Published online May 7, 2013. doi: 10.3748/wjg.v19.i17.2709
Revised: February 28, 2013
Accepted: March 6, 2013
Published online: May 7, 2013
Processing time: 178 Days and 0.1 Hours
AIM: To evaluate the association between HLA-DRB1 alleles and Han and Uyghur ulcerative colitis (UC) patients residing in the Xinjiang Uyghur Autonomous Region of China.
METHODS: In this study, 102 UC patients (53 Han including 22 men and 31 women, and 49 Uyghur patients including 25 men and 24 women; aged 48.07 ± 15.83 years) and 310 age- and sex-matched healthy controls were enrolled in the Department of Gastroenterology, Xinjiang People’s Hospital of China from January 2010 to May 2011. UC was diagnosed based on the clinical, endoscopic and histological findings following Lennard-Jones criteria. Blood samples were collected and genomic DNA was extracted by routine laboratory methods, and both polymerase chain reaction and gene sequencing were used to identify HLA-DRB1 allele variants. The potential association between genetic variation and UC in Han and Uyghur patients was examined. There were no statistical differences in HLA-DRB1 allele frequencies in Han UC patients.
RESULTS: There was no significant difference in the sex ratio between the controls and UC patients (P = 0.740). In Han patients with UC (n = 53), HLA-DRB1 *03, *13 allele frequencies were lower than in healthy controls (n = 161), but not statistically significant, and HLA-DRB1*04*11*14 allele frequencies were higher than in healthy controls, but without statistical significance. Differences between Uyghur UC patients and the control group were observed for HLA-DRB1*04 and HLA-DRB1*13, both showed a greater frequency in UC patients (10.21% vs 2.69%, P = 0.043; 14.29% vs 4.03%, P = 0.019). HLA-DRB1*14 also showed a greater frequency in UC patients (14.29% vs 2.69%, P = 0.006). The frequencies of DRB1*04, *13*14 alleles were increased in Uyghur UC patients compared with normal controls. The frequency of DRB1 * 08 was decreased in Uyghur UC patients compared with normal controls. HLA-DRB1 alleles showed no association with UC in Han patients. There were no statistical differences in HLA-DRB1 allele frequencies in Han UC patients. The frequencies of DRB1*04, *13*14 alleles were increased in Uyghur UC patients compared with normal controls. The frequency of DRB1*08 was decreased in Uyghur UC patients compared with normal controls. Polymorphism of the HLA-DRB1 gene may contribute to the clinical heterogeneity of UC between Han and Uyghur UC patients in China.
CONCLUSION: HLA-DRB1*04*13*14 and DRB1*08 may contribute to the clinical heterogeneity of UC between Han and Uyghur UC patients.
Core tip: This study evaluated the association between HLA-DRB1 alleles and Han and Uyghur ulcerative colitis (UC) patients residing in the Xinjiang Uyghur Autonomous Region of China. The authors found that polymorphism of the HLA-DRB1* gene differed between the Han and Uyghur patients with UC. Polymorphism of the HLA-DRB1 gene may contribute to the clinical heterogeneity of UC between Han and Uyghur UC patients in North-West China.
-
Citation: Aheman A, Gao F, Kuerbanjiang A, Li YX, Abuduhadeer M. Difference in
DRB1 * gene polymorphisms between Han and Uyghur ulcerative colitis patients in China. World J Gastroenterol 2013; 19(17): 2709-2713 - URL: https://www.wjgnet.com/1007-9327/full/v19/i17/2709.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i17.2709
Ulcerative colitis (UC) and Crohn’s disease are often grouped together as inflammatory bowel disease (IBD). IBD is the term used to describe idiopathic disorders associated with chronic inflammation of the gastrointestinal tract[1,2]. Clinical features common to both disorders include abdominal pain, diarrhea, weight loss, and increased risk of developing colorectal cancer[3,4]. The etiology of UC is still not known. However, underlying genetic, environmental, and lifestyle issues can affect an individual’s predisposition to these diseases[5,6]. Genetic factors involved in the regulation of the immune system are thought to play a significant role in the pathogenesis of UC[7]. Human leukocyte antigens (HLA), located on chromosome 6, play an important role in the immune response and several immune-mediated diseases. Several studies have shown that HLA alleles are associated with UC[8]. We previously compared the clinical characteristics of UC in the Han and Uyghur populations residing in the Xinjiang Uyghur Autonomous Region of China. We showed some differences between the Uyghur and Han populations living in the same region, which included a higher prevalence of UC, a younger age of onset, an increased prevalence of the chronic persistent and acute outbreak type, more moderate and severe forms, a higher complication rate, and an increased frequency of positive antineutrophilic cytoplasmic antibodies (ANCA) in the Uyghur population[9]. However, to date, Hardly a research shows that there is association between HLA-DRB1 alleles and UC in the Uyghur population. It would be interesting to know what causes the clinical heterogeneity of UC between Han and Uyghur UC patients in China.
Consecutive patients with UC were recruited from the Department of Gastroenterology, Xinjiang People’s Hospital of China. The diagnosis of UC was made based on clinical, endoscopic, and histological findings in accordance with Lennard-Jones criteria[10]. The extent of disease was assessed by colonoscopy at initial diagnosis and at follow-up. Extensive colitis was defined as lesions located beyond the splenic flexure. Distal colitis was defined as lesions limited to the region distal to the splenic flexure[11,12]. Age- and sex-matched healthy controls were also recruited from the Health Examination Center of Xinjiang People’s Hospital. The study protocol was approved by the Ethics Committee of Xinjiang Uyghur Autonomous Region of China, and individuals selected from the populations sampled were Chinese and Uyghur UC patients from Urumqi, Xinjiang. Informed consent was obtained from all patients.
Genomic DNA was isolated using a QIAamp DNA Blood Mini Kit according to the manufacturer’s instructions (Qiagen, Germany). DNA samples were quantified by ultraviolet measurements at A260.
Polymerase chain reaction (PCR) amplifications were performed in a volume of 20 μL consisting of 20 ng of genomic DNA, PCR buffer (1.5 mmol/L MgCl2, 10 mmol/L Tris-HCl pH 8.4, 50 mmol/L KCl, 0.1 mg/mL of Gelatin, 0.02% of NP-40), 200 mmol/L of each dNTP (Life Technologies, Rockville, MD, United States) and 1 unit of Taq Platinum polymerase (Life Technologies, Grand Island, NY, United States). Seven sense primers and 1 antisense primer were used to amplify HLA-DRB1 alleles (Table 1).
| 5'-CCACAGCACGTTTCTTGGAGTACTCTA-3' |
| 5'-CCAGTTTCTTGTGGCAGCTTAAGTT-3' |
| 5'-TCGTTCCTGTGGCAGGGTAAGTATA-3' |
| 5'-AGCCGTTTCTTGAAGCAGGATAAGTT-3' |
| 5'-CCAAGCACGTTTCTTGGAGGAGG-3' |
| 5'-TCGTTCCTGTGGCAGCCTAAGA-3' |
| 5'-AGCCGTTTCTTGGAGCAGGTTAAAC-3' |
The 3’ amplification primer was 5’-CTGTTACCTCGCCACTGCAC-3’. PCR amplification was performed in an ABI 9700. The DNA was amplified following initial denaturation at 95 °C for 120 s followed by 35 cycles at 95 °C for 30 s, 60 °C for 30 s and 72 °C for 60 s. PCR amplification was confirmed following electrophoresis on a 2.0% agarose gel. Samples were electrophoresed at 10 V/cm for 36 min. PCR products were purified using the High Pure PCR Purification Kit (Roche Diagnostics, Basel, Switzerland).
PCR products were sequenced using BigDye Terminator v3.1 kits (ABI, United States) and automated ABI 3730XL DNA sequencers. The 5’ sequence primer was 5’-TTGCAATTCTTCAATGGGAC-3’ and the 3’ primer was 5’-ACCACCCGGTAGTTGTGTC-3’. The DNA was sequenced following initial denaturation at 95 °C for 120 s followed by 25 cycles at 95 °C for 10 s, 50 °C for 5 s and 60 °C for 60 s. The sequence within the 5′ primer sites was included in the analysis to prevent the mistyping of novel alleles that may have arisen due to recombination within the first variable region. DRB1 genotyping was assigned using Qiagen SBTengine HLA Typing software that compares an unknown sequence against a library of allele sequences (Figure 1).
Hardy-Weinberg disequilibrium was assessed with χ2 tests and clinical records including age were analyzed with t tests. χ2 tests were used to compare genotype and allele frequencies between patients and normal controls. Fisher exact tests with 95%CI were used when the number of samples was less than 5. P values less than 0.05 were considered statistically significant.
DNA was obtained from 102 consecutive UC patients and 310 healthy individuals well matched for sex and age. The main clinical characteristics of the UC patients are summarized in Table 2. UC patients included 47 males and 55 females and the controls included 137 males and 173 females. The average age of UC patients and controls was 48.07 ± 15.83 years and 47.37 ± 14.49 years, respectively. There was no significant difference in the sex ratio between the controls and UC patients (P = 0.740, Table 2).
| Characteristics | UC patients | Controls | P value |
| Total number (n) | 102 | 310 | |
| Han | 53 | 161 | 0.998 |
| Uyghur | 49 | 149 | 0.998 |
| Gender, M/F | 47/55 | 137/173 | 0.740 |
| Age of onset1, yr | 48.07 ± 15.83 | 47.37 ± 14.49 | 0.801 |
| Extensive colitis (Han) | 20 (38) | ||
| Extensive colitis (Uyghur) | 30 (61) | ||
| Distal colitis (Han) | 33 (62) | ||
| Mild and intermediate | 47 (89) | ||
| Severe | 6 (11) | ||
| Distal colitis (Uyghur) | 19 (39) | ||
| Mild and intermediate | 40 (82) | ||
| Severe | 9 (18) |
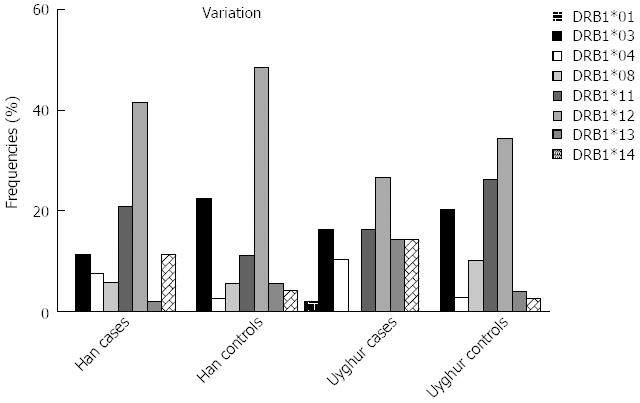
The HLA-DRB1 allele frequencies in the patients and controls are shown in Table 3. In Han patients with UC (n = 53), HLA-DRB1*03, *13 allele frequencies were lower than in healthy controls (n = 161), but not statistically significant (P > 0.05), and HLA-DRB1*04*11*14 allele frequencies were higher than in the healthy controls, but without statistical significance (P > 0.05) (Figure 2).
| Han UC (n = 53) | Controls (n = 161) | χ2 | P value | Uyghur UC (n = 49) | Controls (n = 149) | χ2 | P value | |||||
| n (%) | Genotype frequencies | n (%) | Genotype frequencies | n (%) | Genotype frequencies | n (%) | Genotype frequencies | |||||
| HLA-DRB1**01 | 0 | 0 | 0 | 0 | 1 (2.04) | 0.010 | 0 | 0 | 3.056 | 0.247 | ||
| HLA-DRB1**03 | 6 (11.32) | 0.058 | 36 (22.36) | 0.119 | 3.081 | 0.110 | 8 (16.33) | 0.085 | 30 (20.13) | 0.106 | 0.345 | 0.678 |
| HLA-DRB1**04 | 4 (7.55) | 0.039 | 4 (2.49) | 0.013 | 2.840 | 0.106 | 5 (10.21) | 0.052 | 4 (2.69) | 0.014 | 4.805 | 0.043 |
| HLA-DRB1**08 | 3 (5.66) | 0.029 | 9 (5.59) | 0.028 | 0.000 | 1.000 | 0 | 0 | 15 (10.07) | 0.052 | 5.337 | 0.024 |
| HLA-DRB1**11 | 11 (20.76) | 0.110 | 18 (11.18) | 0.058 | 3.120 | 0.103 | 8 (16.33) | 0.085 | 39 (26.18) | 0.141 | 1.975 | 0.180 |
| HLA-DRB1**12 | 22 (41.51) | 0.235 | 78 (48.45) | 0.282 | 0.771 | 0.429 | 13 (26.53) | 0.143 | 51 (34.23) | 0.189 | 0.999 | 0.380 |
| HLA-DRB1**13 | 1 (1.89) | 0.009 | 9 (5.59) | 0.028 | 1.228 | 0.457 | 7 (14.29) | 0.074 | 6 (4.03) | 0.022 | 6.326 | 0.019 |
| HLA-DRB1**14 | 6 (11.32) | 0.058 | 7 (4.35) | 0.023 | 3.398 | 0.093 | 7 (14.29) | 0.074 | 4 (2.69) | 0.014 | 9.458 | 0.006 |
Differences between Uyghur UC patients and the control group were observed for HLA-DRB1*04 and HLA-DRB1*13, both showed a greater frequency in UC patients (10.21% vs 2.69%, P = 0.043; 14.29% vs 4.03%, P = 0.019). HLA-DRB1*14 also showed a greater frequency in UC patients (14.29% vs 2.69%, P = 0.006), however, HLA-DRB1*08 showed a lower frequency in UC patients than in the controls (0% vs 10.07 %, P = 0.024), Table 3.
UC was previously uncommon in China, however, in the last 10 years more cases have been identified and the incidence of this disease has increased[13]. The clinical characteristics of UC in the Han and Uyghur populations residing in the Xinjiang Uyghur Autonomous Region of China were shown to be different[14]. The genetic factors possibly associated with UC and its clinical characteristics in these two ethnic groups are unknown. A number of HLA alleles have been shown to be associated with variations in immune response diseases (e.g., celiac disease[15], longitudinally extensive transverse myelitis[16], and Behçet’s disease[17]). Several genome-wide scans have shown that the susceptibility locus of UC and Crohn’s disease is on chromosome 16q (IBD1)[18]. In the present study, we evaluated the association between the HLA-DRB1 alleles and UC in Han and Uyghur populations from north-west China. We did not find any association between HLA-DRB1 alleles and UC in the Han population, and these results were consistent with previous Chinese studies. Lü et al[19] showed that polymorphism of the HLA-DRB1 gene did not have a strong association with UC in Chinese patients. Lee et al[20] found that HLA-DQa1c, but not HLA-DRB1 alleles, was associated with ANCA-positive UC in southern China.
We found differences between Uyghur UC patients and healthy controls, in whom HLA-DRB1*04, HLA-DRB1*13 and HLA-DRB1*14 showed a greater frequency in UC patients than in controls. These results were consistent with previous Japanese studies[21]. In contrast to previous Chinese studies HLA-DRB1*08 showed a lower frequency in UC patients than in controls[22]. Polymorphism of the HLA-DRB1 gene may contribute to the clinical heterogeneity of UC between Han and Uyghur UC patients in China. The results in the present study were different probably due to the statistical methods used and the genetic heterogeneity of the ethnic populations. In future studies, we will continue to explore other HLA genes and amplify our sample size to identify the genes associated with UC in different ethnic groups in China and to provide more evidence for the genetic susceptibility of UC.
Previous studies have shown that human leukocyte antigens (HLA) alleles are associated with ulcerative colitis (UC). The clinical characteristics of UC in the Han and Uyghur populations residing in the Xinjiang Uyghur Autonomous Region of China were shown to be different. In this study, the authors examined whether polymorphism of the HLA-DRB1* gene differed between Han and Uyghur patients with UC.
Studies have found that disease distribution and phenotypic appearance differ significantly between ethnic groups and even within populations. The genetic and clinical heterogeneity of UC between the two ethnic groups, Chinese Han and Uyghur, were investigated.
This study found that polymorphism of the HLA-DRB1* gene differed between Han and Uyghur patients with UC. Polymorphism of the HLA-DRB1 gene may contribute to the clinical heterogeneity of UC between Han and Uyghur UC patients in north-west China.
The effects of different genotypes in normal controls and patients with UC are still unknown and likely represent a potentially productive area for future research to better understand the pathogenesis and treatment of UC.
UC is a form of inflammatory bowel disease. It is a refractory, chronic, and nonspecific disease which usually occurs in the rectum and the entire colon. HLA, located on chromosome 6, play an important role in the immune response and several immune-mediated diseases.
The authors investigated the contribution of DRB1* gene polymorphism to the genetic susceptibility and clinical heterogeneity of UC between Han and Uyghur patients. This manuscript contains potentially interesting findings.
P- Reviewer Stemmler MP S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Keyashian K, Annunziata ML, Sakuraba A, Hanauer S. Management of inflammatory bowel disease: past, present and future. Expert Rev Clin Immunol. 2012;8:303-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Zuo S, Wang X, Liu YC, Wang PY. [Therapy progression in surgery of inflammatory bowel diseases]. Zhonghua Wei Chang Wai Ke Zazhi. 2012;15:872-876. [PubMed] |
| 3. | Burri E, Beglinger C. Faecal calprotectin -- a useful tool in the management of inflammatory bowel disease. Swiss Med Wkly. 2012;142:w13557. [PubMed] |
| 4. | Kulaylat MN, Dayton MT. Ulcerative colitis and cancer. J Surg Oncol. 2010;101:706-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Qin X. Etiology of inflammatory bowel disease: a unified hypothesis. World J Gastroenterol. 2012;18:1708-1722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 104] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (2)] |
| 6. | Sun L, Nava GM, Stappenbeck TS. Host genetic susceptibility, dysbiosis, and viral triggers in inflammatory bowel disease. Curr Opin Gastroenterol. 2011;27:321-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Latiano A, Annese V. Genetics and ulcerative colitis: what are the clinical implications? Curr Drug Targets. 2011;12:1383-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Bouzid D, Kammoun A, Amouri A, Mahfoudh N, Haddouk S, Tahri N, Makni H, Masmoudi H. Inflammatory bowel disease: susceptibility and disease heterogeneity revealed by human leukocyte antigen genotyping. Genet Test Mol Biomarkers. 2012;16:482-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Gao F, Liu X, Nu D. Comparisons of clinical characteristics in patients with ulcerative colitis between Uygur and Han nationality in Xinjiang. Zhonghua Xiaohua Neijing Zazhi. 2007;24:4. |
| 10. | Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989;170:2-6; discussion 16-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1396] [Cited by in RCA: 1445] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 11. | Zhao J, Jiang Y, Lei Y, Zou K, Wang C, Huang S, Yi F, Xia B. Functional MICA-129 polymorphism and serum levels of soluble MICA are correlated with ulcerative colitis in Chinese patients. J Gastroenterol Hepatol. 2011;26:593-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Ye X, Jiang Y, Wang H, Chen L, Yuan S, Xia B. Genetic polymorphisms of glutathione S-transferases are associated with ulcerative colitis in central China. Cell Biochem Biophys. 2011;60:323-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Gong W, Lv N, Wang B, Chen Y, Huang Y, Pan W, Jiang B. Risk of ulcerative colitis-associated colorectal cancer in China: a multi-center retrospective study. Dig Dis Sci. 2012;57:503-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Gao F, Liu X, Nu D, Li YX, Jiang XM. Comparisons of clinical characteristics in patients with ulcerative colitis between Uygur and Han nationality in Xinjiang. Zhonghua Xiaohua Neijing Zazhi. 2007;24:423-426. |
| 15. | Krini M, Chouliaras G, Kanariou M, Varela I, Spanou K, Panayiotou J, Roma E, Constantinidou N. HLA class II high-resolution genotyping in Greek children with celiac disease and impact on disease susceptibility. Pediatr Res. 2012;72:625-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Sepúlveda M, Blanco Y, Rovira A, Rio J, Mendibe M, Llufriu S, Gabilondo I, Villoslada P, Castilló J, Corral J. Analysis of prognostic factors associated with longitudinally extensive transverse myelitis. Mult Scler. 2012;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Piga M, Paladini F, Lai S, Erre G, Passiu G, Carcassi C, Sorrentino R, Mathieu A. Genetics of Behçet’s disease in Sardinia: two distinct extended HLA haplotypes harbour the B*51 allele in the normal population and in patients. Clin Exp Rheumatol. 2012;30:S51-S56. [PubMed] |
| 18. | Wild GE, Rioux JD. Genome scan analyses and positional cloning strategy in IBD: successes and limitations. Best Pract Res Clin Gastroenterol. 2004;18:541-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Lü M, Xia B. Polymorphism of HLA-DRB1 gene shows no strong association with ulcerative colitis in Chinese patients. Int J Immunogenet. 2006;33:37-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Lee YT, Sung JJ, Poon P, Lai KN, Li PK. Association of HLA class-II genes and anti-neutrophil cytoplasmic antibodies in Chinese patients with inflammatory bowel disease. Scand J Gastroenterol. 1998;33:623-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Matsumura Y, Kinouchi Y, Nomura E, Negoro K, Kakuta Y, Endo K, Aizawa H, Takagi S, Takahashi S, Shimosegawa T. HLA-DRB1 alleles influence clinical phenotypes in Japanese patients with ulcerative colitis. Tissue Antigens. 2008;71:447-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Lü M, Xia B. Association of HLA2D RB1 alleles with ulcerative colitis in Chinese patients with Han nationality. Zhongguo Mianyixue Zazhi. 2005;21:752-759. |