Published online Jun 7, 2013. doi: 10.3748/wjg.v19.i21.3207
Revised: January 30, 2013
Accepted: March 15, 2013
Published online: June 7, 2013
Processing time: 187 Days and 13.5 Hours
AIM: To analyze the epidemiology, clinical characteristics, treatment patterns and outcome in hepatocellular carcinoma (HCC) patients.
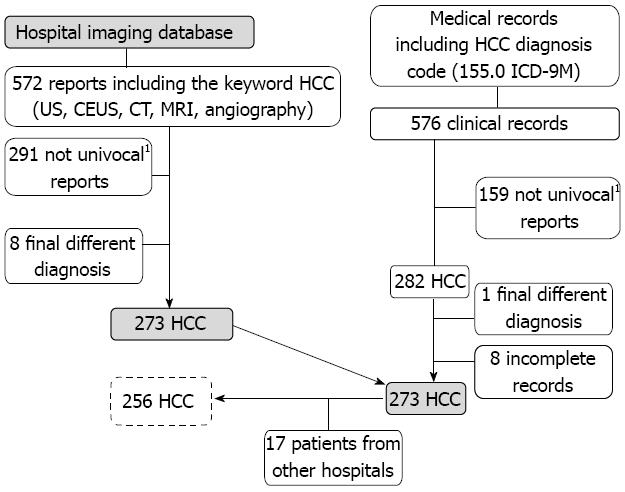
METHODS: We analyzed clinical, pathological and therapeutic data from 256 consecutive patients, examined at S. Croce Hospital in Cuneo-Piedmont, with a diagnosis of HCC between 30th June 2000 and 1st July 2010. We analyzed the hospital imaging database and examined all medical records, including the diagnosis code for HCC (155.0 according to the ICD-9M classification system), both for inpatients and outpatients, and discovered 576 relevant clinical records. After the exclusion of reports relating to multiple admissions for the same patient, we identified 282 HCC patients. Moreover, from this HCC series, we excluded 26 patients: 1 patient because of an alternative final diagnosis, 8 patients because of a lack of complete clinical data in the medical record and 17 patients because they were admitted to different health care facilities, leaving 256 HCC patients. To highlight possible changes in HCC patterns over the ten-year period, we split the population into two five-year groups, according to the diagnosis period: 30th June 2000-30th June 2005 and 1st July 2005-1st July 2010. Patients underwent a 6-mo follow up.
RESULTS: Two hundred and fifty-six HCC patients were included (male/female 182/74; mean age 70 years), 133 in the first period and 123 in the second. Hepatitis C virus (HCV) infection was the most common HCC risk factor (54.1% in the first period, 50.4% in the second; P = 0.63); in the first period, 21.8% of patients were alcoholics and 15.5% were alcoholics in the second period (P > 0.05); the non-viral/non-alcoholic etiology rate was 3.7% in the first period and 20.3% in the second period (P < 0.001). Child class A patients increased significantly in the second period (P < 0.001). Adjusting for age, gender and etiology, there was a significant increase in HCC surveillance during the second period (P = 0.01). Differences between the two periods were seen in tumor parameters: there was an increase in the number of unifocal HCC patients, from 53 to 69 (P = 0.01), as well as an increase in the number of cases where the HCC was < 3 cm [from 22 to 37 (P = 0.01)]. The combined incidence of stage Barcelona Clinic Liver Cancer 0 (very-early) and A (early) HCC was 46 (34.6%) between 2000-2005, increasing to 62 (50.4%) between 2005-2010 (P = 0.01). Of the patients, 62.4% underwent specific treatment in the first group, which increased to 90.2% in the second group (P < 0.001). Diagnosis period (P < 0.01), Barcelona-Clinic Liver Cancer stage (P < 0.01) and treatment per se (P < 0.05) were predictors of better prognosis; surveillance was not related to survival (P = 0.20).
CONCLUSION: This study showed that, between 2000-2005 and 2005-2010, the number of HCV-related HCC decreased, non-viral/non alcoholic etiologies increased and of surveillance programs were more frequently applied.
Core tip: Hepatocellular carcinoma (HCC) is the 5th most common cancer and the 3rd leading cause of cancer mortality worldwide. In recent decades, the incidence of HCC has risen in Europe and the United States. This study showed that, between 2000-2005 and 2005-2010, the number of hepatitis C virus-related HCC decreased, non-viral/non alcoholic etiologies increased and of surveillance programs were more frequently applied.
- Citation: Fenoglio L, Serraino C, Castagna E, Cardellicchio A, Pomero F, Grosso M, Senore C. Epidemiology, clinical-treatment patterns and outcome in 256 hepatocellular carcinoma cases. World J Gastroenterol 2013; 19(21): 3207-3216
- URL: https://www.wjgnet.com/1007-9327/full/v19/i21/3207.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i21.3207
Hepatocellular carcinoma (HCC) is the 5th most common cancer and the 3rd leading cause of cancer mortality worldwide[1]. In recent decades, the incidence of HCC has risen in Europe and the United States; this trend may be explained to several factors, such as increased surveillance programs for HCC-high risk patients, improved management of chronic liver disease, and an epidemic of hepatitis C virus (HCV) infection in the late 1970s[2]. HCC related to HCV infection is a preventable disease whose incidence can be decreased by controlling HCV transmission; in Japan, the incidence of HCC began to decrease by 2000, mainly because of decreased HCV-related cases[3]. Considering the current provision of HCV prevention programs in Europe, can a similar decrease of HCV-related HCC be expected in the future?
HCC occurs two to four times more often among men than women, and within an established background of chronic liver disease (fibrosis or cirrhosis). Hepatitis B virus (HBV) and HCV infections are known independent risk factors for HCC, as well as alcohol consumption[4]. Nevertheless, 5%-30% of patients with HCC lack a defined, identifiable risk factor. Recently, it was suggested that non-alcoholic steatohepatitis (NASH) might account for a substantial portion of cryptogenic cirrhosis and HCC cases[5]. NASH is a more severe form of non-alcoholic fatty liver disease (NAFLD) that is associated with obesity, diabetes, dyslipidemia and insulin resistance[5]. International guidelines recommend surveillance for patients at high risk for HCC[6,7], yet surveillance is not recommended in patients at risk for NASH.
Non-randomized trials and observational studies reported a survival benefit in small HCC recognized within surveillance programs, but these studies had unavoidable biases[8] and the evidence supporting surveillance efficacy in term of improving survival is limited. However, surveillance using 6-mo ultrasound (US) best identifies liver nodules less than 1 cm, and small HCC could benefit from more effective treatment[9]. Certain HCC treatments are potentially curative depending on cancer stage, liver function and performance status, as well as on resources and level of practitioner expertise[10]. The Barcelona Clinic Liver Cancer (BCLC) staging system is widely accepted in clinical practice and was adopted as the reference system by the American Association for the Study of Liver Disease (AASLD) in 2010[7,11]. The BCLC system includes data on patient’s performance status, the number and size of nodules, cancer symptoms and liver function. The BCLC stratifies patients into separate prognostic categories and suggests treatment options according to the stage[11,12]. Patients with localized stage HCC could benefit from curative therapies, such as resection, percutaneous ablation or liver transplantation, whereas patients with intermediate or advanced stages may benefit from non-curative treatments, such as chemoembolization. Recent studies demonstrating that chemoembolization improves survival in well-selected patients with unresectable HCC[13] have led to a greater propensity for chemoembolization treatment, as well as a modification of indication to treatment, such as inclusion of more patients with well-compensated disease and advanced tumors[14].
We created a database of consecutive HCC patients diagnosed in S. Croce General Hospital (Cuneo, Italy) from June 2000 to July 2010. Using this database, we designed the present study to assess clinical characteristics, treatment patterns and outcomes in patients with HCC.
Our population was a consecutive series of patients, examined at S. Croce Hospital in Cuneo, Piedmont, with a diagnosis of HCC between 30th June 2000 to 1st July 2010. All cases with initial HCC diagnosis were considered eligible; incident diagnosis as well as diagnosis was carried out during surveillance.
We analyzed the hospital imaging database and found 572 reports that included the keyword HCC. After exclusion of reports performed for the same patient and reports presenting a different final diagnosis (despite the presence of the keyword), we identified 273 actual HCC patients.
To better identify all the HCC patients referred to our hospital, we examined all medical records that included the diagnosis code for HCC (155.0 according to the ICD-9M classification system), both for inpatients and outpatients; we discovered 576 clinical records, and after exclusion of records presenting the same name (multiple admissions for the same patient), we identified 282 actual HCC patients. Nine patients were excluded from this series: one patient because of an alternative final diagnosis and eight patients because of lack of complete clinical data in the medical record (Figure 1).
To highlight possible changes in HCC patterns over the ten-year period, we split the population into two five-years groups, according to the diagnosis period: 30th June 2000-30th June 2005 and 1st July 2005-1st July 2010.
Patients were classified according to the cause of liver disease as follows: (1) HBV, if patients were hepatitis B surface antigen-positive; (2) HCV, if patients were serum antibody anti-HCV positive; (3) alcoholic, if the daily ethanol intake was > 60 g for women and > 80 g for men for more than 10 years; (4) multi-etiology, if there was a combination of these causative factors; and (5) others, when the cause was different from those cited above (as primary biliary cirrhosis, autoimmune hepatitis, hemochromathosis, etc.).
On admission, clinical (general conditions, presence of ascites, jaundice, etc.), biochemical (routine biochemical tests, liver function tests, alpha-fetoprotein, etc.) and imaging (US, computed tomography, magnetic resonance imaging, hepatic arterial angiography) parameters were assessed.
The HCC database included personal data, etiology of hepatic liver disease, biochemical tests, imaging features (number of nodules, size, presence of ascites, presence of hepatic thrombosis, and presence of metastasis), surveillance application (defined by two US examinations over the last 12 mo)[7] and Child Pugh score[15].
We collected the following data regarding HCC treatment: type, number of procedures, number of admissions, days of hospitalization, treatment complications, presence of post-embolization syndrome (defined by the onset of fever, abdominal pain, moderate degree of ileus, moderate cholestasis and transaminase elevation, self-limited in less than 48 h)[7].
Diagnosis of cirrhosis was made by clinical (endoscopic and/or US signs of portal hypertension, and/or a nodular margin of the liver at US examination) and laboratory features; when possible and/or indicated, cirrhosis was confirmed by histology. The severity of liver dysfunction was scored according to the Child-Pugh classification[15].
Diagnoses of HCC were carried out according to internationally accepted criteria[6,7] and, if required, confirmed by cytology and/or histology.
HCC was classified as unifocal, paucifocal (≤ 3 nodules), multifocal (> 3 nodules), infiltrative and/or massive (infiltrating pattern of growth and/or a huge mass with a diameter > 10 cm and an undefined boundary)[16]. The tumor size of expanding nodules was also measured (in cases of multinodular tumors, the largest one was measured).
HCC stage was scored according to the BCLC staging system[11], recently validated by the AASLD[7].
Univariate associations were tested using the Pearson χ2 test for proportions. Multivariate logistic regression models were fitted. All tests were two-sided with a significance level of P < 0.05.
Survival was calculated from the time of cancer diagnosis to death, with values censored at the date of the last follow up. The Kaplan-Meier method was used to estimate the cumulative probability of survival according to surveillance (yes vs no), BCLC stage (very-early and early vs others) and treatment (yes vs no). Difference between survival curves was assessed using the Log-Rank test; a P value < 0.05 was considered significant.
A Cox proportional hazard model was fitted to test the role of prognostic factors associated with probability of death in the univariate analysis. All statistical analyses were performed using the SAS statistical package (SAS Institute, Cary, NC, United States).
Two hundred and seventy-three consecutive HCC patients was identified over the study period, but 17 patients were admitted to different health care facilities and were excluded because of difficulty in accessing to medical records and clinical data (Figure 1).
Ultimately, 256 HCC patients were included, 133 in the first group (2000-2005) and 123 in the second group (2005-2010).
The median age was 70-year-old (range 32-92 years), with no difference between the two groups. The male/female ratio was 182/74. Chronic liver disease was present in 252 cases (98.4%); cirrhosis in 234 (91.4%) and chronic hepatitis in 18 cases (7%). Four patients presented histologically normal livers.
According to the Child-Pugh score, 159 cirrhotic patients (62.1%) were class A, 83 patients (32.4%) were class B and 14 patients (5.5%) were class C. Comparing the two groups, there were more class A patients in the second period (67 cases vs 92 cases, P < 0.001). The distribution of different Child classes was not related to gender (P = 0.41) or to age (P = 0.37), but Child class B was more represented among alcoholic patients (P = 0.007).
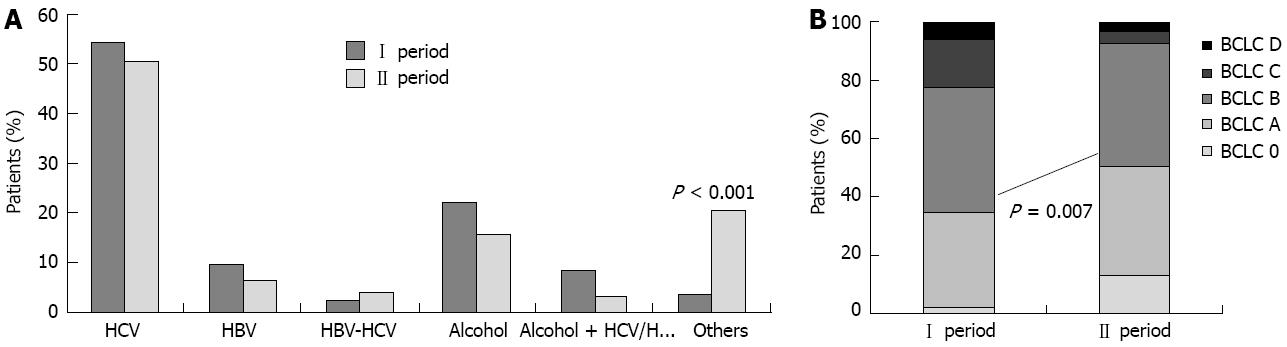
The most common cause of liver disease was HCV infection (134 cases, 52.3%), with a higher incidence in women than in men (P < 0.001). The second most common HCC risk factor was alcoholic liver disease (48 cases, 18.7%), more frequently in men than in women (P = 0.01). HBV infection was identified in 21 cases (8.2%), and an HBV-HCV infection was present in only eight patients (3.1%). Moreover, 15 patients presented a virus infection (6 patients with HBV infection and 9 patients with HCV infection) associated with alcohol consumption. Finally, in 30 cases, the cause of liver disease was different: 4 cases presented a diagnosis of primary biliary cirrhosis (1.6%), 2 cases of autoimmune hepatitis (0.8%), 3 cases of hereditary hemochromathosis (1.2%). Twenty-one cases (8.2%) presented only type 2 diabetes mellitus as potential risk factor for liver disease; however, none of these patients underwent a liver biopsy to confirm the presence of NAFLD-NASH. Comparing the two time periods by etiology (Figure 2A), we noticed a decreasing trend of HCV-related HCCs (72 cases vs 62 cases, P = 0.63) in the second period, with a parallel increase of HCCs not related to viral infection nor to alcohol (5 cases vs 25 cases, P < 0.001); in particular, there were significantly more patients with diabetes and HCC in the second period (3 cases vs 18 cases, P < 0.001).
The diagnosis of HCC was made by histology and/or cytology in 55 patients and by imaging in 201 cases.
Alpha-fetoprotein (AFP) was > 100 ng/dL in 59 cases (23%) and > 200 ng/dL in 74 cases (28.9%), while in the remaining patients, the AFP value was not available.
Sixty-one cases (23.8%) were diagnosed in patients undergoing semiannual US surveillance, while the remaining 195 cases (76.2%) were diagnosed in patients occasionally submitted to US or during an US follow-up at an interval longer than 6 mo. Adjusting for age, gender, etiology and Child-Pugh class, the probability to undergo surveillance was inferior among alcoholic patients (P = 0.002) and patients with “other causes” of liver disease (P < 0.001). Comparing the two groups and after adjusting for age, gender, etiology, surveillance was more relevant in the second period than in the first (P = 0.01).
At the time of diagnosis, most HCCs were unifocal (122 cases, 47.7%), 55 HCCs were paucifocal (21.4%), 62 HCCs were multifocal (24.3%) and 17 HCCs were massive (6.6%). The most common pattern of presentation of HCC over the whole study period was a single nodule more than 3 cm, but less than 5 cm, in diameter (128 cases, 50%). In the multivariate analysis, the probability of discovering a unifocal HCC and an HCC < 3 cm in diameter was higher in patients undergoing surveillance (P = 0.001 and P = 0.01, respectively) as well as in the second group (P = 0.02 and P = 0.001, respectively). Portal thrombosis was detected in 29 patients (11.3%), more commonly in young patients (< 65 years, P = 0.045), in Child class B and C patients (P = 0.017) and in multifocal HCCs (P = 0.02). Extra-hepatic metastases were present in 19 patients (7.4%), whereas ascites were detected in 68 cases (26.6%).
Patients were grouped into five different stages according to the BCLC system (Figure 2B). Nineteen patients (7.4%) belonged to stage BCLC 0 (very-early), 89 (34.7%) to stage BCLC A (early), 109 (42.6%) to stage BCLC B (intermediate), 27 (10.6%) to stage BCLC C (advanced) and 12 (4.7%) to stage BCLC D (terminal). In the multivariate analysis, the probability of discovering a very early/early HCC (BCLC 0 or A) was higher in patients undergoing regular 6-mo surveillance (P = 0.003) and in the second period (P = 0.007).
Sixty-two patients (24.2%) received palliative care or no therapy. The untreated patient cohort presented with more advanced HCC (BCLC B P = 0.003; BCLC C-D P < 0.001) and a more elevated bilirubin value (P = 0.05). There was a slight, but non-significant, trend to treat fewer women than men (P = 0.079), and patients aged > 65 years (P = 0.053). Treatment distribution was not related to etiology.
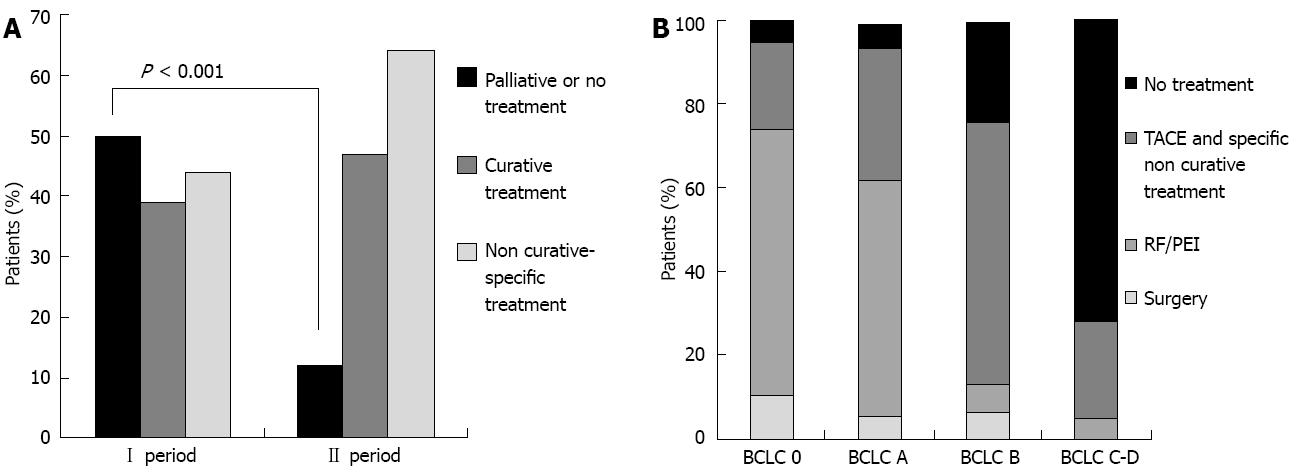
Comparing the two 5-year periods, in the first period 50 patients (37.6%) did not receive any specific treatment for HCC, whereas in the second period, only 12 patients received no specific treatment (9.8%, P < 0.001) (Figure 3A). This decrease was accounted for mainly by HCCs in the intermediate-advanced stage (BCLC B, P = 0.007; BCLC C-D, P = 0.01).
One hundred and ninety-four patients (75.8%) received HCC-specific treatment. During 2000-2005, 83 patients (62.4%) received a specific treatment, compared to 111 patients (90.2%) during 2005-2010. When adjusting for gender, age, etiology, BCLC stage and surveillance, the probability to receive specific treatment was higher in the later time period (P < 0.001).
Among treated patients, 86 (44.3%) underwent curative procedures: surgical resection in 14 cases, percutaneous ablation in 70 cases [percutaneous ethanol injection in five cases, radiofrequency (RF) in 65 cases], and orthotopic liver transplantation (OLT) only in two cases. In three cases it was impossible to perform RF despite the initial indication because of the onset of pain during the procedure (two cases) or HCC location. These three patients underwent trans-arterial chemoembolization (TACE). In 2000-2005, 39 of 83 treated patients (47%) underwent a curative treatment; in the second period it was 47 of 111 treated patients (42.3%, P = 0.61). Interestingly, over the entire study period, only 86 of 108 patients (79.6%) suitable for curative treatment according to BCLC stage effectively underwent it, whereas 15 patients (14%) underwent TACE and seven patients (6.5%) did not receive any specific treatment. In multivariate analysis, curative treatments were less probable in the intermediate-advanced stage (BCLC B, P = 0.01; BCLC C-D, P = 0.007), but there was no difference in curative treatment distribution according to gender, age, etiology and surveillance. In 2000-2005, 69.6% of potentially curable cases underwent a curative treatment, while 21.7% received only TACE and the remaining 8.7% did not receive any specific treatment. In 2005-2010, the proportion of treated patients decreased to 61.3% (P = 0.04), with 32.3% of potentially curable patients receiving TACE and 6.4% not receiving any treatment.
Over the entire study period, among treated patients, 108 (55.6%) underwent non-curative specific treatment: TACE (conventional or Drug-Eluting-Beds TACE) was performed in 90 cases, selective internal radiotherapy (SIRT) in four cases, chemotherapy and/or hormonotherapy in 14 cases. Interestingly, 44 patients who underwent TACE (48.8%) were in BCLC 0-A, i.e., in curable stages (Figure 3B).
At multivariate analysis, TACE application was more common in patients aged < 65 years (P = 0.04), while it was less common in advanced-terminal stage (BCLC C-D P = 0.03) and in patients with bilirubin > 2 mg/dL (P = 0.02); there was no difference in TACE application according to gender, etiology or surveillance.
Thirty-four patients received TACE (77.4% of a total of 44 patients receiving specific non-curative treatment; seven patients underwent chemotherapy and three patients underwent hormonotherapy) in the first period and 56 (87.5% of a total of 64 patients receiving specific non curative treatment; four patients underwent chemotherapy and four patients underwent SIRT) in the second period (P = 0.25). However, after adjusting for gender, age, etiology, BCLC stage and surveillance, the probability to receive TACE was higher in the second period (P = 0.01). TACE application during the second period increased mainly for BCLC B (P = 0.001), but also in BCLC 0-A (P = 0.04).
Only 6 patients were treated with Sorafenib, all of them during the second period.
The prevalence of post-embolization syndrome was 20.4% (50 cases over 245 loco-regional procedures), with no significant differences between the two groups (P = 0.07). Interestingly, in the first period, an antibiotic therapy (without any clinical and/or laboratory suspicion of infectious complication) was started in only six cases (31.6% of all cases), while in the second period, antibiotics treatment was administered to 14 patients (45.2% of all cases) (P = 0.5).
During follow up, nine patients (3.5%; seven patients from the first group and two from the second group) dropped out of the study. By December 31st, 2010, 172 patients (67.2%) had died and 75 patients (29.3%) were still alive.
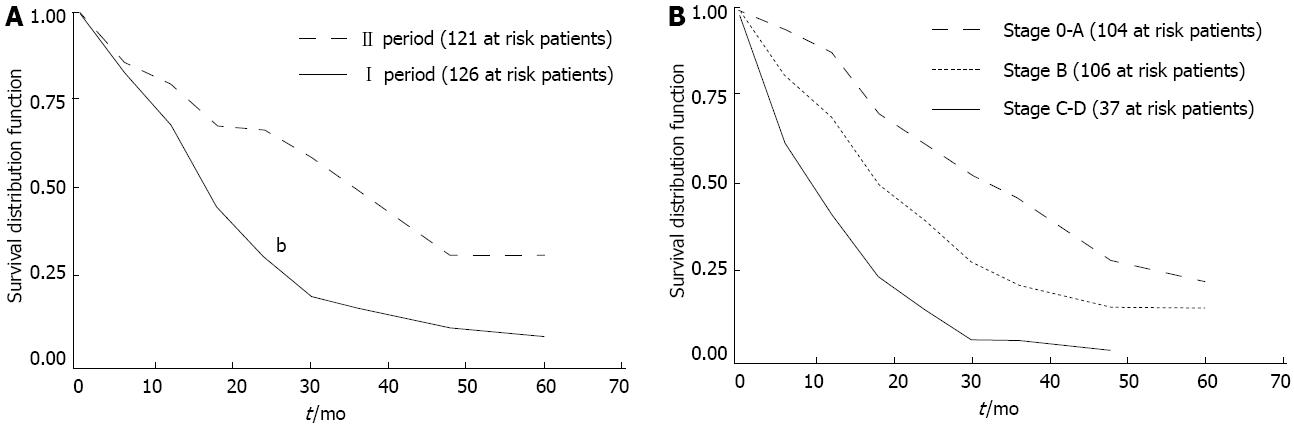
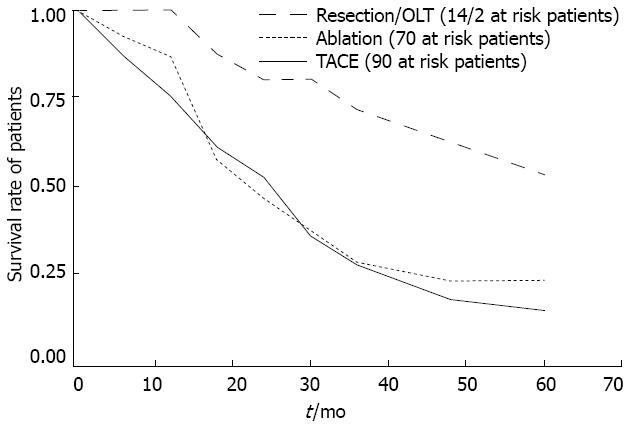
The overall median survival from diagnosis was 22.4 mo (range 0-125 mo). Factors associated with better survival in HCC patients were diagnosis in the second period (P < 0.01) (Figure 4A), early BCLC stage (P < 0.01) (Figure 4B), and treatment (both curative treatments and TACE, P < 0.05) (Figure 5).
On the other hand, age (P = 0.17) and surveillance (P = 0.20) were not significantly associated with a better outcome (Table 1).
| Yes | No | Total | P value | ||
| Gender | Men | 120 (67.8) | 57 (32.2) | 177 | 0.24 |
| Women | 52 (74.3) | 18 (25.7) | 70 | ||
| Age (yr) | ≤ 65 | 39 (61.9) | 24 (38.1) | 63 | 0.17 |
| > 65 | 133 (72.3) | 51 (27.7) | 184 | ||
| Calendar period | 2000-2005 | 116 (92.1) | 10 (7.9) | 126 | < 0.01 |
| 2006-2010 | 56 (46.3) | 65 (53.7) | 121 | ||
| Etiology | Viral infection | 108 (69.7) | 47 (30.3) | 155 | < 0.01 |
| Alcohol | 38 (80.9) | 9 (19.1) | 47 | ||
| Other | 25 (56.8) | 19 (43.2) | 44 | ||
| BCLC stage | A | 59 (56.7) | 45 (43.3) | 104 | < 0.01 |
| B | 79 (74.5) | 27 (25.5) | 106 | ||
| C-D | 34 (91.9) | 3 (8.1) | 37 | ||
| Treatment | No | 60 (93.8) | 4 (6.3) | 64 | 0.04 |
| Yes | 112 (61.2) | 71 (38.8) | 183 | ||
| Surveillance | No | 134 (72.0) | 52 (28.0) | 186 | 0.20 |
| Yes | 38 (62.3) | 23 (37.7) | 61 | ||
| Alpha-fetoprotein | Normal | 104 (59.8) | 70 (40.2) | 174 | < 0.01 |
| Elevated (> 15) | 43 (91.5) | 4 (8.5) | 47 | ||
| Bilirubin | Normal | 48 (57.8) | 35 (42.2) | 83 | < 0.01 |
| Elevated (> 2.1) | 98 (77.8) | 28 (22.2) | 126 |
In the multivariate analysis (Table 2) only treatment (both curative treatments and TACE: HR = 0.37; 95%CI: 0.24-0.57) and BCLC stage (B vs A: HR = 1.50; 95%CI: 1.03-2.19; C-D vs A: HR = 1.75; 95%CI: 1.01-3.03) emerged as significant predictors of survival, while age, gender and surveillance were not significantly associated with a better outcome; patients with a history of alcohol-related liver disease showed a trend toward an increased mortality, but this did not reach the level of statistical significance (alcohol vs viral infection HR = 1.43; 95%CI: 0.96-2.12).
| Multivariate analysis-Cox proportional hazards model | |||
| HR | 95%CI | ||
| Gender | Men | 1 | |
| Women | 1.22 | 0.86-1.72 | |
| Age (yr) | ≤ 65 | 1 | |
| > 65 | 1.20 | 0.82-1.75 | |
| Calendar period | 2000-2005 | 1 | |
| 2006-2010 | 0.67 | 0.46-0.95 | |
| Etiology | Viral infection | 1 | |
| Alcohol | 1.43 | 0.96-2.12 | |
| Other | 0.95 | 0.59-1.53 | |
| BCLC stage | A | 1 | |
| B | 1.50 | 1.03-2.19 | |
| C-D | 1.75 | 1.01-3.03 | |
| Treatment | No | 1 | |
| Yes | 0.37 | 0.24-0.57 | |
| Surveillance | No | 1 | |
| Yes | 1.10 | 0.74-1.65 | |
Elevated bilirubin and alpha-fetoprotein levels were strongly associated with mortality in the univariate analysis (Table 1). However, they were also highly correlated with BCLC stage and the information was not available for about 20% of the patients. Therefore, they were not included in the multivariate model.
This long-term retrospective study was carried out in a large series of consecutive HCCs recruited over ten years at Cuneo Hospital, which is the local referral center for the diagnosis and the treatment of HCC.
We believe that our findings closely represent the “real world” of HCC in Northern Italy. The number of cases identified reflects the current incidence of HCC in Italy, as reported in the latest epidemiological studies[17]. In Italy, HCC incidence is supposed to have started to decrease steadily by 2007[18], as a consequence of reduced HCV infection related cirrhosis. We noticed this trend in our study, with an initial decrease of HCCs in more recent years (133 in the first period vs 123 in the second period), which may be related to a reduction in HCV-related HCCs. This phenomenon could be confirmed in the coming years through continuous monitoring of our population.
Our data confirmed that HCC occurs in a setting of chronic liver disease in 98% of cases[13]. The main risk factor for HCC was HCV infection, as reported in previous Italian series[19-21], followed by alcohol, as in other developed countries[22]. Our data showed only a slight decrease in HCV-related HCC over the study period, although this figure probably would have been more relevant taking into account only the two-year extremes. Furthermore, in the second period there was a significant increase in HCCs unrelated to either viral infection or to alcohol. These cases were mainly patients with type 2 diabetes mellitus. This figure might reflect an increased attention to HCC in this specific population as a consequence of recent studies that reported diabetes and obesity as possible independent risk factors for HCC through the development of NAFLD and NASH[5,23]. To date, there are no evidence-based recommendations as to whether this group should be screened for HCC, as in other HCC high-risk groups[7]. Nonetheless, future data may change this recommendation, and there is increasing interest in this issue in the international literature.
Surveillance programs in HCC high-risk populations have led to the early detection of small tumors eligible for curative therapies[19,24-26], which may lead to improved outcomes[13]. Surveillance with US every 6 mo for detection of early HCC is therefore applied extensively in clinical practice and is recommended by the guidelines for HCC management[6,7].
However, in our study, less than one-third of HCCs (23.8%) were discovered under surveillance, in agreement with other Italian studies[27-29]. On the other hand, surveillance has increased significantly in recent years, probably as a result of increased dissemination of international evidence demonstrating the benefit of surveillance in HCC risk groups. Moreover, in our population, surveillance was inferior in alcoholic patients and in patients with “non-viral/non-alcoholic” cirrhosis. This may be related to poor compliance to strict follow up in the former group, and to a current lack of specific recommendations in the later.
The prognosis in HCC patients is determined by tumor stage, underlying liver function reserve and general health status[6,7,9]. The BCLC staging system[11] takes into account all these factors, and, to date, it is recommended as the reference staging system for HCC[7,12].
As far as tumor stage is concerned, over the entire study period, the most common HCC presentation pattern was a single nodule with a diameter between 3 and 5 cm; notably, in the 2005-2010 period, there was a significant increase in unifocal HCC and in small HCC (< 3 cm of diameter), as a result of the increased use of surveillance programs. In a previous Italian study[30], multifocal HCCs were more common among multi-etiology cirrhotic patients, while unifocal HCCs were more common among HCV-related cirrhosis and among younger patients (< 65 years). In our study, the morphological pattern at the time of diagnosis was not associated with etiology or age, but multifocal HCC was moderately more prevalent among Child B and C class patients than among Child A class patients. With regard to liver function, the majority of our population was in Child class A, in keeping with other Italian studies[19,31]. Moreover, there was an increase in the number of Child class A patients during the second period, which could also be related to the increased use of surveillance.
Finally, as far as HCC staging is concerned, almost half of the population was in BCLC stage B (intermediate stage) at the time of diagnosis. As expected, very-early/early HCCs (BCLC stage 0 and A) were more common in patients who underwent surveillance and, consequently, were present in the 2005-2010 cohort. To date, this is the first Italian study to apply the BCLC staging system; other Italian groups have used the CLIP score[19-21]. Given the complexity of HCC management in clinical practice, we strongly emphasize the need to use a common staging system that can easily define patient groups for different therapies and that can stratify them into separate prognosis categories[7]. The BCLC staging system meets all these characteristics. We stress that the use of guidelines is very important for providing patients with the best treatment options.
In our population, the proportion of HCC patients who underwent specific treatment grew significantly during the last period; however, this growth was not associated with an increased use of curative treatments, as we could expect given the higher number of BCLC 0-A patients in the second period, but may be caused by the increased use of non-curative treatments, such as TACE, in intermediate stage HCCs (BCLC B) in recent years. In fact, in our population, potentially curative therapies were underutilized, even among very early/early HCCs. Similar findings have been obtained in previous studies[21,30,32]. This may have two possible explanations: firstly, the lack of application of BCLC staging system leading to an incorrect allocation of early HCCs to the proper therapeutic approach; and, secondly, the BCLC staging system does not take into account patients’ age, leading to the possibility that, in elderly patients, non-curative treatments were preferred over curative therapies, given the slow HCC growth and the survival benefit provided by non-curative therapies[33].
On the other hand, there was a proportion of patients in our population with intermediate-advanced HCC who underwent curative treatments, namely “overtreated patients”, as reported in another Italian series[30].
Among curative therapies, the most common choice was percutaneous ablation with radiofrequency. This switch from resection to the less invasive and less expensive percutaneous ablation therapy is a current practice supported by the good results achieved in terms of survival. In addition, this preference may be related to the relative high median age of our population (70 years, range 32-92 years); indeed, ablation permits a shorter hospital admission with less severe post-operative complications.
Overall, a large proportion of our population received TACE over the ten-year study period. The use of TACE increased significantly during the last years, mainly in intermediate stage HCCs, as a result of current evidence that TACE improves survival in patients who cannot benefit from curative treatment and who do not have severe impairment in liver function, vascular invasion and extra-hepatic diffusion[13,34,35]. However, our data shows an increased use of TACE in very-early/early HCC stages, and this trend may be explained by the older median age of this patients’ group during the second period, leading to a preference of TACE over curative therapies.
The prevalence of post-embolization syndrome was similar to other series, but in the last years we noticed an increment in antibiotic consumption, despite the absence of signs of infection; this finding underlines the widespread and increasingly frequent inappropriate use of antibiotic treatment in clinical practice.
Liver transplant had a very limited role in the therapy of HCC in our population. Despite a relatively large number of potentially OLT-eligible patients (38 patients, 63% Child-A), only five subjects were submitted to OLT evaluation and two (1%) were definitively transplanted. This low rate of OLT is hard to explain considering that in Piedmont, three liver transplant centers were available at that time. Consequently, at least in our region, OLT should be regarded as a virtual rather than a real therapeutic option.
Notably, almost one-third of HCC patients did not receive any specific treatment; obviously, untreated patients presented more commonly an intermediate-advanced HCC, and there was a non-significant trend in treating fewer patients aged more than 65 years and women. Nonetheless, the rate of untreated patients decreased during the last period, as a result of the parallel increase in treating intermediate-advanced HCCs with non-curative therapies.
As expected, the prognosis of our patients was dictated by liver function, BCLC stage, and diagnosis period.
As far as surveillance is concerned, it was effective in detecting HCCs at an early stage, where curative treatment may be more effective. This fact explains the previously reported survival benefit for patients in surveillance programs[36]. Therefore, the effect of surveillance on survival was usually not significant in multivariate analysis after adjusting for these factors in most studies[37-39]. In addition, at multivariate analysis, surveillance has not proved to be an independent prognostic factor.
Chen et al[40] reported that HCC surveillance resulted in early diagnosis of liver cancer, but not in mortality reduction, because therapy was ineffective. We observed a similar scenario, with an increased rate of HCCs diagnosed at an early stage in the last period, but without a parallel increase in application of curative therapies. Therefore, the surveillance program must be accompanied with appropriate treatment options for patients with newly diagnosed HCC, to improve their survival and justify the expense.
With regard to the therapeutic impact on survival, there was an obvious survival benefit in patients that underwent surgery (OLT plus resection) over the TACE group. Ablation was the most extensively applied curative therapy, but there was only a small survival benefit over the TACE group. This fact may be explained by an inappropriate selection of patients for loco-regional therapy (ablation or TACE). Moreover, as reported by Tseng et al[29], it is possible that in early HCC the two therapies did not differ in terms of survival, but only in terms of recurrence.
Therapy “per se” resulted as an independent favorable prognostic variable in our patients, suggesting that the amenability to treatment identifies a subset of patients with expected better survival.
In conclusion, our cohort study (being in between a population-based and a referral center-based investigation) offers a picture close to what actually occurs in clinical practice. As reported in previous series[19-21,27,28,30], in the real clinical practice, the approach to HCC is far from being adequate, and this tumor remains an undertreated or inappropriately treated complication, despite positive changes that have occurred during recent years. We stress that this state of affairs should be a stimulus for further implementation of surveillance and improved employment of the various therapeutic opportunities available.
Hepatocellular carcinoma (HCC) is the 5th most common cancer and the 3rd leading cause of cancer mortality worldwide. In recent decades, a rising incidence has been reported in Europe and United States. Hepatitis B virus and hepatitis C virus infections are known independent risk factors for HCC, as well as alcohol consumption.
Approaches to HCC treatment are potentially curative depending on cancer stage, liver function and performance status, as well as on resources and the level of practitioner expertise. The Barcelona Clinic Liver Cancer (BCLC) staging system has come to be widely accepted in clinical practice as the reference system to stratify patients into separate prognostic categories and to suggest the treatment options according to the stage.
Data showed a significant increase in HCCs related to neither viral infection nor alcohol; these cases were mainly patients with type 2 diabetes mellitus. This figure might reflect an increased attention in research HCC in this specific population, as a consequence of recent evidence reporting diabetes and obesity as possible independent risk factors for HCC through the development of non-alcoholic steatohepatitis and non-alcoholic fatty liver disease.
The study identified the increased use of surveillance in the latest years, probably as a result of increasing diffusion and knowledge of international evidence about related benefits. However, at multivariate analysis, surveillance has not proved to be an independent prognostic factor. In this population study, potentially curative therapies were underutilized, and this should be a stimulus to a more adequate employment of different therapeutic opportunities.
The authors present an interesting series of patients with HCC treated over 10 years in a single institution. Changes in epidemiology and a lack of strict adherence to the BCLC treatment recommendations follow other recently published series.
P- Reviewer Sangro B S- Editor Gou SX L- Editor Stewart GJ E- Editor Li JY
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2651] [Cited by in RCA: 2599] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 2. | Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705-714. [PubMed] |
| 3. | Chung H, Ueda T, Kudo M. Changing trends in hepatitis C infection over the past 50 years in Japan. Intervirology. 2010;53:39-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 4. | Seeff LB. Introduction: The burden of hepatocellular carcinoma. Gastroenterology. 2004;127:S1-S4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Yasui K, Hashimoto E, Komorizono Y, Koike K, Arii S, Imai Y, Shima T, Kanbara Y, Saibara T, Mori T. Characteristics of patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2011;9:428-433; quiz e50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 322] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 6. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3252] [Cited by in RCA: 3244] [Article Influence: 135.2] [Reference Citation Analysis (0)] |
| 7. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6574] [Article Influence: 469.6] [Reference Citation Analysis (1)] |
| 8. | Sherman M. Surveillance for hepatocellular carcinoma and early diagnosis. Clin Liver Dis. 2007;11:817-837, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2881] [Cited by in RCA: 3088] [Article Influence: 220.6] [Reference Citation Analysis (0)] |
| 10. | El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:1752-1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 768] [Cited by in RCA: 825] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 11. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2877] [Article Influence: 110.7] [Reference Citation Analysis (1)] |
| 12. | Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30:61-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 866] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 13. | Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3241] [Cited by in RCA: 3282] [Article Influence: 149.2] [Reference Citation Analysis (0)] |
| 14. | Giannini EG, Bodini G, Corbo M, Savarino V, Risso D, Di Nolfo MA, Del Poggio P, Benvegnù L, Farinati F, Zoli M. Impact of evidence-based medicine on the treatment of patients with unresectable hepatocellular carcinoma. Aliment Pharmacol Ther. 2010;31:493-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5490] [Cited by in RCA: 5738] [Article Influence: 110.3] [Reference Citation Analysis (2)] |
| 16. | Trevisani F, Caraceni P, Bernardi M, D’Intino PE, Arienti V, Amorati P, Stefanini GF, Grazi G, Mazziotti A, Fornalè L. Gross pathologic types of hepatocellular carcinoma in Italian patients. Relationship with demographic, environmental, and clinical factors. Cancer. 1993;72:1557-1563. [PubMed] |
| 17. | AIRT Working Group. Italian cancer figures--report 2006: 1. Incidence, mortality and estimates. Epidemiol Prev. 2006;30:8-10, 12-28, 30-101 passim. [PubMed] |
| 18. | Bosetti C, Bianchi C, Negri E, Colombo M, La Vecchia C. Estimates of the incidence and prevalence of hepatocellular carcinoma in Italy in 2002 and projections for the years 2007 and 2012. Tumori. 2009;95:23-27. [PubMed] |
| 19. | Sangiovanni A, Del Ninno E, Fasani P, De Fazio C, Ronchi G, Romeo R, Morabito A, De Franchis R, Colombo M. Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance. Gastroenterology. 2004;126:1005-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 429] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 20. | Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35-S50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1691] [Cited by in RCA: 1793] [Article Influence: 85.4] [Reference Citation Analysis (2)] |
| 21. | Trevisani F, Magini G, Santi V, Morselli-Labate AM, Cantarini MC, Di Nolfo MA, Del Poggio P, Benvegnù L, Rapaccini G, Farinati F. Impact of etiology of cirrhosis on the survival of patients diagnosed with hepatocellular carcinoma during surveillance. Am J Gastroenterol. 2007;102:1022-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Nordenstedt H, White DL, El-Serag HB. The changing pattern of epidemiology in hepatocellular carcinoma. Dig Liver Dis. 2010;42 Suppl 3:S206-S214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 404] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 23. | El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460-468. [PubMed] |
| 24. | Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 960] [Cited by in RCA: 945] [Article Influence: 45.0] [Reference Citation Analysis (1)] |
| 25. | Bolondi L, Sofia S, Siringo S, Gaiani S, Casali A, Zironi G, Piscaglia F, Gramantieri L, Zanetti M, Sherman M. Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: a cost effectiveness analysis. Gut. 2001;48:251-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 457] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 26. | Kuo YH, Lu SN, Chen CL, Cheng YF, Lin CY, Hung CH, Chen CH, Changchien CS, Hsu HC, Hu TH. Hepatocellular carcinoma surveillance and appropriate treatment options improve survival for patients with liver cirrhosis. Eur J Cancer. 2010;46:744-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Lencioni R. Surveillance and early diagnosis of hepatocellular carcinoma. Dig Liver Dis. 2010;42 Suppl 3:S223-S227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Santi V, Trevisani F, Gramenzi A, Grignaschi A, Mirici-Cappa F, Del Poggio P, Di Nolfo MA, Benvegnù L, Farinati F, Zoli M. Semiannual surveillance is superior to annual surveillance for the detection of early hepatocellular carcinoma and patient survival. J Hepatol. 2010;53:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 237] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 29. | Tseng PL, Wang JH, Tung HD, Hung CH, Kee KM, Chen CH, Chang KC, Lee CM, Changchien CS, Chen PF. Optimal treatment increased survival of hepatocellular carcinoma patients detected with community-based screening. J Gastroenterol Hepatol. 2010;25:1426-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Farinati F, Sergio A, Baldan A, Giacomin A, Di Nolfo MA, Del Poggio P, Benvegnu L, Rapaccini G, Zoli M, Borzio F. Early and very early hepatocellular carcinoma: when and how much do staging and choice of treatment really matter? A multi-center study. BMC Cancer. 2009;9:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 31. | Borzio M, Colloredo G, Pioltelli P, Quagliuolo M. Epidemiology and outcome of hepatocellular carcinoma in Lombardy. Dig Liver Dis. 2007;39:1011-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | El-Serag HB, Siegel AB, Davila JA, Shaib YH, Cayton-Woody M, McBride R, McGlynn KA. Treatment and outcomes of treating of hepatocellular carcinoma among Medicare recipients in the United States: a population-based study. J Hepatol. 2006;44:158-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 178] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 33. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2207] [Cited by in RCA: 2272] [Article Influence: 103.3] [Reference Citation Analysis (0)] |
| 34. | Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology. 2004;127:S179-S188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 397] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 35. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4508] [Article Influence: 225.4] [Reference Citation Analysis (0)] |
| 36. | Kemp W, Pianko S, Nguyen S, Bailey MJ, Roberts SK. Survival in hepatocellular carcinoma: impact of screening and etiology of liver disease. J Gastroenterol Hepatol. 2005;20:873-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Stravitz RT, Heuman DM, Chand N, Sterling RK, Shiffman ML, Luketic VA, Sanyal AJ, Habib A, Mihas AA, Giles HC. Surveillance for hepatocellular carcinoma in patients with cirrhosis improves outcome. Am J Med. 2008;121:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 38. | Toyoda H, Kumada T, Kiriyama S, Sone Y, Tanikawa M, Hisanaga Y, Yamaguchi A, Isogai M, Kaneoka Y, Washizu J. Impact of surveillance on survival of patients with initial hepatocellular carcinoma: a study from Japan. Clin Gastroenterol Hepatol. 2006;4:1170-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 39. | Chan AC, Poon RT, Ng KK, Lo CM, Fan ST, Wong J. Changing paradigm in the management of hepatocellular carcinoma improves the survival benefit of early detection by screening. Ann Surg. 2008;247:666-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 40. | Chen JG, Parkin DM, Chen QG, Lu JH, Shen QJ, Zhang BC, Zhu YR. Screening for liver cancer: results of a randomised controlled trial in Qidong, China. J Med Screen. 2003;10:204-209. [PubMed] |