Published online May 21, 2013. doi: 10.3748/wjg.v19.i19.2847
Revised: March 22, 2013
Accepted: April 27, 2013
Published online: May 21, 2013
Processing time: 270 Days and 1.8 Hours
The biological properties of tumor cells are known to be regulated by a multitude of cytokines and growth factors, which include epidermal growth factor receptor agonists and members of the transforming growth factor β family. Furthermore, the recent explosion of research in the field of chemokine function as mediators of tumor progression has led to the possibility that these small, immunomodulatory proteins also play key roles in carcinogenesis and may, therefore, be potential targets for novel therapeutic approaches. In this review, we will summarize recently reported findings in chemokine biology with a focus on the gastrointestinal tract.
Core tip: The chemokine network makes an attractive target for therapeutic intervention in many tumor types, including those of the gastrointestinal tract. However, we need to define more selective and specific targets, to minimize systemic side effects during treatment.
- Citation: Miyazaki H, Takabe K, Yeudall WA. Chemokines, chemokine receptors and the gastrointestinal system. World J Gastroenterol 2013; 19(19): 2847-2863
- URL: https://www.wjgnet.com/1007-9327/full/v19/i19/2847.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i19.2847
Cancer is a disease in which normal cells acquire genetic and epigenetic abnormalities[1,2], leading to disorientation of conventional processes for the maintenance of normal cell physiology. These aberrant genetic and epigenetic modifications to the normal cell induce abnormal cell motility, proliferation, and survival, eventually enabling these cells to invade into adjacent tissues and even to migrate to regional or distant organs where they may grow continuously as metastatic lesions[3]. For many cancer patients, metastasis is generally the major cause of disease-related death[4,5]. Therefore, it is indispensable to elucidate the basic molecular mechanisms of tumor development to identify effective therapeutic targets, which can possibly reduce the side-effects of treatment, and define useful molecular markers for early detection and prediction of disease course. Especially, the newly emerged concept of personalized medicine may require in-depth assessment of potential therapeutic molecular target(s) in each individual case through analysis of signaling pathways and subsequent validation of treatment efficacy[6].
Numerous reports have identified molecules that play key roles in development of gastrointestinal cancer. Amongst these, which include growth factors and their receptors[7-9], signaling pathway components[10,11], transcription factors[12,13], matrix remodeling enzymes[14,15] and cytokines[16-18], a milestone finding by Müller et al[19] made chemokines one of the most intensively studied molecular targets to understand the mechanisms of organ-specific metastasis. The chemotactic cytokines or chemokines contribute to the tumor microenvironment by establishing a chemokine gradient, which is important for the process of chemoattraction and subsequent cell motility and infiltration for metastasis[20,21]. In this review, we will summarize the current status of chemokine-related studies in digestive tract cancer.
Recent progress has made it clear that the non-tumor cells, such as stromal fibroblasts and inflammatory cells present in the microenvironment, also play critical roles in establishing the metastatic phenotype of tumor cells[22,23]. Indeed, a role for inflammation in cancer progression is well-recognized, with many different cancer types having an inflammatory component[24]. Tumor cell infiltration is aided by the presence of tumor-associated macrophages (TAMs), dendritic cells and lymphocytes[25-28]. While TAMs are able to kill tumor cells, they also play an important role in enhancing tumor development by secreting matrix metalloproteinases (MMPs)[29,30], growth factors [interleukins (ILs), vascular endothelial growth factors (VEGFs)] and pro-angiogenic factors that support tumor cell growth, neovascularization, and tumor cell invasion through the stromal tissues[31].
Chemokines and chemokine receptors: In humans, the chemokine superfamily is comprised of more than 50 small secreted proteins. These molecules function as immune modulators, chemoattractants and as activators of lymphocytes. They are sub-divided into four major groups based on the relative position of conserved cysteine residues near to the N-terminus: CXC-, CC-, C-, and CX3C-chemokines[32-34]. The CXC-chemokines can be further subdivided according to the presence or absence of a three amino acid motif immediately N-terminal to the first cysteine. Therefore, a glutamic acid-leucine-arginine (ELR) sequence defines the ELR+ CXC-chemokines, which generally function as activators and chemoattractants for neutrophils. In contrast, lymphocytes constitute the target cell type for most ELR- CXC chemokines. The CC-chemokines play both chemoattractant and immunomodulatory roles. CCL5 attracts monocytes, eosinophils and memory T-cells, and is one of the human immunodeficiency virus (HIV)-suppressive factors secreted by CD8+ T-cells[35]. Similarly, CCL3 and the closely related CCL4 are also capable of inhibiting HIV infection of target cells, in addition to their pro-inflammatory and chemoattractant functions[35]. Furthermore, CC-chemokines are also reported to be involved in lymphocyte recirculation and homing to secondary organs (CCL21), as well as T-cell trafficking within the thymus (CCL19)[36-38].
While the major biological functions ascribed to chemokines regulate leukocyte trafficking and recruitment to inflammatory foci, chemokine receptors are also expressed on non-immune cells, and act as key modulators of the biological functions of other cell types. For example, CCL3 is a negative regulator of keratinocyte growth[39], while the CXC-chemokine ligand (CXCL) 5[40], CXCL8[41,42] and other ELR+ chemokines stimulate endothelial cell migration during angiogenesis[43].
To date, 19 chemokine receptors are known to be expressed in mammals[44]. Chemokines activate these specific G-protein-coupled receptors (GPCRs) on the surface of target cells. Chemokine receptors consist of an extracellular ligand binding domain, seven transmembrane spans, and an intracellular carboxyl terminus. The transmembrane domain consists of three intracellular domains and three hydrophobic extracellular domains. Variation of the amino-terminus sequence of these receptors defines the specificity for recognition of chemokine ligands, the binding of which results in phosphorylation of Ser/Thr residues in the intracellular domain and induction of f to the N-terminus of the receptor, specific signal transduction will be induced by the release of these heterotrimeric G-proteins (guanine nucleotide-binding proteins -G/G/G) that are bound to the intracellular carboxyl terminus. Each of these heterotrimeric G-proteins consists of several different subtypes[45]. Chemokine receptors are sub-divided into four types in relation to the class of ligand to which they bind: CXC-chemokine receptors (CXCR), which bind CXC-chemokines; CC-receptors (CCR), for CC-chemokines; and XC-receptors and CX3C-receptors (CX3CR) for XC- and CX3C-chemokines, respectively. Among these receptors and ligands, there is promiscuous binding between several receptors and multiple chemokines (Table 1)[38].
| CC-chemokines | CXC-chemokines | CX3C-chemokine | |||
| Ligands | Receptors | Ligands | Receptors | Ligands | Receptors |
| CCL2 | CCR2 | CXCL1 | CXCR2/CXCR1 | CX3CL1 | CX3CR1 |
| CCL3 | CCR1/CCR5 | CXCL2 | CXCR2 | ||
| CCL4 | CCR5 | CXCL4L1 | CXCR3 | ||
| CCL5 | CCR1/CCR3/CCR5 | CXCL5 | CXCR2 | ||
| CCL7 | CCR1/CCR2/CCR3 | CXCL7 | CXCR2 | ||
| CCL8 | CCR3/CCR5 | CXCL8 | CXCR1 | ||
| CCL13 | CCR2/CCR3 | CXCL9 | CXCR3 | ||
| CCL19 | CCR7 | CXCL10 | CXCR3 | ||
| CCL20 | CCR6 | CXCL11 | CXCR3 | ||
| CCL21 | CCR7 | CXCL12 | CXCR4/R7 | ||
| CCL25 | CCR9 | CXCL14 | Unknown | ||
| CCL27 | CCR10 | CXCL17 | Unknown | ||
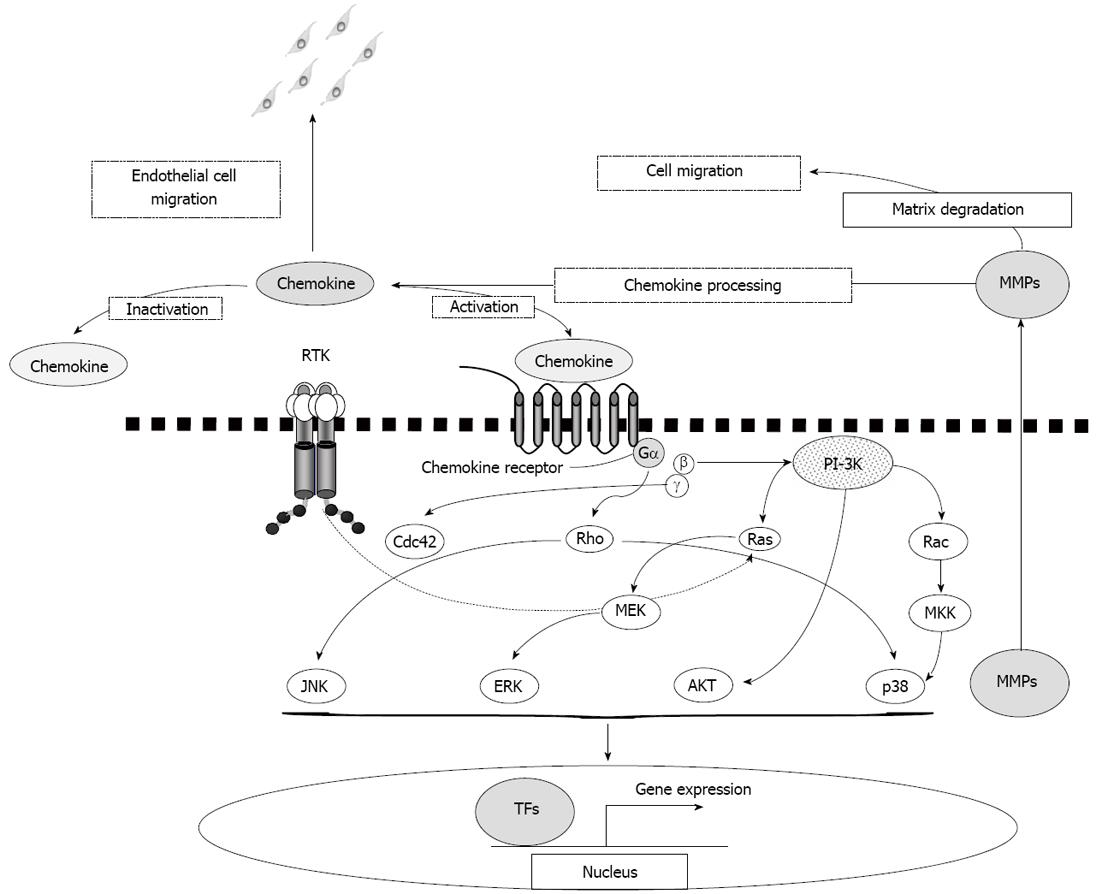
Mechanisms of signal transduction: Binding of chemokines to their innate receptors can activate a number of intracellular signaling pathways that lead to cell proliferation and migration. Downstream mediators identified to date include the small GTPase Ras; extracellular signal-regulated kinases (ERK)[46,47]; phosphatidyl inositide-3-OH kinase (PI-3K)[47-49], and the other small GTPases Rho, Rac and Cdc42[50,51]; and some of these are active in pathways that regulate the actin cytoskeleton. For example, it was shown that migration of Jurkat cells in response to CXCL12 through CXCR4 is mediated by both Rac- and Rho-dependent mechanisms. While Rac is activated by Gi and Gβγ subunits, activation of RhoA occurs via Gα13 and leads to phosphorylation of myosin light chains[52]. Furthermore, CXCL8 (IL-8) signaling through CXCR1 has been reported to mediate migration of leukocytes in a β1-integrin-dependent manner which requires downstream activation of p38 and c-Jun N-terminal kinases[53]. The quality and the quantity of chemokine signaling is not controlled only by the expression volume of chemokine ligand/receptor but, also, by proteolytic processing of chemokine ligands. In this regard, MMPs are major modulators of chemokine signaling. For example, MMP9 can activate CXCL8 by processing its amino terminus, but it also abolishes the function of CXCL1 by cleaving it. Likewise, chemotactic activity of CCL7 is decreased by MMP2, and CXCL12 is decreased by multiple MMPs. However, numerous other proteases have also been documented to act upon chemokines and modulate their activity[54]. Furthermore, recent observations suggest more complexity in signal transduction mechanisms that are induced by heterodimerization of GPCRs[55]. As indicated above, chemokine and chemokine receptor interactions are complex and are connected to multiple combinations of effector proteins and divergent intracellular signaling pathways (Figure 1).
Many growth factors and cytokines act to control cell proliferation, either in a positive or a negative manner[56]. For example, epidermal growth factor (EGF) and related family members activate the EGF receptor (EGFR) and initiate divergent biochemical cascades that result in transcription of genes involved in cell cycle progression and other processes necessary for growth. In contrast, transforming growth factor-beta negatively regulates epithelial cell growth by inhibiting cell cycle transit. Signal transduction pathways regulated by these and other growth factors frequently become altered during tumorigenesis, resulting in deregulated cell growth. It has now become clear that deregulated function of multiple chemokines also contributes to enhanced tumor cell proliferation.
The ELR+ CXC-chemokines play important roles in melanoma cell growth[57,58]. CXCL1 has also been implicated in non-melanoma skin cancers, including tumors of neural origin and squamous cell carcinomas. Zhou et al[59] demonstrated that CXCL1 is highly expressed in anaplastic astrocytomas in vivo, and reported that this enhanced cell growth, motility, adhesion to extracellular matrix, and invasion in vitro, as well as enhanced aggressiveness in vivo. Constitutive expression of CXCL1 in squamous cell carcinomas results in formation of an autocrine growth loop through the CXCR2 receptor, while overexpression of CXCL1, CXCL2 and CXCR2 in esophageal cancer also enhances proliferation[60]. Furthermore, it was found that CXCL2 activates signal transduction through an ERK dependent pathway[61].
Transcriptional upregulation mediated through nuclear factor κB (NFκB)-dependent pathways has been reported to be largely responsible for the enhanced levels of CXCL1 in tumor cells. The tumor promoter okadaic acid, which inhibits protein phosphatases and results in hyperphosphorylation of proteins at serine and threonine residues, activates transcription through two response elements in the CXCL1 promoter, utilizing three distinct NFκB subunits (p65, p52 and c-Rel)[62]. Upregulation of NFκB by the upstream NFκB-inducing kinase is also an important mechanism by which CXCL1 is upregulated[63]. NFκB-dependent induction of CXCL1 is further regulated by poly (ADP-ribose) polymerase-1 (PARP-1). Inactive PARP-1 binds to the CXCL1 promoter and blocks transcription by excluding NFκB. However, activation of PARP-1 causes its promoter binding ability to be lost leading to NFκB upregulation of CXCL1. In melanoma cells, PARP-1 is highly expressed and active[64], and may be a major contributor to melanoma cell proliferation via chemokine-dependent mechanisms, together with constitutively expressed NFκB.
In terms of cancer metastasis, chemokine-dependent mechanisms for targeting to specific secondary sites is now widely recognized after studies showed upregulation of CXCR4 and CCR7 in breast cancer cells and that activation of these receptors could induce actin polymerization, migration and invasion[19]. Importantly, ligands for these receptors were shown to be expressed in organs that represent the primary sites for breast cancer metastasis, strongly suggesting that ligand-receptor “homing” functions in vivo to target tumor cells to sites of secondary growth. Organ-specific metastasis has been reported for different tumor types, including breast[65], ovary[46] and epidermoid carcinomas[60]. Further, more than twenty tumor types have been documented to overexpress CXCR4[66]. Upregulation of CXCR4 expression in tumor cells through the action of VEGF also appears to be an important mechanism to further enhance invasiveness[67].
CXCR4/CXCL12 overexpression is associated with metastasis to lung, liver, lymph nodes and bone marrow. Rearrangement of the actin cytoskeleton and alteration in cell polarity are fundamental processes required for cell motility, regulated at least in some cases by CXCL12-CXCR4 pathways[68]. CXCL12-CXCR4 signaling may also contribute to tumor progression by upregulating protease expression. In prostate cancer cells, various MMPs were shown to be modulated by CXCL12[69]. However, these effects were not consistent for all cell lines examined, suggesting that cell-specific factors may influence the response to CXCL12. In glioma cells, CXCL12 induced expression of MMP15 but not gelatinases. RNA interference studies proved that glioma cell invasiveness in vitro, and tumor aggressiveness in vivo, is due to the upregulation of MMP15 by CXCL12[70]. Chemokine-receptor interactions in skin, as a frequent metastatic site of malignant melanoma, may be another example: CCL27 is highly expressed in skin and the CCR10 receptor for this ligand is frequently upregulated in melanoma cells[71,72].
Furthermore, mounting evidence points to cancer-associated stromal fibroblasts playing important roles in modulating tumor cell behaviour[73,74]. As p53 mutation or loss has been shown to occur in stromal cells[75,76], this may upregulate CXCL12 and enhance proliferation and motility[77].
The majority of metastatic tumor cells fail to colonize secondary lesions successfully, most likely due to induction of programmed cell death[78]. For metastasized lesions to grow, enhancement of growth factors as well as positive molecular interactions with surrounding cells in the lymph node or other organs are indispensable. For example, EGFR signaling can activate survival pathways regulated by protein kinase B (AKT)[79]. However, despite the presence of available growth factors, tumor growth at the secondary lesion may still be uncertain: it may also require other factors and/or pathways in order for cells to survive and proliferate. Studies have also shown that resistance to cell death induced by loss of attachment to the extracellular matrix (anoikis) is an important element at least for certain tumors[80]. Interestingly, some chemokine-receptor combinations, such as CXCL5-CXCR2[48] and CCL19/21-CCR7[81], can activate PI-3K and AKT, key regulators of cell survival. Thus, interaction of chemokines with their receptors could play roles in multiple and complex biological processes that are important for successful survival in metastasized locations. For example, CCL2 is well known for regulating cell migration and is a key mediator of breast cancer cell migration[82]. Also using breast cancer cell lines, Fang et al[83] demonstrated enhanced cell migration and survival along with increased phosphorylation of Smad3 and mitogen-activated protein kinases (MAPKs) in response to CCL2 and, moreover, they found that levels of the innate receptor CCR2 were elevated in breast cancers, accompanied with CCL2 expression. These investigators also suggested that MAPK and Smad3 signaling function as an independent/alternative mechanism for cell survival. Furthermore, they showed that CCL2-induced Smad3 signaling through MAPKs regulates expression and activity of Rho GTPase, thereby facilitating breast cancer cell motility and survival. Therefore, beyond well-described canonical chemokine ligand/receptor signaling, new molecules may need to be considered as critical players in chemokine signaling in cancer. The CXCL12-CXCR4 signaling pathway has been shown to be important for the survival of leukemic B cells in chronic lymphocytic leukemia (CLL) through activation of AKT and ERK1/2[84,85]. Moreover, O’Hayre et al[86] recently identified additional molecular targets and novel phosphoproteins as possible mechanisms for cell survival in CLL. Amongst these is programmed cell death factor 4, found in all CLL cells examined, and also heat shock protein 27, which mediates anti-apoptotic signaling and has previously been linked to chemotherapeutic resistance, which was detected in a subpopulation of CCL patients. If the roles of these cell-survival-related proteins are supported by further future studies, it may be worth considering identifying these and other phosphoproteins whose functions are modified by certain chemokines in specific pathological conditions, such as cancer and/or inflammation in gastrointestinal disease.
Development of microvessels is an another critical event that enables oxygen delivery and nurtures tumor cell survival at both primary and secondary sites[87]. Recently, intensive studies in normal angiogenic development using mouse model systems have revealed the importance of the angiogenic chemokine CXCL12 for the organized development of vessel branching along with neural development (https://intramural.nhlbi.nih.gov/labs/ldbsn/pages/publications.aspx). Furthermore, Komatsu et al[88] reported the importance of the small-G protein R-Ras for the development of abnormal collateral capillary systems which may play critical roles in the survival of localized tumors by supporting nutrient and oxygen delivery,[89]. Nonetheless, it remains unclear how the tumor cells, which express chemokine receptors, respond to chemokines released from these pathological vessels, which are inherently “leaky”. Several different chemokines are known to be pro-angiogenic, notably CXCL5 and CXCL8. Koch et al[90] clearly demonstrated that CXCL8 could induce neovascularization in a rabbit corneal pocket assay. Furthermore, they also showed that the angiogenic activity present in conditioned media derived from macrophages or monocytes from rheumatoid synovial tissues was dependent upon CXCL8. Indeed, macrophages have been reported to induce malignant progression in a breast cancer model by initiating the angiogenic switch[91]. Additionally, CXCL8-CXCR2 signaling facilitates migration and proliferation of endothelial cells[92], and the AKT pathway is important for GPCR-dependent angiogenesis[93] following CXCR1 and CXCR2 activation[49,81]. Also, the human herpesvirus-8, an etiological agent of the highly vascular Kaposi’s sarcoma, induces expression of CXCL8[94], providing further evidence for chemokine involvement in tumorigenesis[95].
Notably, in contrast to the ELR+ chemokines discussed above, most ELR- chemokines have anti-angiogenic or angiostatic activity[96]. Among these, CXCL9, CXCL10 and CXCL11 are inducible by other cytokines, including members of the IL family and interferons. The angiostatic response of these cytokine-inducible chemokines is mediated through the CXCR3 receptor, found on the surface of endothelial cells[97]. Specifically, an alternatively spliced variant of the receptor - CXCR3B - has been shown to mediate this activity[98]. In addition to inhibiting endothelial cell migration, these chemokines also block proliferation. A further critical review focused on the development of tumor angiogenesis is available[99].
Compared to normal cells, many cancer cells overexpress chemokine and chemokine receptors. Ligation of overexpressed chemokine receptors on tumor cells and the specific chemokines released from target organs seem to be critical regulators of metastasis[19,100]. As outlined above, chemokines and their receptors play various important roles in the regulation of invasion, metastasis and dissemination of cancer cells. Here we review chemokine/chemokine receptor interactions, specifically in digestive organs.
In the head and neck region, which includes oral cavity, pharynx, larynx, nasal cavity and paranasal sinuses, the head and neck squamous cell carcinoma (HNSCC) accounts for more than 90% of malignant neoplasms[101]. Despite intensive efforts, survival rates have shown limited improvement over the decades. When primary tumor location is taken into account, the outcome can be even worse, with advanced hypopharyngeal tumors having a 4% five-year survival[102]. Metastasis of HNSCC is generally via the lymphatic system to loco-regional sites. Recently, several different chemokines were shown to be highly expressed in HNSCC derived cell lines and patient tumor samples. For example, in a series of 94 HNSCCs, CXCL1 was found to be overexpressed in around 40% of lesions[103]. Measurement of microvessel density (MVD) in HNSCCs revealed a correlation between CXCL1 expression and angiogenic activity, as well as with nodal metastasis and infiltration of leukocytes. CXCL8 has long been recognized to participate in autocrine (and possibly paracrine) regulation of HNSCC proliferation[60]. Recent studies using global gene expression profiling of primary and synchronous metastatic HNSCC further support previous reports of CXCL8 upregulation[104]. Along with this observation, Chen et al[105] reported that hydrogen sulfide produced by Porphyromonas gingivalis bacteria in the oral cavity induced expression of CXCL8 in gingival and oral epithelial cells. Potentially, this may provide a link between tumor development and the induction of inflammation in periodontal disease, which is associated with persistent bacterial infection, and similar results to this study have also been reported[106].
In addition to CXCL8, another ELR+ angiogenic chemokine, CXCL5, is highly expressed in some HNSCCs. Data suggest that CXCL5 enhances tumor development by stimulating proliferation, cell motility and invasion[107] as knockdown of CXCL5 by siRNA completely inhibited tumorigenicity in a mouse xenograft model.
Furthermore, Delilbasi et al[108] reported upregulated CXCR4 expression in squamous carcinomas of the tongue by an immunohistochemical method. Of interest, their experiment exhibited no difference in expression between primary tumors of early and more advanced stage, although invading cells and those which had metastasized to lymph nodes exhibited higher CXCR4 expression, suggesting in vivo selection for this phenotype with malignant progression. Clatot et al[109] also studied the possible correlation of CXCL12/CXCR4 expression and tumor recurrence and survival in HNSCC patients. They found no meaningful correlation between CXCR4 expression and either recurrence or survival, but a significant difference in CXCL12 expression. Further prospective studies are required to clarify this.
The loss of CCR6 expression in metastatic lesions with concomitant elevation of CCR7 in some HNSCCs was also documented[110]. CCL19 and CCL21, ligands for CCR7, induced migration of metastatic cells in vitro, whereas primary tumor cells responded to the CCR6 ligand, CCL20. Together, these data suggest that CCR7 upregulation might play a role in targeting tumor cells to sites of secondary growth in vivo, by facilitating entry into the lymphatic system and migration to regional lymph nodes. CCR7 signal transduction was also shown to activate cellular invasion and pro-survival pathways by PI-3K and PLCγ-dependent, but EGFR-independent, mechanisms[111].
In the esophagus, several cytokines and chemokines are reported as possible mediators of gastroesophageal reflux, esophagitis, pre-cancerous change (typically Barrett’s esophagus) and adenocarcinoma[112]. Amongst these, CXCL8 is reported as a molecular marker indicative of response to therapeutic procedures. For example, Oh et al[113] compared the expression level of CXCL8 between pre- and postoperation of Nissen fundoplication in reflux esophagitis. They found that CXCL8 expression was significantly reduced postoperatively, as measured by quantitative real-time polymerase chain reaction (qRT-PCR). These authors also reported that CXCL8 expression was higher in patients with reflux compared to those without reflux. Furthermore, they found that patients with the highest CXCL8 expression were those with Barrett’s dysplasia and adenocarcinoma. Chemokines and several other cytokines, such as ILs, CXCL8, and VEGFs were found to be upregulated in cancer-related cachexia, although the underlying mechanism is not understood[114]. Moreover, chemokine expression in tumors is complicated. Verbeke et al[115] screened 51 patients operated on for colon adenocarcinoma, esophageal adenocarcinoma, or esophageal squamous cell carcinoma (SCC) by immunohistochemical staining to examine the expression of CXCL4L1, CXCL8, CXCL10, CXCL12, and VEGF. According to their study, the angiostatic chemokine CXCL4L1 was strongly expressed in colorectal cancer, while there was weaker expression in esophageal cancer. CXCL12 staining was almost negative in esophageal SCC, while stronger staining was observed in adenocarcinoma of the esophagus and colon. VEGF was moderately-to-strongly positive in all 3 types of cancer, but relatively weaker in esophageal adenocarcinoma. Interestingly, not only was the expression of the angiogenic chemokines CXCL8 and CXCL12 heterogeneous in the samples, but so was the expression of the angiostatic chemokines CXCL10 and CXCL4L1. Based on these results, the roles of chemokines are likely complex in these tumors[115]. Recently, in vitro and in vivo studies using AMD3100, a pharmacological CXCR4 inhibitor, and the human HER2 inhibitor, trastuzumab, have indicated CXCR4 to be a positive regulator of human epidermal growth factor receptor 2 (HER2) expression in esophageal cancer. In this study, the authors also suggested a possible complex relationship between HER2 and CXCR4 in tumor development and metastasis[116]. These observations indicate further the involvement of multiple chemokines in molecular events that underpin different stages of cancer development.
In gastric cancer treatment, detection at an early stage of tumor development is still the most effective curable approach. Amongst different types of gastric cancer, peritoneal dissemination is one of the most incurable conditions and no radical and effective treatment is available to date. Hashimoto et al[117] showed that the CXCL12/CXCR4 axis is important in peritoneal dissemination of gastric cancer cells. They found that CXCL12 ligation to CXCR4 on the cancer cell surface strongly and rapidly activates AKT-mammalian target of rapamycin signaling and co-activates production of other metastatic mediators, such as MMPs[117,118]. Graziosi et al[119] also suggested possible involvement of the p38 MAPK pathway in the development of peritoneal dissemination. Using an in vivo mouse system and administering p38 MAPK inhibitors ML3403 or SB203580, they observed decreased tumorigenesis. Microarray studies using these cancer cells identified several downregulated genes, such as CXCR4, fms-related tyrosine kinase 4, non-receptor spleen tyrosine kinase and collagen α2(IV). Interestingly, p38 MAPK inhibitors induced significant downregulation of multidrug resistance-1 gene expression, a well-defined marker of resistance to chemotherapy, which possibly induced susceptibility to the cisplatin treatment in this model[119]. In a separate study, Zhi et al[120] examined the expression of CXCL12 in normal gastric tissue, gastric cancer cell lines, 35 primary gastric carcinomas and corresponding normal gastric tissues. They found that CXCL12 was downregulated in gastric cancer cell lines and patient samples of primary gastric carcinomas compared to normal samples. They provided evidence to show that CXCL12 expression was inversely associated with lymph node metastasis and histological grade. They concluded the possible cause of this downregulation of CXCL12 expression to be hypermethylation of the CXCL12 promoter, as treatment of highly metastatic cancer cell lines with a demethylating agent impaired its invasive phenotype[120]. One possible explanation for this reduction in CXCL12 might be to permit CXCR4-expressing gastric cancer cells to sense a CXCL12 gradient from target lymph nodes or other organs, which would otherwise be masked by CXCL12 expressed by the cancer cells. Together, these reports suggest that the peritoneal dissemination may be induced by the dissemination of CXCR4-expressing gastric cancer cells directed into the peritoneal area, which expresses high levels of CXCL12. Therefore, blocking molecular targets such as AKT and/or CXCR4 could be possible treatment strategies to prevent the activation of signaling events induced by CXCR4 ligation mediated by CXCL12 on the cancer cell surface. Moreover, preventing peritoneal dissemination itself might be possible by developing specific inhibitors that prevent binding between CXCR4 and its ligand. In this regard, Manu et al[121] investigated plumbagin, which is a CXCR4 expression inhibitor, and it was widely effective in downregulating CXCR4 expression in cancer cell lines irrespective of the tissue of origin. The suggested mechanism of CXCR4 downregulation by plumbagin is through inhibition of NFκB. However, regulation of CXCR4 expression in cancer cells may not be so straightforward as initially expected. Bao et al[122] found that HER2, which is frequently overexpressed in gastric cancer, interacts with CD44 and induces CXCR4 expression by blocking expression of microRNA-139. Additionally, EGF signaling may play an important synergistic role in concert with the CXL12/CXCR4 axis in gastric cancer metastasis, as Yasumoto et al[123] found that the EGFR ligands amphiregulin and heparin-binding EGF (HB-EGF)-like growth factor, as well as CXCL12, are highly expressed in malignant ascites. Their work showed that HB-EGF and CXCL12 together enhanced tumor necrosis factor α-converting enzyme-dependent amphiregulin shedding from human gastric carcinoma (NUGC4) cells, which can promote proliferation of NUGC4 cells in animal models. These experiments strongly imply the possibility that several cancer signaling pathways besides the CXCL12/CXCR4 axis are important for development of gastric cancer metastasis.
When they screened 40 gastric cancer patient samples, Zhao et al[124] found that CXCR4 mRNA levels were significantly higher in cases with lymph node metastasis than those without; they also found that the CXCR4 protein level was correlated with poorly differentiated lesions, more advanced tumor stage and lymph node metastasis. Further, they reported higher CXCL12 mRNA in lymph nodes in patients with metastatic gastric cancer. Ingold et al[125] used qRT-PCR to screen CXCL12/CXCR4 mRNA levels in 37 gastric carcinomas, and as well as screening protein levels in 347 gastric carcinomas and 61 matching lymph node metastases using tissue microarrays. They concluded that tumors expressing both CXCL12 in tumor cells and CXCR4 in adjacent microvessels showed a strong correlation with local tumor development and Union for International Cancer Control stages.
Another interesting study recently reported by Xu et al[126] describes the possible activation of lymphangiogenesis pathways in gastric cancer by CXCL1 secretion. A technique was established in an animal model for recovery of lymphatic endothelial cells (LECs) from afferent lymph vessels of sentinel lymph nodes, and the gene expression profile between normal LECs and LECs with lymph node metastasis was compared using microarray analysis. They found that CXCL1 stimulated LEC migration and tube formation through FAK-ERK1/2-RhoA activation and reorganization of F-actin. Importantly, it is well known that CXCR2 expression, which is a CXCL1 receptor, is positively correlated with tumor, node, and metastasis (TNM) stage and lymphatic vessel density[127-130]. Thus, this study may strongly imply a role for CXCL1 in metastasis mediated through effects on LECs. CXCL8 has also been well studied in gastric cancer development. Using the gastric cancer cell line SCG-7901, Ju et al[131] found that CXCL8 can enhance several tumor parameters, such as adhesion to endothelial cells, migration, and invasion. The expression of MMP9, intercellular adhesion molecule (ICAM)-1 and E-cadherin was upregulated in a dose-dependent manner. However, CXCL8 did not affect the proliferation of SCG-7901 cells under these conditions. Several groups have studied polymorphism of the CXCL8 251 allele. Wang et al[132] reported the CXCL8 251 allele AA genotype as a risk factor for gastric cancer in Asian groups but not in Caucasian or Mexicans. Furthermore, statistical analysis revealed that the TA and AA polymorphism is significantly associated with the diffuse type of gastric cancer, and the AA genotype was found to be a risk factor for gastric cardia cancer. Song et al[133] reported screening results of this allele in Helicobacter pylori (H. pylori)-infected Korean populations. They found a significant correlation between MMP9 and disease progression in the AA and AT genotype. Angiopoietin-1, which plays an important role in vascular development, showed upregulation, but not VEGF expression or disease progression in the AA genotype. They predicted that the CXCL8 251 AA genotype may be associated with angiogenesis in gastric carcinogenesis in the H. pylori-infected population. Vinagre et al[134] reported that interaction between CXCL8 251 (AA and AT) allele mutation carriers and the infection of H. pylori strain [phosphoinositide PI4, 5P(2) binding protein (s1m1) cytotoxin-associated gene A product (cagA) positive] may have higher risk for development of gastric adenocarcinoma. In addition, Schneider et al[135] pointed out the complexity and difficulty in selecting in vitro epithelial study models to understand the molecular mechanisms of H. pylori infection. Moreover, a meta-analysis carried out by Liu et al[135] indicated that the variable results reported by many different studies can be affected by differences of histological type, tumor location, H. pylori infection, ethnicity and geographic location[136]. Another CXC chemokine, CXCL5, was also reported recently to be a potential molecular marker for cancer development, especially for late stage gastric cancer[137]. However, it is not yet confirmed if this observation is a consequence of secondary change as a result of gastric cancer progression, or whether it is one of the primary molecular events leading to tumor development. As CXCL5 is linked to tumor development in other organs[77,107,138], further confirmation is needed through the use of in vitro and in vivo model systems in the future. Yanagie et al[139] performed a comparative analysis of differential gene expression related to chemokines/chemokine receptors and cytokines in established gastric cancer cell lines using a cDNA microarray approach. They found that CC-chemokines CCL2, 5, 21 and CXC-chemokines CXCL1, 7, 8, 12, 14 and chemokine receptor CCR6 were upregulated, while CCL3 and CCL25 were downregulated. These chemokines and their receptors may be potential candidates for cancer diagnosis and/or treatment.
Collectively, in gastric cancer development and metastasis, multiple chemokines likely play important roles. Further studies are required to elucidate the many functional roles of these chemokines, especially in synergistic regulation of the signaling pathways that control development of gastric cancers. Another detailed review on the role of chemokines in esophageal and gastric cancer is available[140].
The liver is one of the major metastatic targets of colon cancer and this attributes directly to patient mortality. Many molecules have been proposed as being responsible for the development of hepatic metastases, and accumulated data suggest there are several important signaling pathways involved in the development of both primary and metastatic liver cancer. Among them, the CXCL12-CXCR4, CX3CL1-CX3CR1,and the CCL20-CCR6 axes have received much attention[141].
CXCR4 has been found to be a prognostic marker in various types of cancer as it plays an important role in normal stem cell homing. Cancer stem cells also express CXCR4, which implies that this axis may control the trafficking and metastasis of these cells to organs that express CXCL12, and the liver is one of these[142]. Recently, Li et al[143] reported that the expression of CXCR4 was higher in portal vein tumor thrombus tissue than hepatocellular carcinoma (HCC), and lentivirus-mediated siRNA knockdown of CXCR4 was shown to impair the potential invasiveness of tumor thrombus cells significantly. By screening tissues from 42 HCC patients, including tumor and adjacent regions, and comparing to tissues from cancer-free individuals, Liu et al[128] found that CXCR2 mRNA was significantly higher in HCC than in adjacent or normal liver tissues. Interestingly, TNM staging was not correlated to the level of CXCR2 mRNA but the protein level was relevant to staging, as protein was markedly higher in lesions classified as Stage III/IV. CXCR2 mRNA and protein levels were correlated with intrahepatic metastasis, portal cancer embolus, and low differentiation. Other studies recently reported a possible therapeutic use of CCL2 for prevention of HCC metastasis. In a model of HCC, coupling adenovirus-based CCL2 and the thymidine kinase/ganciclovir expression was shown to prevent intrahepatic metastasis in vivo. This combination was also shown to induce an innate immune response involving monocytes/macrophages and NK cells, leading to prolongation of anti-metastatic effects[144-146]. CCL2 was also identified by Chen et al[147] as a potential target for development of future HCC treatment. Using the recombinant foot-and-mouth disease virus capsid protein VP1 (rVP1), they were able to induce apoptosis of HCC cell lines through deactivation of the AKT pathway and stimulation of caspase cascades via Bax. Furthermore, rVP1 downregulated the expression of CCL2 in an AKT-dependent manner, which can support the survival and migration of HCC tumor cell lines[147]. CXCR7, a CXCL12 receptor, has also been reported to be upregulated in HCC[148]. In another study, shRNA knockdown of CXCR7 expression was found to inhibit many facets of tumor development, such as cell invasion, adhesion, VEGF secretion, endothelial tube formation and tumor growth, although it did not affect metastasis in vivo[149]. CXCR4, an alternative receptor to CXCR7 for CXCL12, is known to play important roles in liver metastasis. CXCL12 is expressed by endothelial cells and likely by Kupffer cells lining the liver sinusoids. The binding of CXCL12 to CXCR4 activates Rho, Rac and Cdc42, enabling tumor cell extravasation without affecting cell adhesion[150].
In addition to direct roles in cancer development, chemokines make important contributions to chronic inflammatory diseases such as chronic hepatitis, which can be a possible precancerous condition. In chronic hepatitis, regulation of lymphocyte motility is controlled by several independent biochemical pathways. However, these signaling events are not well elucidated despite well-documented observations of lymphocyte recruitment to tissues via endothelium. Holt et al[151] suggested that activated human liver myofibroblasts (aLMF) affect the migration and accumulation of lymphocytes within the inflamed liver. Also, when cultured in vitro, aLMF from inflamed human livers and hepatic stellate cells from non-inflamed livers secrete a distinct profile of cytokines and chemokines. The aLMF-conditioned media had chemotactic activity for lymphocytes, which was partially inhibited by pertussis toxin, implying a requirement for GPCR signaling. Additionally, contribution of GPCR-independent lymphocyte chemotaxis by IL-6, hepatocyte growth factor, and VEGF was also reported[151].
As a multitude of studies expand our knowledge of pancreatic disease, the important role of chemokines in the development of pancreatic cancer is becoming evident. CCL20 is well known for its expression in various human cancers[152-155]. In pancreatic adenocarcinoma (PAC) patients, CCL20 mRNA and protein expression was found to be significantly associated with advanced T-stage[154]. It is interesting that CCR6, a canonical CCL20 receptor, is upregulated in chronic pancreatitis, pancreatic cystadenoma and pancreatic carcinoma compared to controls[154]. It has also been reported that, in metastatic pancreatic carcinoma, expression of the angiogenic chemokines CXCL5 and CXCL8 is highly elevated compared to pancreatic cystadenoma or chronic pancreatitis[138]. This observation suggests a potential contribution of these chemokines to the development of metastatic pancreatic cancer. Furthermore, another report suggests that CXCL5 may be a possible prognostic biomarker for pancreatic cancer. Li et al[156] reported that overexpression of CXCL5 is correlated with poorer tumor differentiation, advanced clinical stage, and shorter patient survival. These authors also performed xenograft assays in nude mice, in which they used shRNA downregulation of CXCL5 or antibody-mediated neutralization of CXCR2 and showed attenuation of pancreatic tumor cell growth. They also demonstrated that CXCL5 derived angiogenesis development was ERK, AKT and signal transducer and activator of transcription mediated signaling pathways[156]. Moreover, in a clinical study that analyzed 52 PACs and 52 pancreatic neuroendocrine tumors, Hussain et al[157] found that expression of CXCL8 and its receptors CXCR1/2 were significantly upregulated compared to normal pancreatic tissues, a finding confirmed by immunohistochemistry and qRT-PCR. This may suggest the existence of autocrine and/or paracrine loops that contribute to the development of these tumors. As in other organs, many reports have suggested the importance of the CXCL12-CXCR4 axis in the development of pancreatic cancers. Cui et al[158] compared expression of CXCL12 and CXCR4 between tumor and surrounding tissues. In tumor tissues, CXCL12 expression was significantly lower than that found in paracancerous tissues, normal pancreas, or lymph nodes. In contrast, CXCR4 expression in cancerous tissues was significantly higher than that in normal tissue. Furthermore, expression patterns of the CXCL12/CXCR4 and clinicopathological status showed a strong correlation, including lymph node metastasis. Additionally, CXCL12 expression was significantly associated with MVD but not with microlymphatic vessel density, while CXCR4 expression showed the opposite relationship[158]. These observations imply a significant role for CXCL12/CXCR4 signaling in the development and progression of metastatic pancreatic cancer. Also, in another study, samples from 249 PAC patients were screened by immunohistochemistry and tissue microarray for expression of innate CXCL12 receptors CXCR4 and CXCR7. Expression of CXCR7 was found to be associated with tumor grade, inversely associated with tumor size, and possibly associated with tumor progression and differentiation[159]. Interestingly, though, no significant correlation was found between CXCR4 expression and clinicopathological parameters, which may seem to be inconsistent with previous reports[158]. However, this may be a manifestation of different mechanisms of pathogenesis. Together with these observations, data from many studies suggest CXCL12-CXCR4 signaling may be a rational therapeutic target to prevent the development and metastasis of pancreatic tumors[160,161]. For example, the activation of this axis can be attenuated by suppressing NFκB activity[162]. In pancreatic cancer, CX3CL1 and its cognate receptor CX3CR1, is another possible ligand-receptor combination associated with the pathogenesis of pancreatic cancer. Marchesi et al[163,164] reported involvement of CX3CR1 in perineural invasion and dissemination of neoplastic cells along intra- and extra-pancreatic nerves. Use of CX3CR1 as a possible therapeutic candidate is discussed in detail elsewhere[165]. Pancreatic cancer is one of the most aggressive and intractable malignancies amongst all cancers[166]. Therefore, finding molecular markers to facilitate accurate diagnosis at early stages of disease is an urgent need. From the evidence available, several chemokine-receptor pairs may be good candidates. A combination of several markers, together with these chemokines, may be promising diagnostic tools, such as CXCL17-ICAM2[167] and a classical molecular marker for pancreatic cancer, carbohydrate antigen 19-9, together with CXCL7[168].
Inflammatory bowel disease (IBD) is a chronic inflammatory condition of the colon and small intestine. It is now considered that chemokine-mediated chronic inflammation is a direct cause of colitis-associated cancer (CAC). However, the underlying mechanism of CAC is complex and not well elucidated. CC-chemokines and their receptors have long been recognized as key players in tumor promotion and progression during the course of chronic colitis. In several reports, expression of CC-chemokines, including CCL2, is highly upregulated in the colonic mucosa of IBD patients, as well as in the azoxymethane and dextran sulfate sodium experimental colitis model system[169]. Observations in the D6 receptor knockout mouse provide another important example of the role of chemokines in IBD. D6 is a silent receptor known to bind to a wide array of pro-inflammatory chemokines promiscuously, including CCL2, 5, 8, 7, and 13 in humans. Compared to wild type mice, D6 knockout mice were found to be more susceptible to chemically-induced colitis and failed to recover from the colitis. This may be due to the lack of the D6 receptor, which usually sequesters overexpressed chemokines, failing to stop the development of symptoms[170], and D6 plays a similar role in preventing liver injury[171]. Consistent with a suppressor role for D6, lymphatic vessels expressing D6 were demonstrated in epithelium and connective tissue of both small and large intestine[172]. Using a CCR2 knockout mouse or a CCL2 antagonist, Popivanova et al[173] showed that CCL2 is a crucial mediator of colon cancer development, as mice lacking CCL2 had reduced intracolonic macrophage infiltration and COX-2 expression, attenuated neovascularization, and reduced numbers and size of colon tumors.
B-lymphocytes collected from patients with Crohn’s disease (CD) express toll-like receptor 2 (TLR2) at the cell surface and secrete high levels of CXCL8, and the clinical disease course is well correlated with CXCL8 expression[174]. In contrast, ulcerative colitis (UC) patients also express TLR2, but do not secrete CXCL8 in large amounts. However, CXCL8 is inducible in UC by stimulating TLR2 and its clinical activity correlates inversely with levels of circulating TLR2-positive B cells, converse to CD[174]. In a model of experimental colitis, it was shown that infection with Bacillus polyfermenticus (B. polyfermenticus) affects the biological responses of human intestinal microvascular endothelial cells, including cell migration, tube formation, and permeability through an NFκB/CXCL8 signaling axis[175]. Interestingly, this study also reported that B. polyfermenticus can accelerate the healing process of colitis by stimulating mucosal angiogenesis. Lopez et al[176] found that Lactobacillus rhamnosus GG (LGG), a probiotic, can downregulate the flagellin-induced expression of CXCL8. Although the biological mechanisms of LGG in the maintenance of intestinal homeostasis are largely unknown, it is proposed that LGG may modulate induction of CXCL8 by tumor necrosis factor-α (TNF-α) in the intestinal epithelium[176,177]. In colorectal adenocarcinoma cells HCT-116 and HCT-8, immunohistochemistry revealed that diverse stimuli can upregulate CXCL8 expression[115], with upregulation of CXCL4L1 and synergistic CXCL8 and CXCL10 induction in carcinoma cells by IL-1 and TNF-α or immunoreactive fibronectin. In addition, full-length and N-terminally truncated (more active) CXCL8 was identified in HT-29 colorectal adenocarcinoma cells, as well as strong expression of CXCL4L1 and CXCL12 in patient samples[115]. Moreover, ERK2 and PI-3K/AKT have been identified as candidate pathways for induction of CXCL8 expression in HCT-15 colon cancer cells and MKN-45 gastric adenocarcinoma cells[178].
Arijs et al[179] reported that, in inflamed colonic IBD mucosa, many leukocyte/endothelial cell adhesion molecules (CAMs) and chemokines/chemokine receptors are upregulated, while E-cadherin gene expression was downregulated. Microarray analysis revealed that infliximab (an anti-TNF-α antibody) restores colonic gene expression of endothelial CAMs and most chemokines/chemokine receptors to normal levels of expression, with only CCL20 and CXCL1/2 expression remaining elevated after treatment. In addition to the previously identified 47 integrin-MADCAM1 axis, this study revealed a number of interesting targets for anti-adhesion therapy, including PECAM1, CXCL8, and CCL20, suggesting that anti-TNF-α therapy may work, at least in part, by downregulating certain CAMs[179]. Upregulation of CXCL1, CXCR1 and CXCR2 was reported by Oladipo et al[180] in tumor epithelium compared to normal adjacent tissue collected from patients with stage II and III CRC. In their analysis, no overall association between CXCL1, CXCR1 or CXCR2 expression and prognostic endpoints was found, although survival analysis demonstrated an inverse association between CXCL1 and recurrence-free survival in stage III patients. Interestingly, CXCL8 positivity in the tumor infiltrate correlated with earlier disease stage and improved relapse-free survival in multivariate Cox regression analysis[180]. Schroepf et al[181] screened samples collected from 501 German patients with IBD (336 CD, 165 UC) including 258 children and 243 adults as well as 231 controls. They found CXCR3 pathway-related genes to be significantly overexpressed in inflamed colonic tissue of pediatric CD and UC patients. CXCL9, 10, and 11 are 3 innate ligands for CXCR3, and this study found a correlation between polymorphism in CXCL9 and pediatric CD, while carriers of the hetero- and homozygous genotype variants of CXCL11 rs6817952 were at increased risk for UC in all age groups. Thus, blockade of CXCR3 could be a possible therapeutic avenue in the future[181]. In a study using HT-29 colon cancer cells, Lee et al[182] reported multiple regulatory roles of IL-17 on chemokine expression. Their results indicated a positive effect of IL-17 on chemokines that recruit neutrophils (CXCL8 and CXCL1) and Th17 cells (CCL20). Contrary to this, IL-17 represses expression of CXCL10, CXCL11, and CCL5, three chemokines that selectively recruit Th1 lymphocytes.
Collectively, these findings suggest that synergistic targeting of critical proteins such as chemokines and their receptors may lead to improved treatment outcomes for inflammatory bowel disease and cancer. Table 2 is attached as a summary of published studies related to chemokine/chemokine receptors and gastrointestinal diseases according to their description in this review.
| Organ | Chemokines and receptors | Possible role/observed phenomenon |
| Oral cavity | CXCL1 | Angiogenic activity[103] |
| CXCL8 | Proliferation, metastasis, tumor development and the induction of inflammation in periodontal disease[60,104-106] | |
| CXCL5 | Proliferation, cell motility and invasion[107] | |
| CXCR4 | Enhancement of invasiveness[108] | |
| CXCL12 | Upregulation in metastasis[109] | |
| CCR6, CCR7 | Involvement in metastatic activity[110] | |
| CCR7 | Enhancement of invasion[111] | |
| CCL20 | Upregulated with bacterial infection in OSCC cell lines[152] | |
| Esophagus | CXCL8 | Possible index of inflammation, upregulation in cancer-related cachexia[113,114] |
| CXCR4 | Positive regulator of HER[116] | |
| Stomach | CXCL12, CXCR4 | Metastasis through activation of AKT-mTOR pathway and MMPs, upregulation in lymph node metastasis, strong correlation with tumor development[117,118,124] |
| CXCR4 | Enhancement of metastasis through p38 signaling pathway[119] | |
| CXCL12 | Acquisition of invasive/metastatic phenotype, enhancement of proliferation when coexpressed with other molecules[120,123] | |
| CXCL1 | Activation of lymphangiogenesis by stimulating LECs[126] | |
| CXCR2 | Strong correlation with TNM staging and lymphatic vessel density[127-130] | |
| CXCL8 | Enhancement of tumor development factors, and a possible risk factor as mutant, association with angiogenesis, development of gastric adenocarcinoma[132-134,183] | |
| CXCL5 | Marker for late stage gastric cancer[137] | |
| Other candidates | CC-chemokines (CCL2, 3, 5, 21, 25)/CXC-chemokines (1, 7, 8, 12, 14)/CCR6[139] | |
| Liver | CXCR4 | Metastasis, upregulation in PVTT[142-143] |
| CXCR2 | Upregulation in HCC, especially in late stage[128] | |
| CCL2 | Application to prevent metastasis, application to prevent HCC by deactivating AKT pathway[144-147] | |
| CXCR7 | Upregulation in HCC, functional in tumor development and angiogenesis but not in metastasis[148-149] | |
| CXCL12, CXCR4 | Enhancement of tumor cell extravasation through upregulation of Rho/Rac/Cdc42[150] | |
| CCR6 | Upregulation in metastasis[153] | |
| CCL20 | Enhanced expression in HCC[155] | |
| D6 receptor | Prevention of liver injury[171] | |
| Pancreas | CCL20 | Associated with tumor staging[154] |
| CCR6 | Upregulated in chronic pancreatitis, pancreatic cystadenoma and pancreatic carcinoma[154] | |
| CXCL5, CXCL8 | Upregulation in metastatic pancreatic carcinoma[140] | |
| CXCL5 | Correlated with poorer tumor differentiation, advanced clinical stage, and shorter patient survival, and ERK, AKT and STAT mediated angiogenesis[156] | |
| CXCL8, CXCR1/2 | Upregulation in adenocarcinomas and neuroendocrine tumors[157] | |
| CXCL12, CXCR4 | Downregulation of CXCL12 and upregulation of CXCR4 in tumors. CXCL12 correlated with MVD but not with MLVD, while CXCR4 showed opposite pattern[158] | |
| CXCR4, CXCR7 | CXCR7 associated with tumor grade, inversely associated with tumor size, and possibly associated with tumor progression and differentiation but not with CXCR4[159] | |
| CX3CL1,CX3CR1 | Perineural invasion and dissemination of neoplastic cells along intra- and extra-pancreatic nerves[163,164] | |
| CXCL17 (+ ICAM2) | Diagnostic molecular marker[167] | |
| CXCL7 (+ CA19-9) | Diagnostic molecular marker[168] | |
| Colon | CXCL4L1 | Upregulation in colorectal cancer[115] |
| CCL2 | upregulation in mucosa of IBD[169] | |
| D6 receptor | Plays role of sequestering several chemokines (in mouse colitis model experiment), plays suppressive role in the development and growth of vascular tumors[170,172] | |
| CCL2, CCR2 | important mediator in colon tumor development[173] | |
| CXCL8 | Upregulation along with the development of Crohn's disease, affecting biological responses of human intestinal microvascular endothelial cells in colitis model, positvely correlated with earlier disease stage and improved relapse-free survival[164,175,180] | |
| CXCL10, CXCL41 | Synergistic upregulation with CXCL8 by diverse stimuli, induction by ERK2 and PI-3K/AKT pathway via PAR2[175,178] | |
| CCL20, CXCL1/2, CXCL8 | Remains high even after the treatment with anti-TNF antibody[179] | |
| CXCL1, CXCR1/2 | Upregulation in stage II and III CRC, upregulation in stage II and III CRC[180] | |
| CXCR3 pathway | CXCL9-pediatric Crohn’s disease, CXCL11-UC in all age groups[181] | |
| Other chemokines | IL-17 affects CXCL8, CXCL1, CCL20, CXCL10, CXCL11 and CCR5 in colon cancer cells[182] |
With the recent advances in understanding of the many and varied roles of chemokines and their receptors in tumor development and progression, together with the advent of targeted molecular therapies, excellent opportunities exist to develop novel approaches to treat cancer. This would appear to be of particular relevance for gastrointestinal malignancies, where radical improvements in clinical outcome have so far been elusive.
It is clear that chemokine networks play critical roles in inflammatory diseases and cancer progression, and tumor cells may influence their own proliferation as well as affecting stromal and immune system cells, and vice versa. Therefore, the chemokine network makes an attractive target for therapeutic intervention in many tumor types, including those of the gastrointestinal tract. However, we need to define more selective and specific targets, to minimize systemic side effects during treatment.
P- Reviewer Fernandez TD S- Editor Zhai HH L- Editor A E- Editor Xiong L
| 1. | Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429:457-463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2210] [Cited by in F6Publishing: 2135] [Article Influence: 106.8] [Reference Citation Analysis (0)] |
| 2. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39812] [Cited by in F6Publishing: 44348] [Article Influence: 3411.4] [Reference Citation Analysis (4)] |
| 3. | Christofori G. New signals from the invasive front. Nature. 2006;441:444-450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 719] [Cited by in F6Publishing: 745] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 4. | Alexiou C, Khan OA, Black E, Field ML, Onyeaka P, Beggs L, Duffy JP, Beggs DF. Survival after esophageal resection for carcinoma: the importance of the histologic cell type. Ann Thorac Surg. 2006;82:1073-1077. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Tait CR, Waterworth A, Loncaster J, Horgan K, Dodwell D. The oligometastatic state in breast cancer: hypothesis or reality. Breast. 2005;14:87-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | van ‘t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, Peterse HL, van der Kooy K, Marton MJ, Witteveen AT. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530-536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6904] [Cited by in F6Publishing: 6260] [Article Influence: 284.5] [Reference Citation Analysis (0)] |
| 7. | Golan T, Javle M. Targeting the insulin growth factor pathway in gastrointestinal cancers. Oncology (Williston Park). 2011;25:518-26, 529. [PubMed] [Cited in This Article: ] |
| 8. | Kim EJ, Zalupski MM. Systemic therapy for advanced gastrointestinal stromal tumors: beyond imatinib. J Surg Oncol. 2011;104:901-906. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Belinsky MG, Rink L, Cai KQ, Ochs MF, Eisenberg B, Huang M, von Mehren M, Godwin AK. The insulin-like growth factor system as a potential therapeutic target in gastrointestinal stromal tumors. Cell Cycle. 2008;7:2949-2955. [PubMed] [Cited in This Article: ] |
| 10. | de Sousa EM, Vermeulen L, Richel D, Medema JP. Targeting Wnt signaling in colon cancer stem cells. Clin Cancer Res. 2011;17:647-653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 169] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 11. | Grigoryan T, Wend P, Klaus A, Birchmeier W. Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes Dev. 2008;22:2308-2341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 469] [Cited by in F6Publishing: 453] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 12. | Mosca M, Polentarutti N, Mangano G, Apicella C, Doni A, Mancini F, De Bortoli M, Coletta I, Polenzani L, Santoni G. Regulation of the microsomal prostaglandin E synthase-1 in polarized mononuclear phagocytes and its constitutive expression in neutrophils. J Leukoc Biol. 2007;82:320-326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883-899. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6713] [Cited by in F6Publishing: 7691] [Article Influence: 549.4] [Reference Citation Analysis (0)] |
| 14. | Groblewska M, Mroczko B, Szmitkowski M. [The role of selected matrix metalloproteinases and their inhibitors in colorectal cancer development]. Postepy Hig Med Dosw (Online). 2010;64:22-30. [PubMed] [Cited in This Article: ] |
| 15. | Garcea G, Neal CP, Pattenden CJ, Steward WP, Berry DP. Molecular prognostic markers in pancreatic cancer: a systematic review. Eur J Cancer. 2005;41:2213-2236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 183] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 16. | Sodek KL, Murphy KJ, Brown TJ, Ringuette MJ. Cell-cell and cell-matrix dynamics in intraperitoneal cancer metastasis. Cancer Metastasis Rev. 2012;31:397-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 17. | Nespoli L, Uggeri F, Romano F, Nespoli A, Brivo F, Fumagalli L, Sargenti M, Uggeri F, Gianotti L. Modulation of systemic and intestinal immune response by interleukin-2 therapy in gastrointestinal surgical oncology. Personal experience in the context of current knowledge and future perspectives. Anticancer Res. 2012;32:989-996. [PubMed] [Cited in This Article: ] |
| 18. | Moriasi C, Subramaniam D, Awasthi S, Ramalingam S, Anant S. Prevention of colitis-associated cancer: natural compounds that target the IL-6 soluble receptor. Anticancer Agents Med Chem. 2012;12:1221-1238. [PubMed] [Cited in This Article: ] |
| 19. | Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3911] [Cited by in F6Publishing: 3870] [Article Influence: 168.3] [Reference Citation Analysis (0)] |
| 20. | Ruffini PA, Morandi P, Cabioglu N, Altundag K, Cristofanilli M. Manipulating the chemokine-chemokine receptor network to treat cancer. Cancer. 2007;109:2392-2404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1747] [Cited by in F6Publishing: 1761] [Article Influence: 88.1] [Reference Citation Analysis (0)] |
| 22. | Liao D, Luo Y, Markowitz D, Xiang R, Reisfeld RA. Cancer associated fibroblasts promote tumor growth and metastasis by modulating the tumor immune microenvironment in a 4T1 murine breast cancer model. PLoS One. 2009;4:e7965. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 309] [Cited by in F6Publishing: 363] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 23. | Barnas JL, Simpson-Abelson MR, Yokota SJ, Kelleher RJ, Bankert RB. T cells and stromal fibroblasts in human tumor microenvironments represent potential therapeutic targets. Cancer Microenviron. 2010;3:29-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539-545. [PubMed] [Cited in This Article: ] |
| 25. | Yue ZQ, Liu YP, Ruan JS, Zhou L, Lu Y. Tumor-associated macrophages: a novel potential target for cancer treatment. Chin Med J (Engl). 2012;125:3305-3311. [PubMed] [Cited in This Article: ] |
| 26. | Hagemann T, Biswas SK, Lawrence T, Sica A, Lewis CE. Regulation of macrophage function in tumors: the multifaceted role of NF-kappaB. Blood. 2009;113:3139-3146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 183] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 27. | Melief CJ. Cancer immunotherapy by dendritic cells. Immunity. 2008;29:372-383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 375] [Cited by in F6Publishing: 377] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 28. | Nardin A, Abastado JP. Macrophages and cancer. Front Biosci. 2008;13:3494-3505. [PubMed] [Cited in This Article: ] |
| 29. | Vendramini-Costa DB, Carvalho JE. Molecular link mechanisms between inflammation and cancer. Curr Pharm Des. 2012;18:3831-3852. [PubMed] [Cited in This Article: ] |
| 30. | Almholt K, Johnsen M. Stromal cell involvement in cancer. Recent Results Cancer Res. 2003;162:31-42. [PubMed] [Cited in This Article: ] |
| 31. | Rinderknecht M, Detmar M. Tumor lymphangiogenesis and melanoma metastasis. J Cell Physiol. 2008;216:347-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 32. | Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121-127. [PubMed] [Cited in This Article: ] |
| 33. | Bacon K, Baggiolini M, Broxmeyer H, Horuk R, Lindley I, Mantovani A, Maysushima K, Murphy P, Nomiyama H, Oppenheim J. Chemokine/chemokine receptor nomenclature. J Interferon Cytokine Res. 2002;22:1067-1068. [PubMed] [Cited in This Article: ] |
| 34. | Fernandez EJ, Lolis E. Structure, function, and inhibition of chemokines. Annu Rev Pharmacol Toxicol. 2002;42:469-499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 446] [Cited by in F6Publishing: 448] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 35. | Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811-1815. [PubMed] [Cited in This Article: ] |
| 36. | Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217-242. [PubMed] [Cited in This Article: ] |
| 37. | Kunkel EJ, Butcher EC. Chemokines and the tissue-specific migration of lymphocytes. Immunity. 2002;16:1-4. [PubMed] [Cited in This Article: ] |
| 38. | Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol. 2004;22:891-928. [PubMed] [Cited in This Article: ] |
| 39. | Parkinson EK, Graham GJ, Daubersies P, Burns JE, Heufler C, Plumb M, Schuler G, Pragnell IB. Hemopoietic stem cell inhibitor (SCI/MIP-1 alpha) also inhibits clonogenic epidermal keratinocyte proliferation. J Invest Dermatol. 1993;101:113-117. [PubMed] [Cited in This Article: ] |
| 40. | Rubie C, Frick VO, Wagner M, Schuld J, Gräber S, Brittner B, Bohle RM, Schilling MK. ELR+ CXC chemokine expression in benign and malignant colorectal conditions. BMC Cancer. 2008;8:178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 41. | Singh S, Wu S, Varney M, Singh AP, Singh RK. CXCR1 and CXCR2 silencing modulates CXCL8-dependent endothelial cell proliferation, migration and capillary-like structure formation. Microvasc Res. 2011;82:318-325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 42. | Mestas J, Burdick MD, Reckamp K, Pantuck A, Figlin RA, Strieter RM. The role of CXCR2/CXCR2 ligand biological axis in renal cell carcinoma. J Immunol. 2005;175:5351-5357. [PubMed] [Cited in This Article: ] |
| 43. | Pappa CA, Tsirakis G, Kanellou P, Kaparou M, Stratinaki M, Xekalou A, Alegakis A, Boula A, Stathopoulos EN, Alexandrakis MG. Monitoring serum levels ELR+ CXC chemokines and the relationship between microvessel density and angiogenic growth factors in multiple myeloma. Cytokine. 2011;56:616-620. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 44. | Murphy PM. International Union of Pharmacology. XXX. Update on chemokine receptor nomenclature. Pharmacol Rev. 2002;54:227-229. [PubMed] [Cited in This Article: ] |
| 45. | Hurowitz EH, Melnyk JM, Chen YJ, Kouros-Mehr H, Simon MI, Shizuya H. Genomic characterization of the human heterotrimeric G protein alpha, beta, and gamma subunit genes. DNA Res. 2000;7:111-120. [PubMed] [Cited in This Article: ] |
| 46. | Venkatakrishnan G, Salgia R, Groopman JE. Chemokine receptors CXCR-1/2 activate mitogen-activated protein kinase via the epidermal growth factor receptor in ovarian cancer cells. J Biol Chem. 2000;275:6868-6875. [PubMed] [Cited in This Article: ] |
| 47. | Bonacchi A, Romagnani P, Romanelli RG, Efsen E, Annunziato F, Lasagni L, Francalanci M, Serio M, Laffi G, Pinzani M. Signal transduction by the chemokine receptor CXCR3: activation of Ras/ERK, Src, and phosphatidylinositol 3-kinase/Akt controls cell migration and proliferation in human vascular pericytes. J Biol Chem. 2001;276:9945-9954. [PubMed] [Cited in This Article: ] |
| 48. | Chandrasekar B, Melby PC, Sarau HM, Raveendran M, Perla RP, Marelli-Berg FM, Dulin NO, Singh IS. Chemokine-cytokine cross-talk. The ELR+ CXC chemokine LIX (CXCL5) amplifies a proinflammatory cytokine response via a phosphatidylinositol 3-kinase-NF-kappa B pathway. J Biol Chem. 2003;278:4675-4686. [PubMed] [Cited in This Article: ] |
| 49. | Chandrasekar B, Bysani S, Mummidi S. CXCL16 signals via Gi, phosphatidylinositol 3-kinase, Akt, I kappa B kinase, and nuclear factor-kappa B and induces cell-cell adhesion and aortic smooth muscle cell proliferation. J Biol Chem. 2004;279:3188-3196. [PubMed] [Cited in This Article: ] |
| 50. | Schraufstatter IU, Chung J, Burger M. IL-8 activates endothelial cell CXCR1 and CXCR2 through Rho and Rac signaling pathways. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1094-L1103. [PubMed] [Cited in This Article: ] |
| 51. | Wang D, Sai J, Carter G, Sachpatzidis A, Lolis E, Richmond A. PAK1 kinase is required for CXCL1-induced chemotaxis. Biochemistry. 2002;41:7100-7107. [PubMed] [Cited in This Article: ] |
| 52. | Tan W, Martin D, Gutkind JS. The Galpha13-Rho signaling axis is required for SDF-1-induced migration through CXCR4. J Biol Chem. 2006;281:39542-39549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 116] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 53. | Liu-Bryan R, Pay S, Schraufstatter IU, Rose DM. The CXCR1 tail mediates beta1 integrin-dependent cell migration via MAP kinase signaling. Biochem Biophys Res Commun. 2005;332:117-125. [PubMed] [Cited in This Article: ] |
| 54. | Van Damme J, Struyf S, Opdenakker G. Chemokine-protease interactions in cancer. Semin Cancer Biol. 2004;14:201-208. [PubMed] [Cited in This Article: ] |
| 55. | Muñoz LM, Holgado BL, Martínez-A C, Rodríguez-Frade JM, Mellado M. Chemokine receptor oligomerization: a further step toward chemokine function. Immunol Lett. 2012;145:23-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 56. | Gill GN, Lazar CS. Increased phosphotyrosine content and inhibition of proliferation in EGF-treated A431 cells. Nature. 1981;293:305-307. [PubMed] [Cited in This Article: ] |
| 57. | Dhawan P, Richmond A. Role of CXCL1 in tumorigenesis of melanoma. J Leukoc Biol. 2002;72:9-18. [PubMed] [Cited in This Article: ] |
| 58. | Balentien E, Mufson BE, Shattuck RL, Derynck R, Richmond A. Effects of MGSA/GRO alpha on melanocyte transformation. Oncogene. 1991;6:1115-1124. [PubMed] [Cited in This Article: ] |
| 59. | Zhou Y, Zhang J, Liu Q, Bell R, Muruve DA, Forsyth P, Arcellana-Panlilio M, Robbins S, Yong VW. The chemokine GRO-alpha (CXCL1) confers increased tumorigenicity to glioma cells. Carcinogenesis. 2005;26:2058-2068. [PubMed] [Cited in This Article: ] |
| 60. | Metzner B, Hofmann C, Heinemann C, Zimpfer U, Schraufstätter I, Schöpf E, Norgauer J. Overexpression of CXC-chemokines and CXC-chemokine receptor type II constitute an autocrine growth mechanism in the epidermoid carcinoma cells KB and A431. Oncol Rep. 1999;6:1405-1410. [PubMed] [Cited in This Article: ] |
| 61. | Wang B, Hendricks DT, Wamunyokoli F, Parker MI. A growth-related oncogene/CXC chemokine receptor 2 autocrine loop contributes to cellular proliferation in esophageal cancer. Cancer Res. 2006;66:3071-3077. [PubMed] [Cited in This Article: ] |
| 62. | Feng G, Ohmori Y, Chang PL. Production of chemokine CXCL1/KC by okadaic acid through the nuclear factor-kappaB pathway. Carcinogenesis. 2006;27:43-52. [PubMed] [Cited in This Article: ] |
| 63. | Dhawan P, Richmond A. A novel NF-kappa B-inducing kinase-MAPK signaling pathway up-regulates NF-kappa B activity in melanoma cells. J Biol Chem. 2002;277:7920-7928. [PubMed] [Cited in This Article: ] |
| 64. | Amiri KI, Ha HC, Smulson ME, Richmond A. Differential regulation of CXC ligand 1 transcription in melanoma cell lines by poly(ADP-ribose) polymerase-1. Oncogene. 2006;25:7714-7722. [PubMed] [Cited in This Article: ] |
| 65. | Miller LJ, Kurtzman SH, Wang Y, Anderson KH, Lindquist RR, Kreutzer DL. Expression of interleukin-8 receptors on tumor cells and vascular endothelial cells in human breast cancer tissue. Anticancer Res. 1998;18:77-81. [PubMed] [Cited in This Article: ] |
| 66. | Balkwill F. The significance of cancer cell expression of the chemokine receptor CXCR4. Semin Cancer Biol. 2004;14:171-179. [PubMed] [Cited in This Article: ] |
| 67. | Bachelder RE, Wendt MA, Mercurio AM. Vascular endothelial growth factor promotes breast carcinoma invasion in an autocrine manner by regulating the chemokine receptor CXCR4. Cancer Res. 2002;62:7203-7206. [PubMed] [Cited in This Article: ] |
| 68. | Brown MJ, Nijhara R, Hallam JA, Gignac M, Yamada KM, Erlandsen SL, Delon J, Kruhlak M, Shaw S. Chemokine stimulation of human peripheral blood T lymphocytes induces rapid dephosphorylation of ERM proteins, which facilitates loss of microvilli and polarization. Blood. 2003;102:3890-3899. [PubMed] [Cited in This Article: ] |
| 69. | Singh S, Singh UP, Grizzle WE, Lillard JW. CXCL12-CXCR4 interactions modulate prostate cancer cell migration, metalloproteinase expression and invasion. Lab Invest. 2004;84:1666-1676. [PubMed] [Cited in This Article: ] |
| 70. | Zhang J, Sarkar S, Yong VW. The chemokine stromal cell derived factor-1 (CXCL12) promotes glioma invasiveness through MT2-matrix metalloproteinase. Carcinogenesis. 2005;26:2069-2077. [PubMed] [Cited in This Article: ] |
| 71. | Murakami T, Cardones AR, Finkelstein SE, Restifo NP, Klaunberg BA, Nestle FO, Castillo SS, Dennis PA, Hwang ST. Immune evasion by murine melanoma mediated through CC chemokine receptor-10. J Exp Med. 2003;198:1337-1347. [PubMed] [Cited in This Article: ] |
| 72. | Simonetti O, Goteri G, Lucarini G, Filosa A, Pieramici T, Rubini C, Biagini G, Offidani A. Potential role of CCL27 and CCR10 expression in melanoma progression and immune escape. Eur J Cancer. 2006;42:1181-1187. [PubMed] [Cited in This Article: ] |
| 73. | Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle. 2006;5:1597-1601. [PubMed] [Cited in This Article: ] |
| 74. | Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335-348. [PubMed] [Cited in This Article: ] |
| 75. | Kurose K, Gilley K, Matsumoto S, Watson PH, Zhou XP, Eng C. Frequent somatic mutations in PTEN and TP53 are mutually exclusive in the stroma of breast carcinomas. Nat Genet. 2002;32:355-357. [PubMed] [Cited in This Article: ] |
| 76. | Paterson RF, Ulbright TM, MacLennan GT, Zhang S, Pan CX, Sweeney CJ, Moore CR, Foster RS, Koch MO, Eble JN. Molecular genetic alterations in the laser-capture-microdissected stroma adjacent to bladder carcinoma. Cancer. 2003;98:1830-1836. [PubMed] [Cited in This Article: ] |
| 77. | Yeudall WA, Vaughan CA, Miyazaki H, Ramamoorthy M, Choi MY, Chapman CG, Wang H, Black E, Bulysheva AA, Deb SP. Gain-of-function mutant p53 upregulates CXC chemokines and enhances cell migration. Carcinogenesis. 2012;33:442-451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 78. | Wong CW, Lee A, Shientag L, Yu J, Dong Y, Kao G, Al-Mehdi AB, Bernhard EJ, Muschel RJ. Apoptosis: an early event in metastatic inefficiency. Cancer Res. 2001;61:333-338. [PubMed] [Cited in This Article: ] |
| 79. | Kassenbrock CK, Hunter S, Garl P, Johnson GL, Anderson SM. Inhibition of Src family kinases blocks epidermal growth factor (EGF)-induced activation of Akt, phosphorylation of c-Cbl, and ubiquitination of the EGF receptor. J Biol Chem. 2002;277:24967-24975. [PubMed] [Cited in This Article: ] |
| 80. | Swan EA, Jasser SA, Holsinger FC, Doan D, Bucana C, Myers JN. Acquisition of anoikis resistance is a critical step in the progression of oral tongue cancer. Oral Oncol. 2003;39:648-655. [PubMed] [Cited in This Article: ] |
| 81. | Sánchez-Sánchez N, Riol-Blanco L, de la Rosa G, Puig-Kröger A, García-Bordas J, Martín D, Longo N, Cuadrado A, Cabañas C, Corbí AL. Chemokine receptor CCR7 induces intracellular signaling that inhibits apoptosis of mature dendritic cells. Blood. 2004;104:619-625. [PubMed] [Cited in This Article: ] |
| 82. | Youngs SJ, Ali SA, Taub DD, Rees RC. Chemokines induce migrational responses in human breast carcinoma cell lines. Int J Cancer. 1997;71:257-266. [PubMed] [Cited in This Article: ] |
| 83. | Fang WB, Jokar I, Zou A, Lambert D, Dendukuri P, Cheng N. CCL2/CCR2 chemokine signaling coordinates survival and motility of breast cancer cells through Smad3 protein- and p42/44 mitogen-activated protein kinase (MAPK)-dependent mechanisms. J Biol Chem. 2012;287:36593-36608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 84. | Barretina J, Juncà J, Llano A, Gutiérrez A, Flores A, Blanco J, Clotet B, Esté JA. CXCR4 and SDF-1 expression in B-cell chronic lymphocytic leukemia and stage of the disease. Ann Hematol. 2003;82:500-505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 85. | Burger JA, Tsukada N, Burger M, Zvaifler NJ, Dell’Aquila M, Kipps TJ. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood. 2000;96:2655-2663. [PubMed] [Cited in This Article: ] |
| 86. | O’Hayre M, Salanga CL, Kipps TJ, Messmer D, Dorrestein PC, Handel TM. Elucidating the CXCL12/CXCR4 signaling network in chronic lymphocytic leukemia through phosphoproteomics analysis. PLoS One. 2010;5:e11716. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 87. | Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932-936. [PubMed] [Cited in This Article: ] |
| 88. | Komatsu M, Ruoslahti E. R-Ras is a global regulator of vascular regeneration that suppresses intimal hyperplasia and tumor angiogenesis. Nat Med. 2005;11:1346-1350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 89. | Sawada J, Urakami T, Li F, Urakami A, Zhu W, Fukuda M, Li DY, Ruoslahti E, Komatsu M. Small GTPase R-Ras regulates integrity and functionality of tumor blood vessels. Cancer Cell. 2012;22:235-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 90. | Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, Elner SG, Strieter RM. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798-1801. [PubMed] [Cited in This Article: ] |
| 91. | Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, Qian H, Xue XN, Pollard JW. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238-11246. [PubMed] [Cited in This Article: ] |
| 92. | Heidemann J, Ogawa H, Dwinell MB, Rafiee P, Maaser C, Gockel HR, Otterson MF, Ota DM, Lugering N, Domschke W. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem. 2003;278:8508-8515. [PubMed] [Cited in This Article: ] |
| 93. | Sodhi A, Montaner S, Patel V, Gómez-Román JJ, Li Y, Sausville EA, Sawai ET, Gutkind JS. Akt plays a central role in sarcomagenesis induced by Kaposi’s sarcoma herpesvirus-encoded G protein-coupled receptor. Proc Natl Acad Sci U S A. 2004;101:4821-4826. [PubMed] [Cited in This Article: ] |
| 94. | Sun Q, Matta H, Lu G, Chaudhary PM. Induction of IL-8 expression by human herpesvirus 8 encoded vFLIP K13 via NF-kappaB activation. Oncogene. 2006;25:2717-2726. [PubMed] [Cited in This Article: ] |
| 95. | Montaner S, Kufareva I, Abagyan R, Gutkind JS. Molecular mechanisms deployed by virally encoded G protein-coupled receptors in human diseases. Annu Rev Pharmacol Toxicol. 2013;53:331-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 96. | Strieter RM, Belperio JA, Phillips RJ, Keane MP. CXC chemokines in angiogenesis of cancer. Semin Cancer Biol. 2004;14:195-200. [PubMed] [Cited in This Article: ] |
| 97. | Romagnani P, Annunziato F, Lasagni L, Lazzeri E, Beltrame C, Francalanci M, Uguccioni M, Galli G, Cosmi L, Maurenzig L. Cell cycle-dependent expression of CXC chemokine receptor 3 by endothelial cells mediates angiostatic activity. J Clin Invest. 2001;107:53-63. [PubMed] [Cited in This Article: ] |
| 98. | Lasagni L, Francalanci M, Annunziato F, Lazzeri E, Giannini S, Cosmi L, Sagrinati C, Mazzinghi B, Orlando C, Maggi E. An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J Exp Med. 2003;197:1537-1549. [PubMed] [Cited in This Article: ] |
| 99. | Sakurai T, Kudo M. Signaling pathways governing tumor angiogenesis. Oncology. 2011;81 Suppl 1:24-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 100. | Muller A, Sonkoly E, Eulert C, Gerber PA, Kubitza R, Schirlau K, Franken-Kunkel P, Poremba C, Snyderman C, Klotz LO. Chemokine receptors in head and neck cancer: association with metastatic spread and regulation during chemotherapy. Int J Cancer. 2006;118:2147-2157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 101. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [PubMed] [Cited in This Article: ] |
| 102. | McMahon S, Chen AY. Head and neck cancer. Cancer Metastasis Rev. 2003;22:21-24. [PubMed] [Cited in This Article: ] |
| 103. | Shintani S, Ishikawa T, Nonaka T, Li C, Nakashiro K, Wong DT, Hamakawa H. Growth-regulated oncogene-1 expression is associated with angiogenesis and lymph node metastasis in human oral cancer. Oncology. 2004;66:316-322. [PubMed] [Cited in This Article: ] |
| 104. | Miyazaki H, Patel V, Wang H, Ensley JF, Gutkind JS, Yeudall WA. Growth factor-sensitive molecular targets identified in primary and metastatic head and neck squamous cell carcinoma using microarray analysis. Oral Oncol. 2006;42:240-256. [PubMed] [Cited in This Article: ] |
| 105. | Chen W, Kajiya M, Giro G, Ouhara K, Mackler HE, Mawardi H, Boisvert H, Duncan MJ, Sato K, Kawai T. Bacteria-derived hydrogen sulfide promotes IL-8 production from epithelial cells. Biochem Biophys Res Commun. 2010;391:645-650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 106. | Meyer C, Pries R, Wollenberg B. Established and novel NF-κB inhibitors lead to downregulation of TLR3 and the proliferation and cytokine secretion in HNSCC. Oral Oncol. 2011;47:818-826. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 107. | Miyazaki H, Patel V, Wang H, Edmunds RK, Gutkind JS, Yeudall WA. Down-regulation of CXCL5 inhibits squamous carcinogenesis. Cancer Res. 2006;66:4279-4284. [PubMed] [Cited in This Article: ] |
| 108. | Delilbasi CB, Okura M, Iida S, Kogo M. Investigation of CXCR4 in squamous cell carcinoma of the tongue. Oral Oncol. 2004;40:154-157. [PubMed] [Cited in This Article: ] |
| 109. | Clatot F, Picquenot JM, Choussy O, Gouérant S, Moldovan C, Schultheis D, Cornic M, François A, Blot E, Laberge-Le-Couteulx S. Intratumoural level of SDF-1 correlates with survival in head and neck squamous cell carcinoma. Oral Oncol. 2011;47:1062-1068. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 110. | Wang J, Xi L, Hunt JL, Gooding W, Whiteside TL, Chen Z, Godfrey TE, Ferris RL. Expression pattern of chemokine receptor 6 (CCR6) and CCR7 in squamous cell carcinoma of the head and neck identifies a novel metastatic phenotype. Cancer Res. 2004;64:1861-1866. [PubMed] [Cited in This Article: ] |
| 111. | Wang J, Zhang X, Thomas SM, Grandis JR, Wells A, Chen ZG, Ferris RL. Chemokine receptor 7 activates phosphoinositide-3 kinase-mediated invasive and prosurvival pathways in head and neck cancer cells independent of EGFR. Oncogene. 2005;24:5897-5904. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 112. | Rieder F, Biancani P, Harnett K, Yerian L, Falk GW. Inflammatory mediators in gastroesophageal reflux disease: impact on esophageal motility, fibrosis, and carcinogenesis. Am J Physiol Gastrointest Liver Physiol. 2010;298:G571-G581. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 113. | Oh DS, DeMeester SR, Vallbohmer D, Mori R, Kuramochi H, Hagen JA, Lipham J, Danenberg KD, Danenberg PV, Chandrasoma P. Reduction of interleukin 8 gene expression in reflux esophagitis and Barrett’s esophagus with antireflux surgery. Arch Surg. 2007;142:554-59; discussion 554-59;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 114. | Krzystek-Korpacka M, Matusiewicz M, Diakowska D, Grabowski K, Blachut K, Kustrzeba-Wojcicka I, Banas T. Impact of weight loss on circulating IL-1, IL-6, IL-8, TNF-alpha, VEGF-A, VEGF-C and midkine in gastroesophageal cancer patients. Clin Biochem. 2007;40:1353-1360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 115. | Verbeke H, De Hertogh G, Li S, Vandercappellen J, Noppen S, Schutyser E, El-Asrar AA, Opdenakker G, Van Damme J, Geboes K. Expression of angiostatic platelet factor-4var/CXCL4L1 counterbalances angiogenic impulses of vascular endothelial growth factor, interleukin-8/CXCL8, and stromal cell-derived factor 1/CXCL12 in esophageal and colorectal cancer. Hum Pathol. 2010;41:990-1001. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 116. | Gros SJ, Kurschat N, Drenckhan A, Dohrmann T, Forberich E, Effenberger K, Reichelt U, Hoffman RM, Pantel K, Kaifi JT. Involvement of CXCR4 chemokine receptor in metastastic HER2-positive esophageal cancer. PLoS One. 2012;7:e47287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 117. | Hashimoto I, Koizumi K, Tatematsu M, Minami T, Cho S, Takeno N, Nakashima A, Sakurai H, Saito S, Tsukada K. Blocking on the CXCR4/mTOR signalling pathway induces the anti-metastatic properties and autophagic cell death in peritoneal disseminated gastric cancer cells. Eur J Cancer. 2008;44:1022-1029. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 118. | Koizumi K, Kato S, Sakurai H, Hashimoto I, Yasumoto K, Saiki I. Therapeutics target of CXCR4 and its downstream in peritoneal carcinomatosis of gastric cancer. Front Biosci (Schol Ed). 2012;4:269-276. [PubMed] [Cited in This Article: ] |
| 119. | Graziosi L, Mencarelli A, Santorelli C, Renga B, Cipriani S, Cavazzoni E, Palladino G, Laufer S, Burnet M, Donini A. Mechanistic role of p38 MAPK in gastric cancer dissemination in a rodent model peritoneal metastasis. Eur J Pharmacol. 2012;674:143-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 120. | Zhi Y, Chen J, Zhang S, Chang X, Ma J, Dai D. Down-regulation of CXCL12 by DNA hypermethylation and its involvement in gastric cancer metastatic progression. Dig Dis Sci. 2012;57:650-659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 121. | Manu KA, Shanmugam MK, Rajendran P, Li F, Ramachandran L, Hay HS, Kannaiyan R, Swamy SN, Vali S, Kapoor S. Plumbagin inhibits invasion and migration of breast and gastric cancer cells by downregulating the expression of chemokine receptor CXCR4. Mol Cancer. 2011;10:107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 122. | Bao W, Fu HJ, Xie QS, Wang L, Zhang R, Guo ZY, Zhao J, Meng YL, Ren XL, Wang T. HER2 interacts with CD44 to up-regulate CXCR4 via epigenetic silencing of microRNA-139 in gastric cancer cells. Gastroenterology. 2011;141:2076-2087.e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 123. | Yasumoto K, Yamada T, Kawashima A, Wang W, Li Q, Donev IS, Tacheuchi S, Mouri H, Yamashita K, Ohtsubo K. The EGFR ligands amphiregulin and heparin-binding egf-like growth factor promote peritoneal carcinomatosis in CXCR4-expressing gastric cancer. Clin Cancer Res. 2011;17:3619-3630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 124. | Zhao BC, Wang ZJ, Mao WZ, Ma HC, Han JG, Zhao B, Xu HM. CXCR4/SDF-1 axis is involved in lymph node metastasis of gastric carcinoma. World J Gastroenterol. 2011;17:2389-2396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 53] [Cited by in F6Publishing: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 125. | Ingold B, Simon E, Ungethüm U, Kuban RJ, Müller BM, Lupp A, Neumann U, Ebert MP, Denkert C, Weichert W. Vascular CXCR4 expression - a novel antiangiogenic target in gastric cancer? PLoS One. 2010;5:e10087. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 126. | Xu J, Zhang C, He Y, Wu H, Wang Z, Song W, Li W, He W, Cai S, Zhan W. Lymphatic endothelial cell-secreted CXCL1 stimulates lymphangiogenesis and metastasis of gastric cancer. Int J Cancer. 2012;130:787-797. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 127. | Han L, Jiang B, Wu H, Wang X, Tang X, Huang J, Zhu J. High expression of CXCR2 is associated with tumorigenesis, progression, and prognosis of laryngeal squamous cell carcinoma. Med Oncol. 2012;29:2466-2472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 128. | Liu Z, Yang L, Xu J, Zhang X, Wang B. Enhanced expression and clinical significance of chemokine receptor CXCR2 in hepatocellular carcinoma. J Surg Res. 2011;166:241-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 129. | Kitadai Y, Kodama M, Cho S, Kuroda T, Ochiumi T, Kimura S, Tanaka S, Matsumura S, Yasui W, Chayama K. Quantitative analysis of lymphangiogenic markers for predicting metastasis of human gastric carcinoma to lymph nodes. Int J Cancer. 2005;115:388-392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 130. | Gao P, Zhou GY, Zhang QH, Su ZX, Zhang TG, Xiang L, Wang Y, Zhang SL, Mu K. Lymphangiogenesis in gastric carcinoma correlates with prognosis. J Pathol. 2009;218:192-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 131. | Ju D, Sun D, Xiu L, Meng X, Zhang C, Wei P. Interleukin-8 is associated with adhesion, migration and invasion in human gastric cancer SCG-7901 cells. Med Oncol. 2012;29:91-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 132. | Wang J, Pan HF, Hu YT, Zhu Y, He Q. Polymorphism of IL-8 in 251 allele and gastric cancer susceptibility: a meta-analysis. Dig Dis Sci. 2010;55:1818-1823. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 133. | Song JH, Kim SG, Jung SA, Lee MK, Jung HC, Song IS. The interleukin-8-251 AA genotype is associated with angiogenesis in gastric carcinogenesis in Helicobacter pylori-infected Koreans. Cytokine. 2010;51:158-165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 134. | Vinagre RM, Corvelo TC, Arnaud VC, Leite AC, Barile KA, Martins LC. Determination of strains of Helicobacter pylori and of polymorphism in the interleukin-8 gene in patients with stomach cancer. Arq Gastroenterol. 2011;48:46-51. [PubMed] [Cited in This Article: ] |
| 135. | Schneider S, Carra G, Sahin U, Hoy B, Rieder G, Wessler S. Complex cellular responses of Helicobacter pylori-colonized gastric adenocarcinoma cells. Infect Immun. 2011;79:2362-2371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 136. | Liu L, Zhuang W, Wang C, Chen Z, Wu XT, Zhou Y. Interleukin-8 -251 A/T gene polymorphism and gastric cancer susceptibility: a meta-analysis of epidemiological studies. Cytokine. 2010;50:328-334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 137. | Park JY, Park KH, Bang S, Kim MH, Lee JE, Gang J, Koh SS, Song SY. CXCL5 overexpression is associated with late stage gastric cancer. J Cancer Res Clin Oncol. 2007;133:835-840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 138. | Frick VO, Rubie C, Wagner M, Graeber S, Grimm H, Kopp B, Rau BM, Schilling MK. Enhanced ENA-78 and IL-8 expression in patients with malignant pancreatic diseases. Pancreatology. 2008;8:488-497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 139. | Yanagie H, Hisa T, Ono M, Eriguchi M. [Chemokine and chemokine receptor related to cancer metastasis]. Gan To Kagaku Ryoho. 2010;37:2052-2057. [PubMed] [Cited in This Article: ] |
| 140. | Verbeke H, Geboes K, Van Damme J, Struyf S. The role of CXC chemokines in the transition of chronic inflammation to esophageal and gastric cancer. Biochim Biophys Acta. 2012;1825:117-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 141. | Huang F, Geng XP. Chemokines and hepatocellular carcinoma. World J Gastroenterol. 2010;16:1832-1836. [PubMed] [Cited in This Article: ] |
| 142. | Furusato B, Mohamed A, Uhlén M, Rhim JS. CXCR4 and cancer. Pathol Int. 2010;60:497-505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 224] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 143. | Li N, Guo W, Shi J, Xue J, Hu H, Xie D, Wu M, Cheng S. Expression of the chemokine receptor CXCR4 in human hepatocellular carcinoma and its role in portal vein tumor thrombus. J Exp Clin Cancer Res. 2010;29:156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 144. | Tsuchiyama T, Kaneko S, Nakamoto Y, Sakai Y, Honda M, Mukaida N, Kobayashi K. Enhanced antitumor effects of a bicistronic adenovirus vector expressing both herpes simplex virus thymidine kinase and monocyte chemoattractant protein-1 against hepatocellular carcinoma. Cancer Gene Ther. 2003;10:260-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 145. | Sakai Y, Kaneko S, Nakamoto Y, Kagaya T, Mukaida N, Kobayashi K. Enhanced anti-tumor effects of herpes simplex virus thymidine kinase/ganciclovir system by codelivering monocyte chemoattractant protein-1 in hepatocellular carcinoma. Cancer Gene Ther. 2001;8:695-704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 146. | Kakinoki K, Nakamoto Y, Kagaya T, Tsuchiyama T, Sakai Y, Nakahama T, Mukaida N, Kaneko S. Prevention of intrahepatic metastasis of liver cancer by suicide gene therapy and chemokine ligand 2/monocyte chemoattractant protein-1 delivery in mice. J Gene Med. 2010;12:1002-1013. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 147. | Chen TA, Wang JL, Hung SW, Chu CL, Cheng YC, Liang SM. Recombinant VP1, an Akt inhibitor, suppresses progression of hepatocellular carcinoma by inducing apoptosis and modulation of CCL2 production. PLoS One. 2011;6:e23317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 148. | Monnier J, Boissan M, L’Helgoualc’h A, Lacombe ML, Turlin B, Zucman-Rossi J, Théret N, Piquet-Pellorce C, Samson M. CXCR7 is up-regulated in human and murine hepatocellular carcinoma and is specifically expressed by endothelial cells. Eur J Cancer. 2012;48:138-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 149. | Zheng K, Li HY, Su XL, Wang XY, Tian T, Li F, Ren GS. Chemokine receptor CXCR7 regulates the invasion, angiogenesis and tumor growth of human hepatocellular carcinoma cells. J Exp Clin Cancer Res. 2010;29:31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 150. | Gassmann P, Haier J, Schlüter K, Domikowsky B, Wendel C, Wiesner U, Kubitza R, Engers R, Schneider SW, Homey B. CXCR4 regulates the early extravasation of metastatic tumor cells in vivo. Neoplasia. 2009;11:651-661. [PubMed] [Cited in This Article: ] |
| 151. | Holt AP, Haughton EL, Lalor PF, Filer A, Buckley CD, Adams DH. Liver myofibroblasts regulate infiltration and positioning of lymphocytes in human liver. Gastroenterology. 2009;136:705-714. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 152. | Abiko Y, Nishimura M, Kusano K, Nakashima K, Okumura K, Arakawa T, Takuma T, Mizoguchi I, Kaku T. Expression of MIP-3alpha/CCL20, a macrophage inflammatory protein in oral squamous cell carcinoma. Arch Oral Biol. 2003;48:171-175. [PubMed] [Cited in This Article: ] |
| 153. | Ghadjar P, Coupland SE, Na IK, Noutsias M, Letsch A, Stroux A, Bauer S, Buhr HJ, Thiel E, Scheibenbogen C. Chemokine receptor CCR6 expression level and liver metastases in colorectal cancer. J Clin Oncol. 2006;24:1910-1916. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 154. | Rubie C, Frick VO, Ghadjar P, Wagner M, Grimm H, Vicinus B, Justinger C, Graeber S, Schilling MK. CCL20/CCR6 expression profile in pancreatic cancer. J Transl Med. 2010;8:45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 155. | Rubie C, Frick VO, Wagner M, Rau B, Weber C, Kruse B, Kempf K, Tilton B, König J, Schilling M. Enhanced expression and clinical significance of CC-chemokine MIP-3 alpha in hepatocellular carcinoma. Scand J Immunol. 2006;63:468-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 156. | Li A, King J, Moro A, Sugi MD, Dawson DW, Kaplan J, Li G, Lu X, Strieter RM, Burdick M. Overexpression of CXCL5 is associated with poor survival in patients with pancreatic cancer. Am J Pathol. 2011;178:1340-1349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 157. | Hussain F, Wang J, Ahmed R, Guest SK, Lam EW, Stamp G, El-Bahrawy M. The expression of IL-8 and IL-8 receptors in pancreatic adenocarcinomas and pancreatic neuroendocrine tumours. Cytokine. 2010;49:134-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 158. | Cui K, Zhao W, Wang C, Wang A, Zhang B, Zhou W, Yu J, Sun Z, Li S. The CXCR4-CXCL12 pathway facilitates the progression of pancreatic cancer via induction of angiogenesis and lymphangiogenesis. J Surg Res. 2011;171:143-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 159. | Gebauer F, Tachezy M, Effenberger K, von Loga K, Zander H, Marx A, Kaifi JT, Sauter G, Izbicki JR, Bockhorn M. Prognostic impact of CXCR4 and CXCR7 expression in pancreatic adenocarcinoma. J Surg Oncol. 2011;104:140-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 160. | Yadav VR, Sung B, Prasad S, Kannappan R, Cho SG, Liu M, Chaturvedi MM, Aggarwal BB. Celastrol suppresses invasion of colon and pancreatic cancer cells through the downregulation of expression of CXCR4 chemokine receptor. J Mol Med (Berl). 2010;88:1243-1253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 161. | Singh S, Srivastava SK, Bhardwaj A, Owen LB, Singh AP. CXCL12-CXCR4 signalling axis confers gemcitabine resistance to pancreatic cancer cells: a novel target for therapy. Br J Cancer. 2010;103:1671-1679. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 201] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 162. | Chua AW, Hay HS, Rajendran P, Shanmugam MK, Li F, Bist P, Koay ES, Lim LH, Kumar AP, Sethi G. Butein downregulates chemokine receptor CXCR4 expression and function through suppression of NF-κB activation in breast and pancreatic tumor cells. Biochem Pharmacol. 2010;80:1553-1562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 163. | Marchesi F, Locatelli M, Solinas G, Erreni M, Allavena P, Mantovani A. Role of CX3CR1/CX3CL1 axis in primary and secondary involvement of the nervous system by cancer. J Neuroimmunol. 2010;224:39-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 164. | Marchesi F, Piemonti L, Mantovani A, Allavena P. Molecular mechanisms of perineural invasion, a forgotten pathway of dissemination and metastasis. Cytokine Growth Factor Rev. 2010;21:77-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 184] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 165. | D’Haese JG, Demir IE, Friess H, Ceyhan GO. Fractalkine/CX3CR1: why a single chemokine-receptor duo bears a major and unique therapeutic potential. Expert Opin Ther Targets. 2010;14:207-219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 166. | Pierantoni C, Pagliacci A, Scartozzi M, Berardi R, Bianconi M, Cascinu S. Pancreatic cancer: progress in cancer therapy. Crit Rev Oncol Hematol. 2008;67:27-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 167. | Hiraoka N, Yamazaki-Itoh R, Ino Y, Mizuguchi Y, Yamada T, Hirohashi S, Kanai Y. CXCL17 and ICAM2 are associated with a potential anti-tumor immune response in early intraepithelial stages of human pancreatic carcinogenesis. Gastroenterology. 2011;140:310-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 168. | Matsubara J, Honda K, Ono M, Tanaka Y, Kobayashi M, Jung G, Yanagisawa K, Sakuma T, Nakamori S, Sata N. Reduced plasma level of CXC chemokine ligand 7 in patients with pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:160-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 169. | Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, Oshima M, Fujii C, Mukaida N. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118:560-570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 443] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 170. | Vetrano S, Borroni EM, Sarukhan A, Savino B, Bonecchi R, Correale C, Arena V, Fantini M, Roncalli M, Malesci A. The lymphatic system controls intestinal inflammation and inflammation-associated Colon Cancer through the chemokine decoy receptor D6. Gut. 2010;59:197-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 171. | Berres ML, Trautwein C, Zaldivar MM, Schmitz P, Pauels K, Lira SA, Tacke F, Wasmuth HE. The chemokine scavenging receptor D6 limits acute toxic liver injury in vivo. Biol Chem. 2009;390:1039-1045. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 172. | Nibbs RJ, Kriehuber E, Ponath PD, Parent D, Qin S, Campbell JD, Henderson A, Kerjaschki D, Maurer D, Graham GJ. The beta-chemokine receptor D6 is expressed by lymphatic endothelium and a subset of vascular tumors. Am J Pathol. 2001;158:867-877. [PubMed] [Cited in This Article: ] |
| 173. | Popivanova BK, Kostadinova FI, Furuichi K, Shamekh MM, Kondo T, Wada T, Egashira K, Mukaida N. Blockade of a chemokine, CCL2, reduces chronic colitis-associated carcinogenesis in mice. Cancer Res. 2009;69:7884-7892. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 149] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 174. | Noronha AM, Liang Y, Hetzel JT, Hasturk H, Kantarci A, Stucchi A, Zhang Y, Nikolajczyk BS, Farraye FA, Ganley-Leal LM. Hyperactivated B cells in human inflammatory bowel disease. J Leukoc Biol. 2009;86:1007-1016. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 175. | Im E, Choi YJ, Kim CH, Fiocchi C, Pothoulakis C, Rhee SH. The angiogenic effect of probiotic Bacillus polyfermenticus on human intestinal microvascular endothelial cells is mediated by IL-8. Am J Physiol Gastrointest Liver Physiol. 2009;297:G999-G1008. [PubMed] [Cited in This Article: ] |
| 176. | Lopez M, Li N, Kataria J, Russell M, Neu J. Live and ultraviolet-inactivated Lactobacillus rhamnosus GG decrease flagellin-induced interleukin-8 production in Caco-2 cells. J Nutr. 2008;138:2264-2268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 177. | Zhang L, Li N, Caicedo R, Neu J. Alive and dead Lactobacillus rhamnosus GG decrease tumor necrosis factor-alpha-induced interleukin-8 production in Caco-2 cells. J Nutr. 2005;135:1752-1756. [PubMed] [Cited in This Article: ] |
| 178. | Tanaka Y, Sekiguchi F, Hong H, Kawabata A. PAR2 triggers IL-8 release via MEK/ERK and PI3-kinase/Akt pathways in GI epithelial cells. Biochem Biophys Res Commun. 2008;377:622-626. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 179. | Arijs I, De Hertogh G, Machiels K, Van Steen K, Lemaire K, Schraenen A, Van Lommel L, Quintens R, Van Assche G, Vermeire S. Mucosal gene expression of cell adhesion molecules, chemokines, and chemokine receptors in patients with inflammatory bowel disease before and after infliximab treatment. Am J Gastroenterol. 2011;106:748-761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 180. | Oladipo O, Conlon S, O’Grady A, Purcell C, Wilson C, Maxwell PJ, Johnston PG, Stevenson M, Kay EW, Wilson RH. The expression and prognostic impact of CXC-chemokines in stage II and III colorectal cancer epithelial and stromal tissue. Br J Cancer. 2011;104:480-487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 181. | Schroepf S, Kappler R, Brand S, Prell C, Lohse P, Glas J, Hoster E, Helmbrecht J, Ballauff A, Berger M. Strong overexpression of CXCR3 axis components in childhood inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:1882-1890. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 182. | Lee JW, Wang P, Kattah MG, Youssef S, Steinman L, DeFea K, Straus DS. Differential regulation of chemokines by IL-17 in colonic epithelial cells. J Immunol. 2008;181:6536-6545. [PubMed] [Cited in This Article: ] |
| 183. | Ju DW, Wei PK, Lin HM, Sun DZ, Yu S, Xiu LJ. [Effects of Xiaotan Sanjie Decoction on expressions of interleukin-8 and its receptors in gastric tumor xenografts and gastric tissue adjacent to the tumor in mice]. Zhongxiyi Jiehe Xuebao. 2010;8:74-79. [PubMed] [Cited in This Article: ] |