Published online Jun 7, 2013. doi: 10.3748/wjg.v19.i21.3324
Revised: March 23, 2013
Accepted: April 18, 2013
Published online: June 7, 2013
Processing time: 124 Days and 21.5 Hours
AIM: To investigate whether the reduction of stem cell factor (SCF) is mediated by decreased endogenous insulin-like growth factor (IGF)-1 in diabetic rat colon smooth muscle.
METHODS: Sixteen Sprague-Dawley rats were randomly divided into two groups: control group and streptozotocin-induced diabetic group. After 8 wk of streptozotocin administration, colonic motility function and contractility of circular muscle strips were measured. The expression of endogenous IGF-1 and SCF was tested in colonic tissues. Colonic smooth muscle cells were cultured from normal adult rats. IGF-1 siRNA transfection was used to investigate whether SCF expression was affected by endogenous IGF-1 expression in smooth muscle cells, and IGF-1 induced SCF expression effects were studied. The effect of high glucose on the expression of endogenous IGF-1 and SCF was also investigated.
RESULTS: Diabetic rats showed prolonged colonic transit time (252 ± 16 min vs 168 ± 9 min, P < 0.01) and weakness of circular muscle contraction (0.81 ± 0.09 g vs 2.48 ± 0.23 g, P < 0.01) compared with the control group. Endogenous IGF-1 and SCF protein expression was significantly reduced in the diabetic colonic muscle tissues. IGF-1 and SCF mRNA expression also showed a paralleled reduction in diabetic rats. In the IGF-1 siRNA transfected smooth muscle cells, SCF mRNA and protein expression was significantly decreased. IGF-1 could induce SCF expression in a concentration and time-dependent manner, mainly through the extracellular-signal-regulated kinase 1/2 signal pathway. High glucose inhibited endogenous IGF-1 and SCF expression and the addition of IGF-1 to the medium reversed the SCF expression.
CONCLUSION: Myopathy may resolve in colonic motility dysfunction in diabetic rats. Deficiency of endogenous IGF-1 in colonic smooth muscle cells leads to reduction of SCF expression.
Core tip: Endogenous insulin-like growth factor (IGF)-1 levels in diabetic rat colonic tissues were decreased. Hyperglycemia may be involved in initiating this change. In colonic smooth muscle cells, IGF-1 had a direct effect on increasing stem cell factor mRNA and protein levels mediated by extracellular-signal-regulated kinase 1/2 signaling.
- Citation: Wang Y, Xu XY, Tang YR, Yang WW, Yuan YF, Ning YJ, Yu YJ, Lin L. Effect of endogenous insulin-like growth factor and stem cell factor on diabetic colonic dysmotility. World J Gastroenterol 2013; 19(21): 3324-3331
- URL: https://www.wjgnet.com/1007-9327/full/v19/i21/3324.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i21.3324
Approximately 75% of diabetic patients with any type of diabetes mellitus (DM) present gastrointestinal symptoms[1,2], particularly those who have poor glycemic control. Constipation and the use of laxatives are relatively common in patients with DM[3]. This disorder involves the depletion of interstitial cells of Cajal (ICCs), dystrophic changes of smooth muscle cells (SMCs), and impairment of enteric nervous systems[4-6]. ICCs are electrical active cells that generate and propagate electrical slow waves and serve as a bidirectional interface between nerves and smooth muscle[5-7]. ICCs damage has been shown in the stomach, jejunum, and colon of patients suffering from either type 1 or type 2 diabetes. ICCs depletion has been demonstrated in both patients with DM and laboratory animal models[8], and ICCs depletion is a consequence of stem cell factor deficiency[9].
It is well established that ICCs survival and function depend on the activation of c-kit, a receptor tyrosine kinase integral to ICCs. SCF, as a c-kit ligand, is produced locally within the tunica muscularis[10-12].
In vitro experiments showed insulin-like growth factor-1 (IGF-1) prevented ICC deletion in long-term cultured smooth muscle[9]. In intact tissues, a significant proportion of tissue IGF-1 is actually produced by SMCs[13]. Therefore, local IGF-1 signaling may play a significant role in SCF expression. The aim of this research is to study the local colonic IGF-1 expression in DM rats and the endogenous IGF-1 effects on SCF production.
Male SD rats (weighing 200-250 g) were used for the experiments. Smooth muscle cells were obtained from SD rat colons. Diabetic rats (streptozotocin, STZ-D, n = 8) were induced by a single intraperitoneal injection of streptozotocin (STZ, 60 mg/kg body weight) dissolved in a citrate buffer, and age-matched control rats (n = 8) received equal volumes of buffer by ip injection. Diabetes was confirmed 1 wk later by measurement of tail vein blood glucose levels with an AccuChek Compact Plus glucometer (Roche, IN, United States). Rats with final blood glucose levels > 16.7 mmol/L were included in the study. At 8 wk after STZ administration, all the experimental rats were sacrificed by cervical dislocation. Food and water were given ad libitum. All animals were maintained in a controlled environment with alternating 12 h light/dark cycles. All animal care, use, and experimental protocols were approved by the Institutional Animal and Use Committee of Nanjing Medical University.
Eight weeks after STZ injection, colonic transit was measured using a bead expulsion test as described[14]. A glass bead (5 mm in diameter) was inserted through the anus, and pushed with a plastic rod into the distal colon for a distance of 3 cm, while each rat was under transient ethyl ether anesthesia. The time until bead expulsion was measured.
Eight weeks after STZ injection, freshly obtained full-thickness distal colon tissues (about 5 cm from the anus) from control and diabetic rats were stored on ice for less than 1 h and then immersed in warm, oxygenated (95% O2 and 5% CO2) Krebs solution (in mmol/L: 118 NaCl, 4.7 KCl, 2.5 CaCl2, 1 NaH2PO4, 1.2 MgCl2, 11 D-glucos and 25 NaHCO3). The mucosal layers were removed by micro-dissection under a magnifying glass and discarded. Circular muscle strips (2 mm × 10 mm) were prepared. After connecting to an isometric force transducer (AlcottBiotech), the strips were allowed to equilibrate for 30 min under an initial tension of 0.5 g. The bath temperature was maintained at 37 °C. Contractile responses to acetylcholine (Ach, 1 μmol/L, Sigma-Aldrich, St. Louis, MO, United States) were obtained. At least three muscle strips from the distal colon of each rat were used for muscle experiments. The mean of the three or more muscle strips exposed to Ach 1 μmol/L was determined for each rat.
SMCs were isolated from colonic tissues and cultured as described previously[15]. Briefly, whole SD rat colons were dissected, and mucosa and serosa were quickly removed from muscle tissues. After digestion with type II collagenase, SMCs were cultured in DMEM medium (Gibco) with 10% fetal bovine serum. Cells of passage 2 or 3 were used for the studies. Before the experiments, cells were serum starved for 24 h and then stimulated with different concentrations of IGF-1 (R and D Systems, Minneapolis, MN, United States) for various time periods. Phosphorylated extracellular-signal-regulated kinase 1/2 (ERK1/2) and phosphorylated Akt were measured after IGF-1 (100 ng/mL) treatment for 15 min. ERK1/2 inhibitor PD98059 (50 μmol/L) and phosphoinositide 3-kinase inhibitor (PI3K) inhibitor LY294002 (50 μmol/L) (Cell Signaling, Danvers, MA, United States) were added to the medium 30 min before IGF-1 treatment. Culture medium containing 25 mmol/L D-glucose was used for high glucose stimulation, and mannitol (5 mmol/L D-glucose plus 19.5 mmol/L mannitol) was used for osmotic controls.
IGF-1 siRNA was synthesized by GenePharma (Shanghai GenePharmaCo, China). The sequence for IGF-1 siRNA was as follows: anti-IGF-1-sense 5’-GCAGGAAACAAGACCUACATT-3’, and anti-IGF-1-antisense 5’-UGUAGGUCUUGUUUCCUGCTT-3’. Negative control siRNA: sense 5’-UUCUCCGAACGUGUCACGUTT-3’, and antisense 5’-ACGUGACACGUUCGGAGAATT-3’. Primary colonic smooth muscle cells were grown in six-well plates to 30% confluence, washed with serum-free medium immediately before transfection, and 800 μL serum-free medium was added to each well. siRNA (4 μg) was mixed with 10 μL of X-tremeGENE siRNA transfection reagent (Roche) in 200 μL serum-free medium. The mixture was incubated for 20 min at room temperature and then added to cells, according to the manufacturer’s instructions. Serum was added 4 h later to a final concentration of 10%. Cells were harvested at 72 h after transfection.
Distal colonic tissues were dissected from the mucosa and serosa. The remaining tissues were mainly muscle layers and used for Western blotting[16]. Tissues and cultured cells were lysed and centrifuged at 12000 g for 20 min. Protein samples were run on a 12% polyacrylamide gel in Tris-HCl. Proteins were then transferred to nitrocellulose membranes for 1 h at 100 V. The membranes were blocked with 5% (w/v) skim milk for 1 h at room temperature, and probed with primary antibodies at 4 °C overnight, and then washed in Tris-buffered saline with 0.1% Tween 20 for 5 min three times. The membranes were probed with corresponding horseradish peroxidase- conjugated secondary anti-rabbit and anti-goat antibodies at 1:2000 dilutions. Protein bands were detected with enhanced chemiluminescence Western blotting reagents (Thermo Fisher Scientific, Rockford, IL, United States). The resulting bands were scanned with an Epson 2400 printer and analyzed using the ImageJ program. All the antibodies used in this study, except for IGF-1 (Abcam, Cambridge, MA, United States) and SCF (Santa Cruz, CA, United States), were from Cell Signaling Technology.
Total RNA was isolated from colonic muscle layers and isolated SMCs, and quantified using Spectrophotometer Nanodrop 2000 (Thermo Scientific). RNA (500 ng) was submitted for cDNA synthesis using Prime Script RT Master Mix (Takara). The levels of isolated mRNA were measured by real-time polymerase chain reaction (PCR) using SYBR Premix Ex Taq (Takara) according to the manufacturer’s instructions. PCR was initiated at 95 °C for 30 s, followed by 40 cycles of denaturing at 95 °C for 5 s, annealing and extending at 60 °C for 30 s. After PCR, a dissociation curve was constructed at 60-95 °C. PCR was performed in triplicate. The oligonucleotide primers used were as follows: IGF-1, forward, GGCATTGTGGATGAGTGTTG, reverse, GTCTTGGGCATGTCAGTGTG; SCF, forward, TTCGCTTGTAATTGGCTTTGC; reverse, CAACTGCCCTTGTAAGAC TTGCA; and 18S rRNA, forward, GCGAAAGCATTTGCCAAGAA, reverse, GGCATCGTTTATGGTCGGAAC. Results were shown to be relative to 18S rRNA mRNA.
The results are expressed as the mean ± SE. The SPSS statistical package (version 14.0; SPSS Inc, Chicago, IL, United States) was used for statistical analysis. The differences between the two groups were analyzed using Student’s t test, and differences between the three groups were analyzed using analysis of variance. A P value < 0.05 was considered statistically significant.
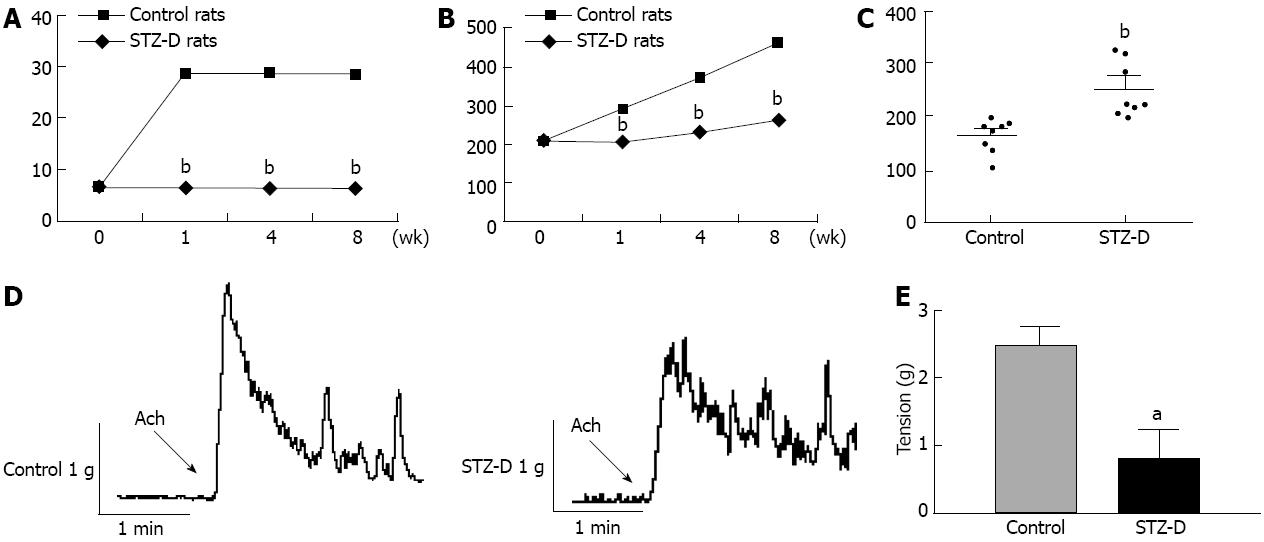
All STZ-induced rats showed hyperglycemia at the time of experiments, with their blood glucose concentrations being significantly higher than those of the age-matched rats which were not injected with STZ (n = 8) (Figure 1A). The body weight of STZ-treated (STZ-D) rats was significantly lower than the age-matched normal rats (n = 8) (Figure 1B).
The distal colon transit time was significantly increased in STZ-D group 8 wk after induction of diabetes (Figure 1C). Colonic circular smooth muscle strips from STZ-D rats showed weak contractility to Ach (Figure 1D). The maximum contractile tension of the STZ-D group was significantly reduced compared with control group (n = 8 each group) (Figure 1E).
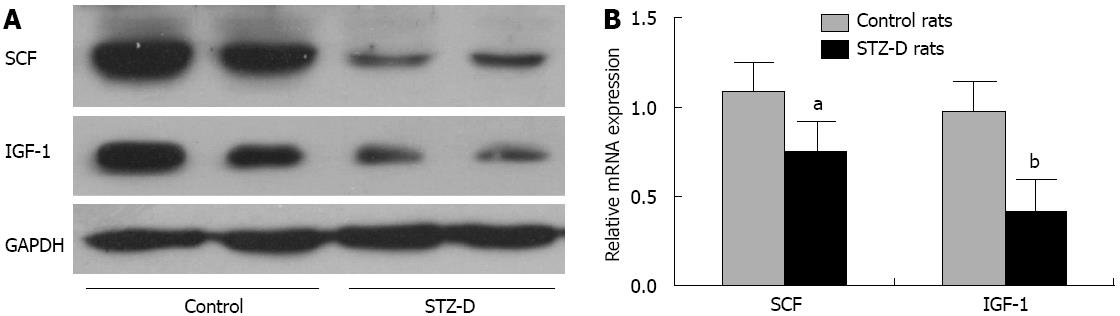
Eight weeks after STZ administration, the rats were sacrificed and distal colonic tissues were harvested. Because the mucosa/submucosa and serosa had been removed, the protein and mRNA were mainly from the muscle layer. The protein levels of SCF and IGF-1 were significantly decreased in STZ-D rat distal colons (Figure 2A). SCF and IGF-1 mRNA levels were consistent with the protein results. SCF mRNA levels of diabetic rats were lower than those of control rats, and IGF-1 mRNA levels were also decreased in diabetic rat colonic tissues (Figure 2B).
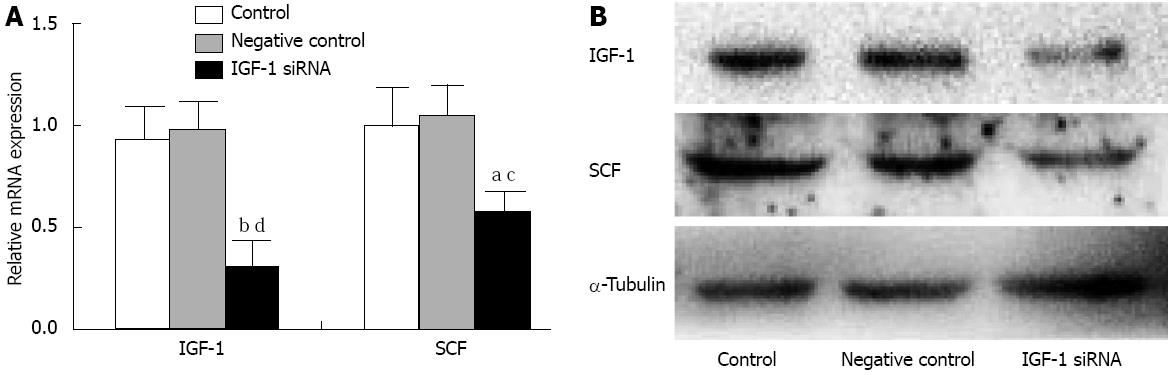
siRNA knockdown of endogenous IGF-1 reduced SCF mRNA levels in SMCs. and SCF protein levels also showed a significant parallel reduction of IGF-1 levels compared with the controls (Figure 3).
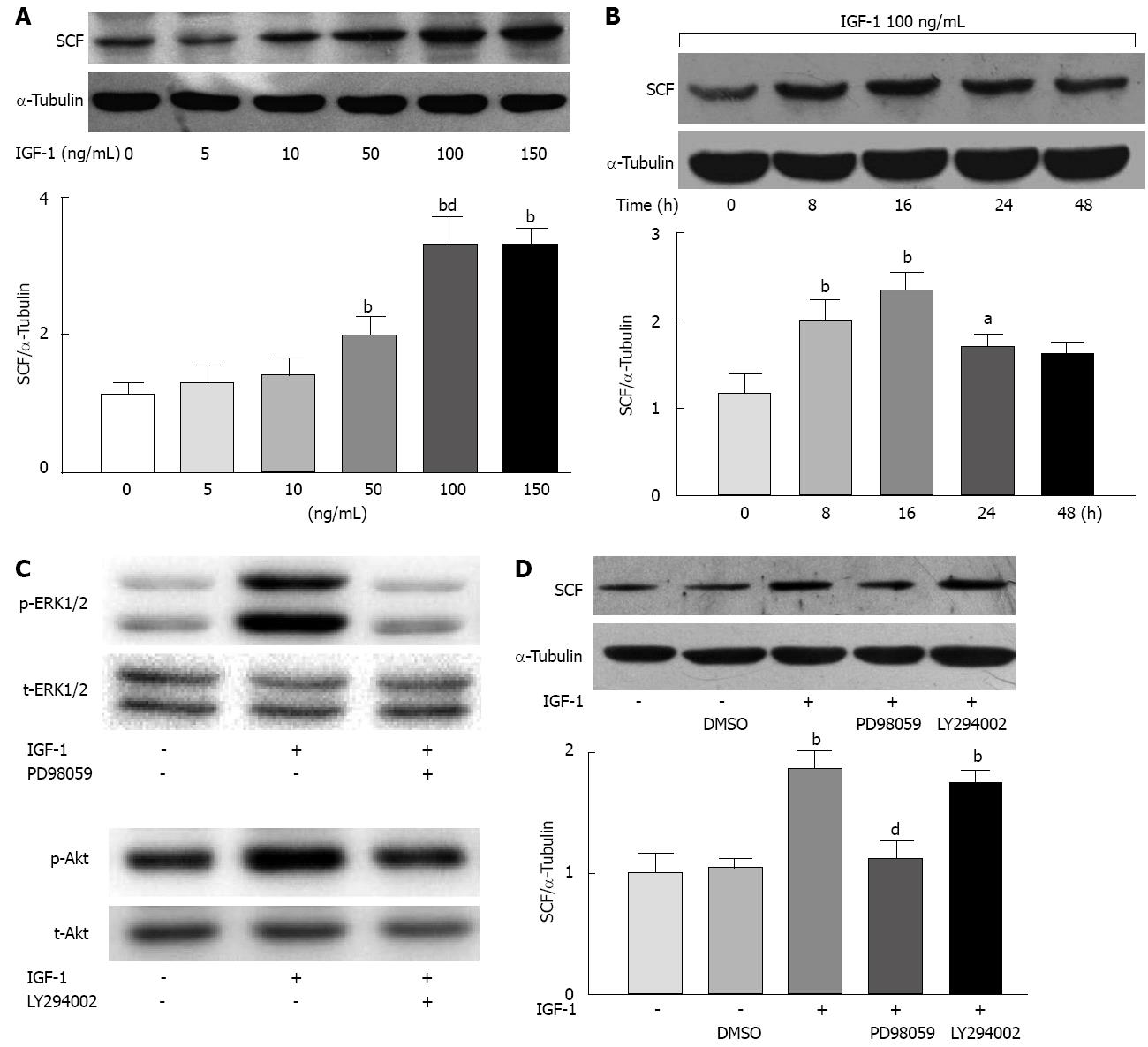
IGF-1 elicited a dose-dependent increase in the expression of SCF in rat colonic SMCs. The concentration of 50 ng/mL significantly increased the expression of SCF (P < 0.01). One hundred ng/mL and 150 ng/mL IGF-1 showed a maximal effect on the expression of SCF (P < 0.01); therefore, 100 ng/mL was used in subsequent experiments (Figure 4A).
IGF-1 induced maximum SCF expression in SMCs at 16 h; while at 24 and 48 h, the stimulating effect of IGF-1 on SCF gradually diminished. By 48 h, the expression levels had almost returned to baseline (0 h) (Figure 4B).
To determine whether IGF-1-induced SCF expression was mediated by Akt-dependent or ERK1/2-dependent mechanisms, we measured the phosphorylation of Akt and ERK1/2. Both of them could be phosphorylated by IGF-1 (100 ng/mL). We then used a PI3K/Akt inhibitor (LY-294002, 50 μmol/L) and an ERK1/2 inhibitor (PD-98059, 50 μmol/L) before IGF-1 (100 ng/mL) was added to the media. Sixteen hours after IGF-1 stimulation, the ERK1/2 inhibitor significantly inhibited IGF-1-induced SCF expression (P < 0.01) while the PI3K/Akt inhibitor showed no effect (Figure 4C).
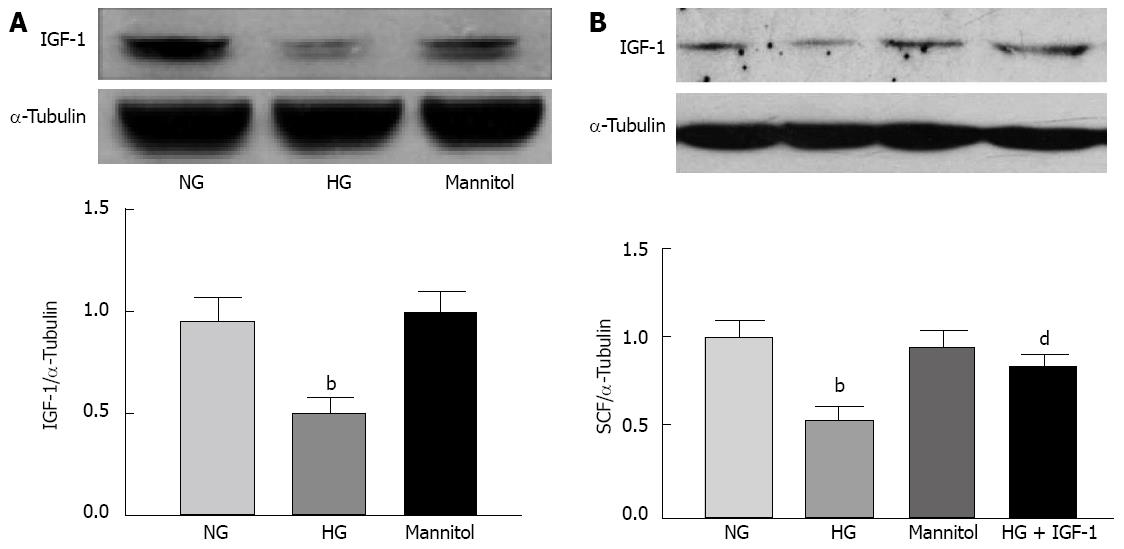
Compared with cultures for 24 h in normal glucose (5 mmol/L) medium, cultures in DMEM containing 25 mmol/L glucose significantly inhibited IGF-1 expression in cultured colonic SMCs. Mannitol was used as an osmotic control which did not have similar inhibitive effect (Figure 5A). High glucose concentrations also suppressed SCF expression, which was reversed by the addition of exogenous IGF-1 (100 ng/mL) (Figure 5B).
Intestinal transit is often disturbed in diabetes (rapid or slow)[17], and symptoms such as diarrhea and constipation occur more frequently than in the general population[18]. The prevalence of GI symptoms was found to be more common in patients who had long histories of diabetes[19]. In diabetic animal models, it has been proven that 2 mo of DM is adequate to impair gastrointestinal motility. The spontaneously diabetic NOD/LtJ mouse, as a type 1 DM model, developed gastroparesis 2 mo after the onset of diabetes[20], and a type 2 DM model db/db mouse developed disturbed motility of the stomach, small intestine, and colon 7 wk after the development of DM[21].
Intestinal contractility is dependent on not only intact smooth muscle but also trophic factors, such as SCF and IGF-1, which protect the gastrointestinal slow wave generating ICCs[9], and promote gastrointestinal growth[22], respectively. In the present study, we found that SCF levels in colonic tissue of diabetic rats were significantly reduced, which is consistent with previous reports; in which NOD/LtJ mice had significantly reduced gastric mRNA levels of SCF[9] and diabetic db/db mice had lower mRNA levels of SCF in the small intestine and colon compared with age-matched control mice 7 wk after the development of type 2 DM[21]. Reduction of SCF production in the gastrointestinal tract of diabetic rats appears to play a significant role in decreasing ICCs network densities that have been described in human diabetes[9]. SCF has been shown to be produced locally by SMC and neurons within the tunica muscularis[9,23]. Decreases in IGF-1 signaling have been considered to be responsible for the decrease in SCF levels. Horváth et al[9,24] have shown that IGF-1 treatment of murine gastric tunica muscularis tissues could reverse SCF reductions in long-term organ culture, and decreased levels of SCF were correlated with smooth-muscle atrophy. The observed pro-survival effects of insulin and IGF-1 on mature ICCs were probably indirect because ICCs do not express insulin or IGF-1 receptors[9], while SMCs express both insulin and IGF-1 receptors. Therefore, both insulin and IGF-1 may induce SMCs to produce SCF.
IGF-1 is an endogenous growth factor that plays a central role in the growth and development of visceral and vascular smooth muscle[25,26]. Existing evidence suggests that mesenchymal cells, including α-smooth muscle actin-positive myofibroblasts and smooth muscle cells, are primary sources of locally expressed IGF-1 in the intestine[27,28]. Local intestinal IGF-1 has autocrine effects on the growth of human intestinal muscle cells[29]. In this study, we found that endogenous IGF-1 was decreased in diabetic colonic tissues. Meanwhile, SCF expression in colonic tissue was paralleled by endogenous IGF-1, which suggested that a lack of endogenous IGF-1 might lead to low levels of SCF expression.
IGF-1 binds to the cellular membrane IGF-1 receptor leading to stimulation of proliferation and inhibition of apoptosis through PI3K/Akt and mitogen-activated protein kinase pathways[15,30]. In the present study, IGF-1 could cause both ERK1/2 and Akt phosphorylation, but IGF-1 promoted SCF expression mainly through ERK1/2-dependent signaling, not the PI3K/Akt pathway.
Both type 1 and type 2 diabetes patients have hyperglycemia as a basic feature, and chronic hyperglycemia is associated with dysfunction, damage, and failure of several organs[31]. This study showed that high glucose impaired the production of trophic factors in colonic SMCs. Whether the mechanism underlying the effects is oxidative stress caused by high glucose remains to be determined.
The current study is an extension of those described by Horváth et al[9]. The significant differences between that study and the current one are: (1) we showed evidence supporting the presence of dysfunction of smooth muscle contractility in animals, which is more compelling evidence of myopathy than just a reduction of myh11 mRNA levels; (2) in cell culture studies, we demonstrated that IGF-1 had a direct effect on increasing SCF mRNA and protein levels, and this effect was mediated by ERK1/2 signaling; additionally; and (3) we found that endogenous IGF-1 levels in diabetic rat colonic tissues were decreased, and that hyperglycemia may be involved in initiating this change.
In conclusion, we have demonstrated that the myopathy may resolve in colonic motility dysfunction in diabetic rats. Deficiency of endogenous IGF-1 in SMCs caused reduction in SCF expression, which is a critical developmental, growth, and survival factor for ICCs.
Most diabetic patients experience gastrointestinal symptoms, particularly those who are under poor glycemic control. Constipation is relatively common in patients with diabetes mellitus, and involves the depletion of interstitial cells of Cajal (ICCs), dystrophic changes of smooth muscle cell, and impairment of enteric nervous system.
ICCs are the primary electrical pacemakers for rhythmic contractile activity. ICC depletion has been demonstrated in both patients with diabetes mellitus and laboratory animal models, and is a consequence of stem cell factor (SCF) deficiency. Cell culture experiments have shown that addition of insulin-like growth factor-1 (IGF-1) prevented ICC deletion in long-term cultured smooth muscle cells.
This study showed evidence supporting the presence of dysfunction of smooth muscle contractility in animals, which is compelling evidence of myopathy. In cell culture studies, the authors demonstrated that IGF-1 had a direct effect on increasing SCF mRNA and protein levels, and this effect was mediated by extracellular-signal-regulated kinase 1/2 signaling; and that endogenous IGF-1 levels in diabetic rat colonic tissues were decreased, and that hyperglycemia may be involved in initiating this change.
The results of this study suggest that compensation of endogenous IGF-1 is a potential therapeutic option that could prevent the development of diabetic colonic dysmotility.
SCF is a cytokine that binds to the c-Kit receptor. SCF can exist both as a transmembrane protein and a soluble protein. This cytokine plays an important role in hematopoiesis, spermatogenesis, and melanogenesis. In gastrointestinal tract, SCF is mainly produced by smooth muscle cells and neurons.
This is a good descriptive study in which authors showed that myopathy may resolve in colonic motility dysfunction in diabetic rats, and deficiency of endogenous IGF-1 in colonic smooth muscle cells caused reduction of SCF expression. The results are interesting and suggest that endogenous IGF-1 is a potential therapeutic target for diabetic colonic dysmotility.
P- Reviewers Kawakami K, Torres-Aleman I S- Editor Gou SX L- Editor A E- Editor Ma S
| 1. | Folwaczny C, Riepl R, Tschöp M, Landgraf R. Gastrointestinal involvement in patients with diabetes mellitus: Part I (first of two parts). Epidemiology, pathophysiology, clinical findings. Z Gastroenterol. 1999;37:803-815. [PubMed] |
| 2. | Verne GN, Sninsky CA. Diabetes and the gastrointestinal tract. Gastroenterol Clin North Am. 1998;27:861-874, vi-vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Rodrigues ML, Motta ME. Mechanisms and factors associated with gastrointestinal symptoms in patients with diabetes mellitus. J Pediatr (Rio J). 2012;88:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Pasricha PJ, Pehlivanov ND, Gomez G, Vittal H, Lurken MS, Farrugia G. Changes in the gastric enteric nervous system and muscle: a case report on two patients with diabetic gastroparesis. BMC Gastroenterol. 2008;8:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Farrugia G. Interstitial cells of Cajal in health and disease. Neurogastroenterol Motil. 2008;20:54-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 256] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 6. | Huizinga JD, Lammers WJ. Gut peristalsis is governed by a multitude of cooperating mechanisms. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1-G8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 257] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 7. | Huizinga JD. Gastrointestinal peristalsis: joint action of enteric nerves, smooth muscle, and interstitial cells of Cajal. Microsc Res Tech. 1999;47:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Ordög T, Hayashi Y, Gibbons SJ. Cellular pathogenesis of diabetic gastroenteropathy. Minerva Gastroenterol Dietol. 2009;55:315-343. [PubMed] |
| 9. | Horváth VJ, Vittal H, Lörincz A, Chen H, Almeida-Porada G, Redelman D, Ordög T. Reduced stem cell factor links smooth myopathy and loss of interstitial cells of cajal in murine diabetic gastroparesis. Gastroenterology. 2006;130:759-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 177] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 10. | Ordög T, Ward SM, Sanders KM. Interstitial cells of cajal generate electrical slow waves in the murine stomach. J Physiol. 1999;518:257-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 189] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Wu JJ, Rothman TP, Gershon MD. Development of the interstitial cell of Cajal: origin, kit dependence and neuronal and nonneuronal sources of kit ligand. J Neurosci Res. 2000;59:384-401. [PubMed] |
| 12. | Huizinga JD, Berezin I, Sircar K, Hewlett B, Donnelly G, Bercik P, Ross C, Algoufi T, Fitzgerald P, Der T. Development of interstitial cells of Cajal in a full-term infant without an enteric nervous system. Gastroenterology. 2001;120:561-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Williams KL, Fuller CR, Fagin J, Lund PK. Mesenchymal IGF-I overexpression: paracrine effects in the intestine, distinct from endocrine actions. Am J Physiol Gastrointest Liver Physiol. 2002;283:G875-G885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Million M, Maillot C, Saunders P, Rivier J, Vale W, Taché Y. Human urocortin II, a new CRF-related peptide, displays selective CRF(2)-mediated action on gastric transit in rats. Am J Physiol Gastrointest Liver Physiol. 2002;282:G34-G40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Kuemmerle JF. IGF-I elicits growth of human intestinal smooth muscle cells by activation of PI3K, PDK-1, and p70S6 kinase. Am J Physiol Gastrointest Liver Physiol. 2003;284:G411-G422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Park C, Yan W, Ward SM, Hwang SJ, Wu Q, Hatton WJ, Park JK, Sanders KM, Ro S. MicroRNAs dynamically remodel gastrointestinal smooth muscle cells. PLoS One. 2011;6:e18628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Keshavarzian A, Iber FL. Intestinal transit in insulin-requiring diabetics. Am J Gastroenterol. 1986;81:257-260. [PubMed] |
| 18. | Bytzer P, Talley NJ, Leemon M, Young LJ, Jones MP, Horowitz M. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: a population-based survey of 15,000 adults. Arch Intern Med. 2001;161:1989-1996. [PubMed] |
| 19. | Oh JH, Choi MG, Kang MI, Lee KM, Kim JI, Kim BW, Lee DS, Kim SS, Choi H, Han SW. The prevalence of gastrointestinal symptoms in patients with non-insulin dependent diabetes mellitus. Korean J Intern Med. 2009;24:309-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Ordög T, Takayama I, Cheung WK, Ward SM, Sanders KM. Remodeling of networks of interstitial cells of Cajal in a murine model of diabetic gastroparesis. Diabetes. 2000;49:1731-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Yamamoto T, Watabe K, Nakahara M, Ogiyama H, Kiyohara T, Tsutsui S, Tamura S, Shinomura Y, Hayashi N. Disturbed gastrointestinal motility and decreased interstitial cells of Cajal in diabetic db/db mice. J Gastroenterol Hepatol. 2008;23:660-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Kuemmerle JF, Bushman TL. IGF-I stimulates intestinal muscle cell growth by activating distinct PI 3-kinase and MAP kinase pathways. Am J Physiol. 1998;275:G151-G158. [PubMed] |
| 23. | Torihashi S, Yoshida H, Nishikawa S, Kunisada T, Sanders KM. Enteric neurons express Steel factor-lacZ transgene in the murine gastrointestinal tract. Brain Res. 1996;738:323-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Horváth VJ, Vittal H, Ordög T. Reduced insulin and IGF-I signaling, not hyperglycemia, underlies the diabetes-associated depletion of interstitial cells of Cajal in the murine stomach. Diabetes. 2005;54:1528-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Vrieling A, Voskuil DW, Bosma A, Majoor DM, van Doorn J, Cats A, Depla AC, Timmer R, Witteman BJ, Wesseling J. Expression of insulin-like growth factor system components in colorectal tissue and its relation with serum IGF levels. Growth Horm IGF Res. 2009;19:126-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Conti E, Carrozza C, Capoluongo E, Volpe M, Crea F, Zuppi C, Andreotti F. Insulin-like growth factor-1 as a vascular protective factor. Circulation. 2004;110:2260-2265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 184] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 27. | Pucilowska JB, McNaughton KK, Mohapatra NK, Hoyt EC, Zimmermann EM, Sartor RB, Lund PK. IGF-I and procollagen alpha1(I) are coexpressed in a subset of mesenchymal cells in active Crohn’s disease. Am J Physiol Gastrointest Liver Physiol. 2000;279:G1307-G1322. [PubMed] |
| 28. | Winesett DE, Ulshen MH, Hoyt EC, Mohapatra NK, Fuller CR, Lund PK. Regulation and localization of the insulin-like growth factor system in small bowel during altered nutrient status. Am J Physiol. 1995;268:G631-G640. [PubMed] |
| 29. | Kuemmerle JF. Autocrine regulation of growth in cultured human intestinal muscle by growth factors. Gastroenterology. 1997;113:817-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Kuemmerle JF. Endogenous IGF-I protects human intestinal smooth muscle cells from apoptosis by regulation of GSK-3 beta activity. Am J Physiol Gastrointest Liver Physiol. 2005;288:G101-G110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35:S64-S71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1005] [Cited by in RCA: 1114] [Article Influence: 85.7] [Reference Citation Analysis (0)] |