March 1, 2013
Accepted: March 15, 2013
Published online: June 7, 2013
Processing time: 139 Days and 19.3 Hours
Solid pseudopapillary neoplasm (SPN) is a rare and low-grade malignant pancreatic neoplasm composed of poorly cohesive monomorphic neoplastic cells forming solid and pseudopapillary structures with frequent hemorrhagic-cystic degeneration. Intraductal papillary mucinous neoplasm (IPMN) is a pancreatic exocrine tumor composed of intraductal papillary growth of mucin containing neoplastic cells in the main pancreatic duct or its major branches. In the case presented here, a 53-year-old, Japanese man was found to have multiple cystic lesions and dilatation of the main pancreatic duct in the neck of the pancreas. Histological examination revealed a main-duct and branch-duct type IPMN, of the gastric-type, involving the neck of the pancreas, associated with a 0.5 cm SPN in the caudal side of the IPMN. We diagnosed this case as synchronous SPN and IPMN. As far as we know, only one other case of synchronous SPN and IPMN has been reported. Both the present case and the previously reported case showed abnormal nuclear expression of β-catenin in SPN, whereas IPMN showed no abnormal nuclear expression. These results suggest that β-catenin abnormality is not a common pathogenetic factor of synchronous SPN and IPMN.
Core tip: We report the second case of synchronous solid pseudopapillary neoplasm (SPN) and intraductal papillary mucinous neoplasm (IPMN) of the pancreas in a 53-year-old Japanese man. IPMN was classified as a “combined” type due to the involvement of both the main and branch ducts, and it showed a gastric subtype with low-grade dysplasia. Adjacent to the caudal side of IPMN, a 0.5-cm solid SPN was present. The SPN showed abnormal nuclear expression of β-catenin in SPN, whereas IPMN showed no abnormal nuclear expression. This result suggested that β-catenin abnormality is not a common pathogenetic factor of synchronous SPN or IPMN.
- Citation: Hirabayashi K, Zamboni G, Ito H, Ogawa M, Kawaguchi Y, Yamashita T, Nakagohri T, Nakamura N. Synchronous pancreatic solid pseudopapillary neoplasm and intraductal papillary mucinous neoplasm. World J Gastroenterol 2013; 19(21): 3358-3363
- URL: https://www.wjgnet.com/1007-9327/full/v19/i21/3358.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i21.3358
Solid pseudopapillary neoplasm (SPN) is a rare and low-grade malignant pancreatic neoplasm composed of poorly cohesive, monomorphic cells forming solid and pseudopapillary structures with frequent hemorrhagic-cystic degeneration, which predominantly occurs in young women. Immunohistochemically, SPN usually expresses vimentin, α-1-antitrypsin, CD56, progesterone receptor, CD10, and nuclear/cytoplasmic β-catenin[1-3].
Intraductal papillary mucinous neoplasm (IPMN) is a macroscopic pancreatic exocrine tumour that grows primarily within the main pancreatic duct or its major branches, and mainly occurs in older men. IPMNs are composed of intraductal papillary growth of mucin-containing neoplastic cells that are classified into 4 major subgroups: gastric, intestinal, pancreatobiliary, and oncocytic types[4,5]. Gastric-type IPMN consists of innocuous, tall columnar cells that resemble gastric foveolar epithelium and express mucin (MUC) 5AC, but are negative for MUC1, MUC2, and CDX2[4,5]. Intestinal-type IPMN consists of neoplastic cells that resemble intestinal villous neoplasms with MUC2, MUC5AC, and CDX2 expression, but without MUC1 expression[4,5]. Pancreatobiliary-type IPMN consists of thin, branching papillae with high-grade dysplasia and is at least focally positive for MUC1 and MUC5AC, but is negative for MUC2 or CDX2[4,5]. Oncocytic-type IPMN is characterised by complex and arborizing papillae lined by cuboidal cells with granular eosinophilic cytoplasm, which usually express MUC5AC and MUC6, but are negative for MUC2 and CDX2[4,5].
Although IPMNs have been reported to occur synchronously with a variety of primary pancreatic neoplasms such as pancreatic ductal adenocarcinomas, endocrine neoplasms, or serous cyst adenomas[6-9], the synchronous occurrence of SPN with other pancreatic neoplasms has only been reported in a single case report associated with an IPMN[10]. In the current study, we present the histological and clinical features of a second case of synchronous “early” SPN and IPMN, with a review of the literature.
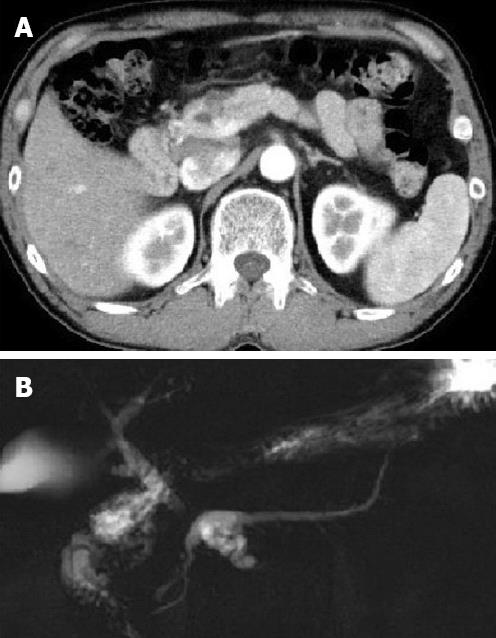
A 53-year-old Japanese man with multiple pancreatic cystic lesions, which were detected at a local hospital, was referred to the Tokai University Hospital for further examination. Upon inspection, he had no complaints, and no abnormal physical findings were reported. Laboratory data, including tumour markers, carcinoembryonic antigen, or carbohydrate antigen 19-9, showed no abnormal findings. Computed tomography showed multiple cystic lesions measuring 2.3 cm in the neck of the pancreas (Figure 1A). Magnetic resonance cholangiopancreatography imaging showed multiple cystic dilations of the pancreatic branch ducts connecting to the dilated main pancreatic duct in the neck of the pancreas (Figure 1B). Endoscopic retrograde pancreaticography showed a dilated main pancreatic duct in the neck of the pancreas, measuring 6 mm in diameter, with multiple nodular defects. Distal pancreatectomy and splenectomy were performed. At present, the patient is alive and well 25 mo after the operation.
The resected specimen was fixed in 10% formalin, routinely embedded in paraffin, sectioned at 4-μm, and stained with haematoxylin and eosin.
The sections were deparaffinised in xylene and rehydrated in a graded ethanol series. The primary antibodies and their dilutions and sources are summarized in Table 1. If necessary, antigen retrieval was performed by autoclaving the samples for 15 min in a sodium citrate buffer at pH 6.0 (β-catenin, CD56, chromogranin A, E-cadherin, MUC1, MUC2, MUC5AC, MUC6, vimentin, and Ki67) or by treatment with 0.1% trypsin at 37 °C for 30 min (synaptophysin). The antibodies were detected using EnVision Plus (Dako, Glostrup, Denmark; for β-catenin, CD56, chromogranin A, E-cadherin, MUC1, MUC2, MUC5AC, MUC6, vimentin, synaptophysin, and Ki67), or Horseradish Peroxidase-conjugated polyclonal goat anti-rabbit immunoglobulin (Dako; for alpha-1-antitryosin), with 3,3’-diaminobenzidine as the chromogen. Assays for pancytokeratin, progesterone receptor (PR), and CD10 were performed using Ventana Benchmark XT (Ventana Medical Systems, Tucson, AZ, United States) equipped with the Ultra View Detection Kit. Antigen retrieval was performed by treatment with CC1 (Ventana; for PR and CD10) or protease 1 (Ventana; for pancytokeratin).
| Clone | Source | Dilution | IPMN | SPN | |
| Pancytokeratin | AE1, AE3, and PCK26 | Ventana | Prediluted | (+) | (-) |
| Alpha-1 anti-trypsin | Polyclonal | Dako | 1:100 | (-) | (+) |
| CD10 | 56C6 | Leica | 1:50 | (-) | (+) |
| CD56 | 1B6 | Leica | 1:30 | (-) | (+) |
| MUC1 | Ma695 | Leica | 1:50 | (-) | (-) |
| MUC2 | Ccp58 | Leica | 1:100 | (-) | (-) |
| MUC5AC | CLH2 | Leica | 1:100 | (+) | (-) |
| MUC6 | CLH5 | Leica | 1:100 | (+) | (-) |
| Vimentin | V9 | Dako | 1:100 | (-) | (+) |
| Synaptophysin | 27G12 | Leica | 1:50 | (-) | (-/+) |
| Chromogranin A | DAK-A3 | Dako | 1:20 | (-) | (-) |
| β-catenin1 | 17C2 | Leica | 1:100 | (-) | (+) |
| E-cadherin | 36B5 | Leica | 1:20 | (+) | (-) |
| Progesterone receptor | 1E2 | Ventana | Prediluted | (-) | (+) |
| Ki67 | MIB1 | Dako | 1:50 | < 1% | < 1% |
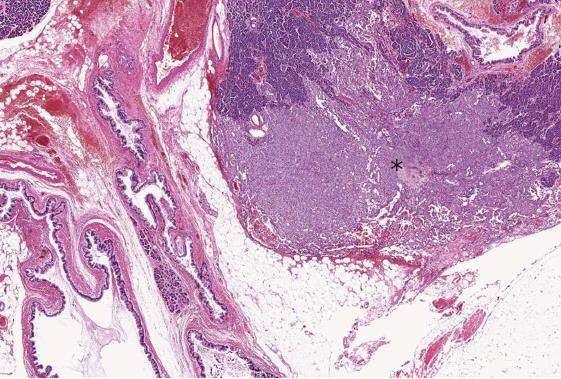
On gross examination, the distal pancreatectomy specimen showed a dilatation of the main pancreatic duct, with multiple cystic dilatations of the branch ducts, filled with mucus and containing papillae, in the neck of the pancreas. A whitish and solid lesion measuring 0.5 cm × 0.3 cm in size, located adjacent to the caudal side of the cystically dilated branch ducts, was identified.
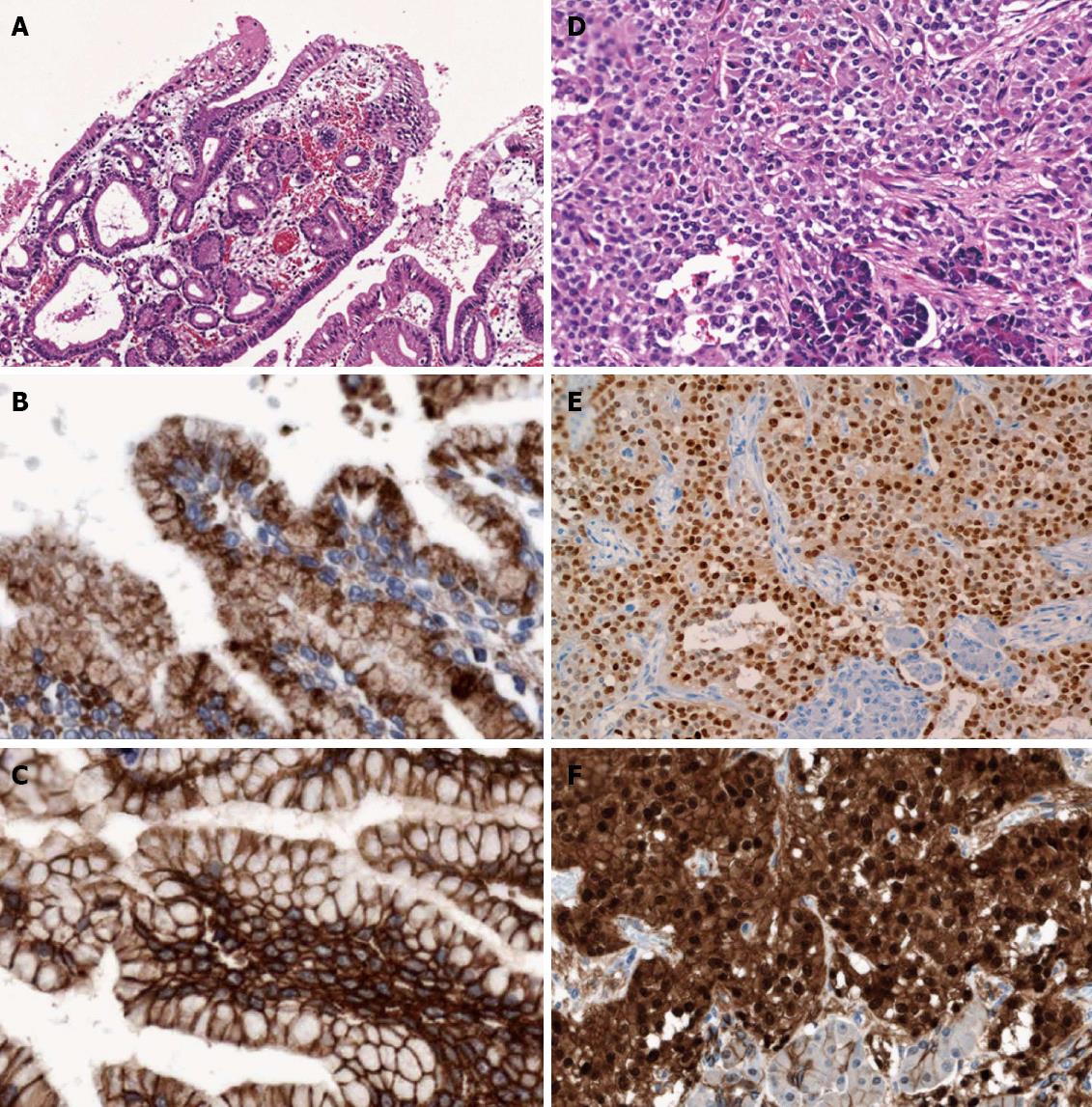
Microscopically, 2 distinct primary pancreatic neoplasms were present (Figure 2): (1) a typical central and branch-duct type IPMN, with the cyst lined by tall and pale columnar cells with only low-grade dysplasia, and pyloric-gland adenoma-like papillary projections (Figure 3A). Immunohistochemically, the neoplastic cells showed gastric-type phenotype with MUC5AC (Figure 3B) and MUC6 positivity, and MUC1 and MUC2 negativity. β-catenin immunostaining showed membranous expression, but did not show abnormal nuclear expression (Figure 3C). On the basis of current World Health Organization (WHO) criteria[4], we diagnosed the patient with gastric-type IPMN with low-grade dysplasia; and (2) a small, SPN, adjacent to the IPMN, which showed an ill-defined demarcation, with focal extension to the pancreatic parenchyma. The tumor showed a prevalent solid pattern, with focal degenerative changes and rough pseudopapillary structures (Figure 3D). The neoplastic cells had eosinophilic cytoplasm and small, round nuclei with finely dispersed chromatin. We failed to find perineural infiltration, peripancreatic invasion, or calcification. Immunohistochemically, the neoplastic cells expressed CD56, vimentin, alpha-1-antitrypsin, CD10, progesterone receptor (Figure 3E), nuclear/cytoplasmic β-catenin (Figure 3F), focal positivity for synaptophysin and negativity for chromogranin A, and E-cadherin and cytokeratin AE1 and 3. The immunohistochemical profiles of the 2 neoplasms are summarized in Table 1.
As our results show, the SPN was located near the IPMN. However, neither transition nor collision between IPMN and SPN were identified. Therefore, we diagnosed the present case as synchronous SPN and IPMN of the pancreas.The surgical margins were free of both types of tumors. No lymph node metastasis was identified.
We reported a case of synchronous small, radiologically undetected SPN, in a patient operated on for a radiologically evident IPMN of the pancreas. To our knowledge, this association has only been previously reported by Imamura et al[10]. They reported a patient who presented with a 3-cm, solid SPN in the head of the pancreas that invaded the mesocolon with a synchronous IPMN constituted by 2 multilocular cystic lesions in the head of the pancreas. In other words, the 2 cases showed mirror clinicopathologic traits.
In general, SPN occurs predominantly in young women (91%) and is rare in men (9%)[11]. Interestingly, however, both our case and Imamura’s occurred in men[10]. Several authors have reported the clinicopathologic correlations between genders and SPN. Takahashi et al[12] reported that the cases of SPN in men tend to exhibit solid components without prominent degenerative changes. Tien et al[13] reported no significant differences in patient age, size, neoplasm location, or malignancy rate between the genders in SPN. By contrast, Machado et al[14] reported that cases of SPN in men are more aggressive and that the patients are older. According to the literature review of 1014 SPN patients (women, 877; men, 137) by Lin et al[15], men were on average 5 years older, had a twofold higher incidence of metastases (women, 4.3%; men, 10.2%) and invasive malignancy (women, 12.4%; men, 24.4%), and showed a threefold higher death rate (women, 3.6%; men, 11.4%). There is no difference in immunohistochemical profiles between the genders in SPN cases[12,13].
The SPN reported by Imamura et al[10] showed a solid appearance with calcification, but no cystic degeneration. Although our case showed a prevalent solid pattern, cystic degeneration with haemorrhage was only observed microscopically; whereas foci of calcification were not present. The Imamura’s SPN case was considered malignant for the presence of perineural infiltration, the invasion of the anterior peripancreatic soft tissue, adipose tissue of the transverse mesocolon, and the duodenum[10]. By contrast, our case was limited to the pancreas, without peripancreatic extension or perineural infiltration. However, since it was not proven to be a predictor of malignancy, the current WHO classification considers all SPNs as low-grade malignant neoplasms[4].
SPNs usually form large masses, with mean diameter of 6 cm and range of 0.5-34.5 cm[11]. Our case, measuring 0.5 cm in diameter, is one of the smallest reported SPNs[11]. Small SPNs generally show less cystic change, less sharp demarcation, and frequently no capsule[11]. Our case, indicating a prevalent solid pattern and ill-demarcated margins, fits well with the concept of an “early” stage of development. Nonetheless, it presents foci of cystic degeneration with papillary formations.
According to the current WHO classification[4], the IPMN in the present case should be classified as low-grade dysplasia. Imamura et al[10] diagnosed the IPMN component in their case as intraductal papillary mucinous adenoma (IPMA). Under the current WHO classification guidelines, IPMA is now designated as IPMN with low-grade dysplasia[4]. Imamura et al[10] did not mention the subtype of IPMN applicable to their case. We assume that it might have been a gastric type, based on the features of their figures and the MUC5AC positivity, and MUC1 and MUC2 negativity.
In both our case and Imamura’s case, the SPNs were adjacent to the IPMNs, without any transition or collision between the 2 neoplasms[10].
The occurrence of SPN and IPMN offers an interesting opportunity to investigate the role of β-catenin gene mutation in the pathogenesis of both lesions. In fact, almost all SPNs harbour somatic point mutations in exon 3 of CTNNB1, the gene that encodes β-catenin, which results in abnormal nuclear expression of β-catenin on immunohistochemistry[1]. The role of β-catenin gene mutations in IPMN is more controversial. β-catenin nuclear protein accumulation has been reported in one patient with familial adenomatous polyposis[16] and in a small series of IPMNs. In the latter study, 7 of 18 IPMNs indicated abnormal β-catenin nuclear protein accumulation (1 of 8 adenomas, 2 of 3 borderline, and 4 of 7 carcinomas)[17]. Both the present case and Imamura et al[10] showed abnormal nuclear expression of β-catenin in SPN, whereas IPMN showed only membranous expression, but no abnormal nuclear expression. These results suggest that, at least in these 2 patients, there is no correlation between the β-catenin abnormalities, exclusively found in SPNs, and the development of synchronous IPMNs.
In conclusion, we present a second case of synchronous SPN and IPMN of the pancreas. Unlike SPNs that usually occur in adolescent girls or young women, the 2 synchronous SPNs and IPMNs occurred in old, male patients (53 and 66 years, respectively). It is still unknown whether SPN or IPMN neoplasms occur independently or are in some way linked. From the comparison of the 2 cases, it appears that the β-catenin gene abnormalities play a role exclusively in the development of SPN, whereas a different molecular pathogenesis has to be considered for the IPMN counterpart.
The authors thank Mr. Hiroyuki Oyamada (Division of Diagnostic Pathology, Tokai University Hospital) for his valuable technical assistance.
P- Reviewers Lee KT, Schlitter AM S- Editor Gou SX L- Editor A E- Editor Li JY
| 1. | Abraham SC, Klimstra DS, Wilentz RE, Yeo CJ, Conlon K, Brennan M, Cameron JL, Wu TT, Hruban RH. Solid-pseudopapillary tumors of the pancreas are genetically distinct from pancreatic ductal adenocarcinomas and almost always harbor beta-catenin mutations. Am J Pathol. 2002;160:1361-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 317] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 2. | Notohara K, Hamazaki S, Tsukayama C, Nakamoto S, Kawabata K, Mizobuchi K, Sakamoto K, Okada S. Solid-pseudopapillary tumor of the pancreas: immunohistochemical localization of neuroendocrine markers and CD10. Am J Surg Pathol. 2000;24:1361-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 168] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 3. | Zamboni G, Bonetti F, Scarpa A, Pelosi G, Doglioni C, Iannucci A, Castelli P, Balercia G, Aldovini D, Bellomi A. Expression of progesterone receptors in solid-cystic tumour of the pancreas: a clinicopathological and immunohistochemical study of ten cases. Virchows Arch A Pathol Anat Histopathol. 1993;423:425-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 63] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. 4th ed. Lyon: International Agency for Research on Cancer 2010; . |
| 5. | Furukawa T, Klöppel G, Volkan Adsay N, Albores-Saavedra J, Fukushima N, Horii A, Hruban RH, Kato Y, Klimstra DS, Longnecker DS. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch. 2005;447:794-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 474] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 6. | Chahal P, Saad AJ, Jeyarajah RD. An unusual case of a coexistent serous cystadenoma and intraductal papillary mucinous neoplasm of pancreas. EUS to the rescue! JOP. 2011;12:244-246. [PubMed] |
| 7. | Larghi A, Stobinski M, Galasso D, Lecca PG, Costamagna G. Concomitant intraductal papillary mucinous neoplasm and pancreatic endocrine tumour: Report of two cases and review of the literature. Dig Liver Dis. 2009;41:759-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Yamaguchi K, Kanemitsu S, Hatori T, Maguchi H, Shimizu Y, Tada M, Nakagohri T, Hanada K, Osanai M, Noda Y. Pancreatic ductal adenocarcinoma derived from IPMN and pancreatic ductal adenocarcinoma concomitant with IPMN. Pancreas. 2011;40:571-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 192] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 9. | Ishida M, Shiomi H, Naka S, Tani T, Okabe H. Concomitant intraductal papillary mucinous neoplasm and neuroendocrine tumor of the pancreas. Oncol Lett. 2013;5:63-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Imamura N, Chijiiwa K, Ohuchida J, Hiyoshi M, Takahashi N, Yorita K, Kataoka H. Synchronous solid pseudopapillary neoplasm and intraductal papillary mucinous neoplasm of the pancreas: report of a case. Surg Today. 2011;41:865-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Papavramidis T, Papavramidis S. Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. J Am Coll Surg. 2005;200:965-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 536] [Article Influence: 26.8] [Reference Citation Analysis (1)] |
| 12. | Takahashi Y, Hiraoka N, Onozato K, Shibata T, Kosuge T, Nimura Y, Kanai Y, Hirohashi S. Solid-pseudopapillary neoplasms of the pancreas in men and women: do they differ? Virchows Arch. 2006;448:561-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Tien YW, Ser KH, Hu RH, Lee CY, Jeng YM, Lee PH. Solid pseudopapillary neoplasms of the pancreas: is there a pathologic basis for the observed gender differences in incidence? Surgery. 2005;137:591-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Machado MC, Machado MA, Bacchella T, Jukemura J, Almeida JL, Cunha JE. Solid pseudopapillary neoplasm of the pancreas: distinct patterns of onset, diagnosis, and prognosis for male versus female patients. Surgery. 2008;143:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 160] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 15. | Lin MY, Stabile BE. Solid pseudopapillary neoplasm of the pancreas: a rare and atypically aggressive disease among male patients. Am Surg. 2010;76:1075-1078. [PubMed] |
| 16. | Chetty R, Salahshor S, Bapat B, Berk T, Croitoru M, Gallinger S. Intraductal papillary mucinous neoplasm of the pancreas in a patient with attenuated familial adenomatous polyposis. J Clin Pathol. 2005;58:97-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Chetty R, Serra S, Salahshor S, Alsaad K, Shih W, Blaszyk H, Woodgett JR, Tsao MS. Expression of Wnt-signaling pathway proteins in intraductal papillary mucinous neoplasms of the pancreas: a tissue microarray analysis. Hum Pathol. 2006;37:212-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |