Published online Apr 21, 2013. doi: 10.3748/wjg.v19.i15.2348
Revised: November 20, 2012
Accepted: November 24, 2012
Published online: April 21, 2013
Processing time: 16 Days and 9.5 Hours
AIM: To assess advanced neuroendocrine tumor (NET) treatment patterns and resource utilization by tumor progression stage and tumor site in the United States.
METHODS: United States Physicians meeting eligibility criteria were provided with online data extraction forms to collect patient chart data on recent NET patients. Resource utilization and treatment pattern data were collected over a baseline period (after diagnosis and before tumor progression), as well as initial and secondary progression periods, with progression defined according to measureable radiographic evidence of tumor progression. Resource categories used in the analysis include: Treatments (e.g., surgery, chemotherapy, radiotherapy, targeted therapies), hospitalizations and physician visits, diagnostic tests (biomarkers, imaging, laboratory tests). Comparisons between categories of resource utilization and tumor progression status were examined using univariate (by tumor site) and multivariate analyses (across all tumor sites).
RESULTS: Fifty-five physicians were included in the study and completed online data extraction forms using the charts of 110 patients. The physician sample showed a relatively even distribution for those affiliated with academic versus community hospitals (46% vs 55%). Forty (36.3%) patients were reported to have pancreatic NET (pNET), while 70 (63.6%) patients had gastrointestinal tract (GI)/Lung as the primary NET site. Univariate analysis showed the proportion of patients hospitalized increased from 32.7% during baseline to 42.1% in the progression stages. While surgeries were performed at similar proportions overall at baseline and progression, pNET patients, were more likely than GI/Lung NET patients to have undergone surgery during the baseline (33.3% vs 25.0%) and any progression periods (26.7% vs 23.4%). While peptide-receptor radionuclide and targeted therapy utilization was low across NET types and tumor stages, GI/Lung types exhibited greater utilization of these technologies compared to pNET. Chemotherapy utilization was also greater among GI/Lung types. Multivariate analysis results demonstrated that patients in first progression period were over 3 times more likely to receive chemotherapy when compared to baseline (odds ratio: 3.31; 95%CI: 1.46-7.48, P = 0.0041). Further, progression was associated with a greater likelihood of having a study physician visit [relative risk (RR): 1.54; 95%CI: 1.10-2.17, P = 0.0117], and an increased frequency of other physician visits (RR: 1.84; 95%CI: 1.10-3.10, P = 0.0211).
CONCLUSION: Resource utilization in advanced NET in the United States is significant overall and data suggests progression has an impact on resource utilization regardless of NET tumor site.
- Citation: Strosberg J, Casciano R, Stern L, Parikh R, Chulikavit M, Willet J, Liu Z, Wang X, Grzegorzewski KJ. United States-based practice patterns and resource utilization in advanced neuroendocrine tumor treatment. World J Gastroenterol 2013; 19(15): 2348-2354
- URL: https://www.wjgnet.com/1007-9327/full/v19/i15/2348.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i15.2348
Neuroendocrine tumors (NET) are a group of diverse neoplasms, commonly originating in the gastrointestinal tract (GI), lungs, and pancreas. The prevalence of NET is estimated to be 35.0 per 100000 in the United States, and the age-adjusted incidence of NET has increased from an estimated 1.1 per 100000 persons in 1973 to 5.3 per 100000 persons in 2004[1]. The specific factors responsible for this rise in incidence are not know; however, improved classification of tumors and widespread use of endoscopy as a screening tool are likely contributors to this increase[1].
Pancreatic NET (pNET) are often classified based on hormone produced (e.g., gastrinoma, insulinoma)[2]. GI and lung NET types have traditionally been classified by site of origin, and morphologic pattern. Newer classifications, however, have been formulated to reflect the considerable variability in histopathology and presentation within each site of origin[3]. Patients with ileocecal NET typically present with carcinoid syndrome, which results from an over production of serotonin. Symptoms and complications include diarrhea, hot flashes, bronchoconstriction, and right-sided valvular heart disease[3]. NET is commonly perceived as indolent[1]; however, due to the variability and uncertainty of symptoms associated with the disease, NET is often diagnosed in an advanced stage whereby the prognosis is poor (65% 5-year mortality rate). This is particularly true of pNET, where the 5-year mortality rate has been reported to be as high as 73%[1].
Patients with localized NETs and those with resectable oligometastases are often managed surgically. Advanced unresectable tumors are often treated with somatostatin analogs (SSA), either for control of symptoms or inhibition of tumor growth[4]. Other treatment options include streptozocin or temozolomide-based chemotherapy, or targeted therapies such as sunitinib or everolimus[5,6]. Platinum and etoposide-based regiments are typically used for poorly differentiated tumors. While guidelines to aid treatment decisions have been published[5,6], little is known about how disease progression and tumor type influence NET treatment decisions among United States physicians in a real-world setting. Therefore, the aim of the current study is to assess advanced NET treatment patterns and resource utilization by tumor progression stage and tumor site in the United States.
In a United States-based sub-analysis of a global study (details of which have been published elsewhere[7]) Physicians (gastroenterologists, endocrinologists, and oncologists) were contacted to take part in the research from December 2010 to January 2011. A total of 4100 physicians were identified in a market research database, and were recruited via an online invitation. A convenience sampling method was applied in order to achieve a final global study sample of 197 physicians, with a target sample of 55 physicians in the United States sub-study. Eligibility criteria included the following: practicing medicine for at least 3 years (but no more than 30 years) prior to the study date, spending at least 50% of one’s working time on patient care, treating at least 3 NET patients in the past year, and specializing in gastroenterology, endocrinology, or oncology.
Physicians were instructed to complete internet-based data extraction forms, referring to clinical charts of patients. They were asked to refer only to charts on their most recent patients who were diagnosed with advanced NET of the GI tract, lung, or pancreas - at least one patient must have experienced tumor progression. Additionally, selected patients had to have confirmed well - to moderately-differentiated tumor histology, assessed as per the 2000 World Health Organization criteria[8]. Data regarding patients with poorly differentiated tumors were not selected for this study. Proportions, frequencies, and means (respectively) were compared by NET progression stage and tumor site for the following measures:
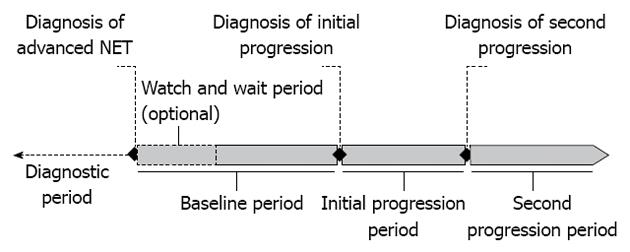
NET progression: The main variable of interest was 3-level tumor progression stage. The baseline period, or first stage, was defined as the time between diagnosis of NET and diagnosis of tumor progression with measurable or radiographic evidence as reported by physicians. Initial progression, or second stage, was defined as the time period during which tumor progression was first diagnosed and treated. Secondary progression, or third stage, was defined as the time point when further measurable or radiographic evidence for progression was found, and the following period of treatment. Since not all patients had a second progression, physicians were asked to project resource use during this period, assuming a duration of 12 mo for all patients. The date of the last resource use served as a proxy for patients who had no recorded first progression or second progression date. Initial and secondary progression, in aggregate, were referred to as any progression (i.e., the any progression period encompasses both the initial and secondary progression period, assessing resources accrued in both; Figure 1).
NET type: The patient sample was further stratified into two groups, one with primary NET location as either the lung or GI, and the other with primary NET location as the pancreas. It should be noted that no pre-specified distribution among NET sub-types was implemented; physicians were asked only to report data on their most recent patients.
Patient baseline attributes: Include age, site of metastasis, ECOG performance status, tumor histology and grade, performance status, comorbidities, and tumor symptoms.
Physician attributes: Include primary specialty, whether they practiced in an academic or community hospital, number of years of training and practice, and proportion of time spent in direct patient care.
Medical resources: Chemotherapy, peptide-receptor radionuclide therapy (PRRT), SSA, and other pharmaceutical therapies including targeted therapies; as well as study-physician and other physician visits, hospitalizations, surgical procedures such as radiofrequency ablation, microwave ablation, cryoablation, radiotherapy, hepatic arterial embolization, and transplant; Biomarker tests including neuron specific enolase, chromogranin A, pancreatic polypeptide, neurotensin, plasma serotonin, vasoactive intestinal polypeptide, Ghrelin, human chorionic gonadotropin, Ki-67, 5-hydroxyndoleacetic acid, plasma substance P, total and free T4; other lab tests including CBC, BUN, serum glucose, Darkfield microscopy, serum creatinine, and lipid profile; and imaging test including ultrasounds, computed tomography (CT) scans, helical scans, and others.
Proportions and frequencies (for dichotomous) as well as means and medians (for count outcomes) were assessed for the study resource use categories (listed above) were compared according to NET progression stage and tumor site. Resources were assessed for any progression and any time. Any progression was measured at an event level, where first progression and second progression were considered as separate events. Thus, it included both first progression and second progression resource-use information, which may have resulted in multiple events per patient. For any progression, utilization rates were reported as a proportion of patients per event for each specific resource. Any time included resources used at least once during baseline, first progression, or second progression. A given patient is counted only once if a particular resource is received in two or more time periods. Practice patterns for any time were analyzed as the proportion of patients utilizing each resource.
Multivariable models were conducted to compare resource use across disease progression stages. As the study was not powered to ascertain statistically significant differences, these analyses were considered secondary. Some patients did not have baseline or first progression data (Measures), and repeated measurement of outcomes over tumor stage progression produced correlated outcomes. Therefore generalized estimating equations (GEE) were employed. GEE are generalized linear models which estimate the average response over the population, as opposed to predicting responses for individuals. Utilizing such an approach, models were computed for binary (yes/no) outcomes assuming a binomial distribution. For count outcomes a Poisson distribution was assumed. All models adjusted for the following covariates: primary NET location, age, country, physician specialty, tumor histology, ECOG performance status, and patient follow-up time. It should be noted that the resource use category study physician visits was assessed as a binary outcome (i.e., did a patient have ≥ 1 visit or not); however, due to the issue of convergence, a Poisson distribution was assumed for this variable rather than a binomial distribution. A Bonferroni correction on P value was calculated to determine an appropriate alpha value for the statistical significance threshold (0.05/9 = 0.0055).
Out of the total sample of physicians (n = 55), 13% (n = 7) primarily practiced medical oncology, 29% (n = 16) hematology/oncology, 29% (n = 16) gastroenterology, and 29% (n = 16) endocrinology. The physician sample showed a relatively even distribution for those affiliated with academic versus community hospitals (46% vs 55%). Each physician reported data on 2 patients (n = 110 total). Baseline data were not available for 6 United States patients because their date of diagnosis of advanced NET and first progression were the same; however, 61 patients had at least initial progression.
Forty (36.3%) patients were reported to have pNET, while 70 (63.6%) patients had GI/Lung as the primary NET site (GI/Lung NET). The mean duration of baseline period was found to be 14.0 mo for pNET, 13.3 mo for GI/Lung, and 13.6 mo for both tumor types. The mean duration of initial progression was 5.7 mo for pNET, 7.9 mo for GI/Lung, and 7.1 mo for both tumor types. Sixty three (57.3%) patients had well differentiated tumor histology, while 47 (42.7%) patients had moderately differentiated tumors. Furthermore, 63 (57.3%) patients were found to have symptoms and 47 (42.7%) showed no symptoms. ECOG Performance varied as follows: 44 (40.0%) patients showed a score of 0-1, 24 showed a score of 2, 12 showed a score of 3, 5 showed a score of 4, and 25 patients had no recorded score.
Resource utilization at any time and across all NET sub-types shows SSAs to be the treatment used in the highest proportion of patients (78.2%), followed by surgery and chemotherapy (used in 45.5% and 38.2% of patients, respectively). Any time resource utilization also indicates high proportions of patients undergoing hospitalization (56.4%) as well as diagnostics such as CT scans (71.8%), biomarkers (66.4%) and other lab tests (63.6%). pNET patients were proportionately less likely than GI/Lung NET patients to receive chemotherapy (30.0% vs 42.9%), CT scans (65.0% vs 75.7%), and PRRT (0.0% vs 8.6%). pNET patients were more likely to have received ultrasound (50.0% vs 31.4%) and other laboratory tests (72.5% vs 58.6%), and to have undergone surgery (50.0% vs 42.9%), when compared to GI/Lung NET patients. Utilization of other resources were found to be similar between the two groups: SSA (80.0% vs 77.1%), targeted therapy (5.0% vs 7.1%), hospitalization (55.0% vs 57.1%), helical scans (37.5% vs 38.6%), other imaging tests (57.5% vs 58.6%), and biomarker tests (65.0% vs 67.1%; Table 1).
| All NET (n = 110) | GI/lung NET (n = 70) | pNET (n = 40) | |
| Treatments | |||
| Surgery | 50 (45.45) | 30 (42.86) | 20 (50.00) |
| Chemotherapy2 | 42 (38.18) | 30 (42.86) | 12 (30.00) |
| PRRT | 6 (5.45) | 6 (8.57) | 0 (0.00) |
| Somatostatin | 86 (78.18) | 54 (77.14) | 32 (80.00) |
| Targeted therapy3 | 7 (6.36) | 5 (7.14) | 2 (5.00) |
| Resources | |||
| Hospitalizations | 62 (56.36) | 40 (57.14) | 22 (55.00) |
| Ultrasounds | 42 (38.18) | 22 (31.43) | 20 (50.00) |
| CT scans | 79 (71.82) | 53 (75.71) | 26 (65.00) |
| Helical scans | 42 (38.18) | 27 (38.57) | 15 (37.50) |
| Other imaging tests4 | 64 (58.18) | 41 (58.57) | 23 (57.50) |
| Bio marker5 | 73 (66.36) | 47 (67.14) | 26 (65.00) |
| Other lab tests6 | 70 (63.64) | 41 (58.57) | 29 (72.50) |
| Physician visit | 109 (99.09) | 40 (100.00) | 69 (98.57) |
Resource utilization by progression state and by tumor site is summarized in Table 2. First, chemotherapy utilization followed markedly different patterns between pNET and GI/Lung NET patients. No chemotherapy use was observed among pNET patients at baseline; however, it increased to greater than 26.7% during progression. Doxorubicin, streptozocin, and temozolomide were the most frequently used chemotherapies. Among GI/Lung NET patients chemotherapy use gradually increased from baseline to second progression; cisplatin, 5-fluorouracil, etoposide and doxorubicin being the most frequently used chemotherapies. Second, targeted therapies were not widely used among pNET patients (2.8% at baseline; 1.7% during progression). GI/Lung NET patients showed marginally higher use of targeted therapies, with increased utilization during second progression (5.7%) over baseline (2.9%) and initial progression (2.4%). Third, PRRT was not utilized among pNET patients; however, a small number of GI/Lung NET patients received PRRT (4.4% during baseline, 4.5% during progression).
| Baseline | Any progression | |||||
| All NET | GI/Lung | pNET | All NET | GI/Lung | pNET | |
| Treatments | ||||||
| Chemotherapy1 | 17 (16.4) | 17 (25.0) | 0 (0.0) | 46 (26.9) | 30 (27.0) | 16 (26.7) |
| Targeted therapies2 | 3 (2.9) | 2 (2.9) | 1 (2.8) | 6 (3.5) | 5 (4.5) | 1 (1.7) |
| PRRT | 3 (2.9) | 3 (4.4) | 0 (0.0) | 39 (6.1) | 5 (4.5) | 0 (0.0) |
| Somatostatin analogs | 66 (63.5) | 43 (63.2) | 23 (63.9) | 83 (48.5) | 52 (46.9) | 31 (51.7) |
| Surgery | 29 (27.9) | 17 (25.0) | 12 (33.3) | 42 (24.6) | 26 (23.4) | 16 (26.7) |
| Resources | ||||||
| Hospitalizations | 34 (32.7) | 23 (33.8) | 11 (30.6) | 72 (42.1) | 44 (39.6) | 28 (46.7) |
| Ultrasound | 38 (36.5) | 20 (29.4) | 18 (50.0) | 34 (19.9) | 22 (19.8) | 12 (20.0) |
| CT scans (conventional) | 62 (59.6) | 43 (63.2) | 19 (52.8) | 109 (63.7) | 72 (64.9) | 37 (61.8) |
| CT scans (helical or spiral) | 23 (22.1) | 15 (22.1) | 8 (22.2) | 51 (29.8) | 33 (29.7) | 18 (30.0) |
| Other imaging3 | 51 (49.0) | 31 (45.6) | 20 (55.6) | 52 (30.5) | 33 (29.7) | 19 (31.7) |
| Biomarkers4 | 66 (63.4) | 44 (64.7) | 22 (61.1) | 84 (49.1) | 55 (49.6) | 29 (48.3) |
| Lab tests5 | 61 (58.7) | 36 (52.9) | 25 (69.4) | 84 (49.1) | 50 (45.1) | 34 (56.7) |
| Study physician visit | 102 (98.08) | 66 (97.06) | 36 (100.00) | 165 (96.49) | 107 (96.40) | 58 (96.67) |
| Other physician visit | 77 (74.04) | 50 (73.53) | 27 (75.00) | 143 (83.62) | 92 (82.88) | 51 (85.00) |
Similar SSA use was observed among both pNET and GI/Lung NET patients. At baseline 63.5% of all NET patients received SSA. Utilization at first progression was marginally lower (62.3%), while second progression utilization decreased to 40.9%, with an average of 48.5% at any progression. The proportion of patients who were hospitalized increased between baseline and any progression periods (32.7%-42.1% overall). Proportions of patients utilizing CT Scans increased upon progression. Ultrasound, biomarker, laboratory tests and other imaging decreased upon progression. Lastly, surgeries were performed at similar proportions overall at baseline and progression. pNET patients, however, were more likely than GI/Lung NET patients to have undergone surgery during the baseline (33.3% vs 25.0%) and any progression periods (26.7% vs 23.4%).
Patients in first progression were observed to be over 3 times more likely to receive chemotherapy when compared to baseline (odds ratio: 3.31; 95%CI: 1.46-7.48; Table 3). Patients in first progression were also more likely to have a study physician visit [relative risk (RR): 1.54; 95%CI: 1.10-2.17], as well as an increased frequency of other physician visits (RR: 1.84; 95%CI: 1.10-3.10; Table 4).
The aim of this study was to assess the resource utilization and treatment patterns of NET by tumor progression stage and by tumor site. The results suggest that there is significant resource utilization associated with United States NET patients regardless of tumor site, particularly with respect to hospitalizations, surgeries, imaging and lab tests, chemotherapy, and SSA (Table 1). As may be expected, disease progression is associated with a decrease in utilization rates for certain diagnostics including ultrasound, biomarker, laboratory tests and other imaging. However, disease progression is associated with an increase in other resources, such as other (non-study) physician visits, hospitalizations, chemotherapy, and CT scans (Table 2). In keeping with these results, the multivariate analysis demonstrates that NET patients are more likely to receive chemotherapy and visit physicians when disease progresses.
While overall resource utilization increases with disease progression irrespective of tumor site, there were variations in practice patterns depending on whether patients had GI/Lung or pNET. For instance, pNET patients were found to be less likely than GI/Lung patients to be administered targeted, chemo-, or peptide-receptor radionuclide therapies, and more likely to have undergone surgical procedures than the GI/Lung patients. Although the heterogeneous nature of NET makes inferences about whether physicians treated patients according to current guidelines difficult, some comparisons can be made with caution[5,6]. The high utilization of surgical procedures and SSA observed here is consistent with NCCN guidelines[5]. It is possible that utilization of targeted therapies (everolimus and sunitinib) in pNET was low because of their novelty, limited availability, and restricted reimbursement by managed care organizations during the patient data collection time period (December 2010-January 2011). Furthermore, recent clinical trial data supporting the use of these therapies were not available when the study was conducted[9,10]. Inferences regarding the observed differences in utilization of targeted and PRRT therapies between pNET and GI/Lung types are inconclusive.
The low use of targeted therapies overall should be considered more closely. Recent clinical trial data suggest sunitinib and everolimus improve progression-free survival by 6-7 mo compared to placebo plus best supportive care[9-11]. While more research is necessary to elucidate differences in adverse event reporting (and quality of life more generally) between patients receiving chemotherapy versus targeted agents, these trials suggest that targeted therapies are associated with relatively low rates of adverse events, and may be more tolerable than chemotherapy[9,11].
Rates of chemotherapy use are higher than one might expect (42.86% in GI/Lung NET and 30.00% in pNET); especially given that chemotherapy is approved only in well- to moderately-differentiated pNET. While the reasons for these relatively high rates cannot be known for certain, it is possible that physicians are using chemotherapy regimens off-label, as a result of having few alternative treatments available for the advanced GI/Lung and pancreatic NET populations.
Interestingly, we found similar baseline durations for GI/Lung and pNET types; with marginally shorter first progression duration in pNET (secondary progression duration could not be ascertained). As pNET has been reported to have a more aggressive disease course[1], caution should be taken in interpreting these results. It is plausible that the operational definition of disease progression in the current study is not sensitive to changes in the tumor pathology which lead to the mean differences in survival and progression, generally observed elsewhere[1].
In comparison to results from a global analysis[7], this United States-specific sub-analysis shows several differences in resource utilization and practice patterns. Specifically, cross-sectional resource utilization is lower in the United States for certain categories including chemotherapy, PRRT, and notably hospitalizations. Multivariate analyses are in line with those from the global analysis, showing an increase in chemotherapy and physician visits associated with progression. However, while the global analysis also showed an increase in SSA use with progression, the United States sub-analysis data do not.
Due to possible selection bias and the exclusion criteria used to identify physicians for study eligibility, the sample of participating physicians may differ from the general population of physicians in ways that may differentially affect the study results. Some patients are missing baseline data (n = 6), and for those patients with initial progression data but not secondary progression data, hypothetical resource use projections were made by the study physicians. Because of the cross-sectional nature of the study, recall bias may have affected the observed results. Additionally, the self-reported nature of the data limited the ability to assess the variety of symptoms associated with NET clinical syndromes, ancillary treatments used to palliate hormonal symptoms. Targeted therapy utilization was likely under-reported due to the structure of the data collection form, which relied on open-ended responses for this treatment category. As noted above, the study findings show high rates of chemotherapy usage. While the reported results may represent off-label usage, it is also possible that patients with poorly-differentiated NET were included, as etoposide- and cisplatin- based regimens are approved in this indication.
Overall study results confirm that advanced NET in the United States is associated with significant resource use regardless of tumor site. Resource utilization follows a consistent pattern across NET tumor types as the disease progresses, suggesting progression has an impact on resource utilization regardless of tumor site. Targeted therapy use (everolimus and sunitinib) was reported to be relatively low compared to other treatments, likely due to pending regulatory approval at the time of the study. However, with the regulatory approvals in place, targeted therapy use is expected to increase in the future. Further research involving larger patient populations is warranted to fully depict the nature of NET resource utilization and related treatment patterns and to define the real world economic impact of NET disease progression.
Editorial assistance was provided by Jan-Samuel Wagner, who received compensation from LA-SER Analytica for his work.
Neuroendocrine tumors (NET) are a group of diverse neoplasms, commonly originating in the gastrointestinal tract, lungs, and pancreas. The prevalence of NET is estimated to be 35.0 per 100000 in the United States.
While guidelines to aid treatment decisions have been published, little is known about how disease progression and tumor type influence NET treatment decisions among United States physicians in a real-world setting.
This study assessed the resource utilization and treatment patterns of NET by tumor progression stage and by tumor site. Overall study results confirm that advanced NET in the United States is associated with significant resource use regardless of tumor site.
Further research involving larger patient populations is warranted to fully depict the nature of NET resource utilization and related treatment patterns and to define the real world economic impact of NET disease progression.
Resource categories used in the resource utilization analysis include: Treatments (e.g., surgery, chemotherapy, radiotherapy, targeted therapies), hospitalizations and physician visits, diagnostic tests (biomarkers, imaging, laboratory tests).
This is a well-written article summarizing resource utilization of treatments for neuroendocrine tumors in the United States based on anonymous physician surveys. While such a study is inherently subject to recall bias, the manuscript lists this potential pitfall in the discussion section.
P- Reviewer Strosberg J S- Editor Gou SX L- Editor A E- Editor Li JY
| 1. | Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063-3072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3022] [Cited by in RCA: 3246] [Article Influence: 190.9] [Reference Citation Analysis (0)] |
| 2. | Batcher E, Madaj P, Gianoukakis AG. Pancreatic neuroendocrine tumors. Endocr Res. 2011;36:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Pinchot SN, Holen K, Sippel RS, Chen H. Carcinoid tumors. Oncologist. 2008;13:1255-1269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 177] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 4. | Rinke A, Müller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, Mayer C, Aminossadati B, Pape UF, Bläker M. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27:4656-4663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1609] [Cited by in RCA: 1742] [Article Influence: 108.9] [Reference Citation Analysis (0)] |
| 5. | National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Neuroendocrine Tumors Version 1. 2011;. |
| 6. | Kulke MH, Anthony LB, Bushnell DL, de Herder WW, Goldsmith SJ, Klimstra DS, Marx SJ, Pasieka JL, Pommier RF, Yao JC. NANETS treatment guidelines: well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas. 2010;39:735-752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 467] [Cited by in RCA: 398] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 7. | Casciano R, Wang X, Stern L, Parikh R, Chulikavit M, Willet J, Liu Z, Strosberg J, Cadiot G, Riechelmann R. International Practice Patterns and Resource Utilization in the Treatment of Neuroendocrine Tumors. Pancreas. 2013;Jan 25; Epub ahead of print. [PubMed] |
| 8. | Bodei L, Ferone D, Grana CM, Cremonesi M, Signore A, Dierckx RA, Paganelli G. Introduction: Neuroendocrine Tumors. 2008; Available from: http: //dissertations.ub.rug.nl/FILES/faculties/medicine/2009/l.bodei/01c1.pdf. |
| 9. | Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2039] [Cited by in RCA: 2117] [Article Influence: 151.2] [Reference Citation Analysis (0)] |
| 10. | Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2032] [Cited by in RCA: 1829] [Article Influence: 130.6] [Reference Citation Analysis (0)] |
| 11. | Kulke MH, Bendell J, Kvols L, Picus J, Pommier R, Yao J. Evolving diagnostic and treatment strategies for pancreatic neuroendocrine tumors. J Hematol Oncol. 2011;4:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |