Published online Feb 21, 2012. doi: 10.3748/wjg.v18.i7.646
Revised: September 28, 2011
Accepted: October 5, 2011
Published online: February 21, 2012
AIM: To observe the synergistic effects of hyperthermia in oxaliplatin-induced cytotoxicity in human colon adenocarcinoma Lovo cells.
METHODS: The human colon adenocarcinoma cell line Lovo was obtained from Sun Yat-Sen University. Cells were sealed with parafilm and placed in a circulating water bath, and was maintained within 0.01 °C of the desired temperature (37 °C, 39 °C, 41 °C, 43 °C and 45 °C). Thermal therapy was given alone to the negative control group while oxaliplatin was administered to the treatment group at doses of 12.5 μg/mL and 50 μg/mL. Identification of morphological changes, 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide assay, flow cytometry and Western blotting were used to investigate the effect of thermochemotherapy on human colon adenocarcinoma Lovo cells, including changes in the signal pathway related to apoptosis.
RESULTS: A temperature-dependent inhibition of cell growth was observed after oxaliplatin exposure, while a synergistic interaction was detected preferentially with sequential combination. Thermochemotherapy changed the morphology of Lovo cells, increased the inhibition rate of the Lovo cells (P < 0.05) and enhanced cellular population in the G0/G1 phase (16.7% ± 4.8 % in phase S plus 3.7% ± 2.4 % in phase G2/M, P < 0.05). Thermochemotherapy increased apoptosis through upregulating p53, Bax and downregulating Bcl-2. Protein levels were elevated in p53, Bax/Bcl-2 in thermochemotherapy group as compared with the control group (P < 0.05).
CONCLUSION: Thermochemotherapy may play an important role in apoptosis via the activation of p53, Bax and the repression of Bcl-2 in Lovo cells.
- Citation: Zhang XL, Hu AB, Cui SZ, Wei HB. Thermotherapy enhances oxaliplatin-induced cytotoxicity in human colon carcinoma cells. World J Gastroenterol 2012; 18(7): 646-653
- URL: https://www.wjgnet.com/1007-9327/full/v18/i7/646.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i7.646
Colorectal cancer (CRC) is one of the most common types of cancer worldwide[1,2]. Over the past decades, the incidence of CRC has been increasing in China, with an increased mortality due to early metastases[3]. Despite surgical resection, more than 40% of the CRC patients die of the metastases ultimately[4-6]. The poor prognosis is associated with difficulties in early diagnosis at a curable stage. Therefore, the urgent need to develop new therapeutic strategies in order to significantly improve the prognosis of the metastatic CRC patients is not overstated.
Oxaliplatin, a new third-generation platinum coordination complex of the 1,2-diaminocyclohexane family, has shown promising activity for CRC[7-9]. Its spectrum of antitumor activities in tumor models differs from that of cisplatin and carboplatin. Oxaliplatin is active in a broad range of cancer cell lines and does not produce cross-resistance of cisplatin and carboplatin Its side effects are also distinct from other platinum drugs-it induces no renal or hepatic toxicity but causes both a reversible acute, cold-related dysesthesia and a dose-limiting cumulative peripheral sensory neuropathy that usually rapidly regresses after treatment withdrawal[10].
It is well known that hyperthermia is a strong factor that increases tumor sensitivity to chemotherapy[11,12]. Cells in the DNA synthetic phase of the cell cycle are relatively resistant to chemotherapy, but are especially sensitive to hyperthermia. Thus, a combined treatment may, in some circumstances, be an advantage[13]. Previous data have shown that the cytotoxicity and anti-tumor effect of cisplatin are greatly enhanced at elevated temperatures[14]. It is also reported that the effect of oxaliplatin on colon cells is thermally enhanced at 42 °C when compared with 37 °C[15].
A key mechanism associated with cancer cell growth is the control of apoptosis. Apoptosis is defined as programmed cell death, which occurs in response to disruption of normal homeostatic mechanisms. It is a critical parameter in tumor surveillance of abnormal cells[16]. Induction of apoptosis of cancer cells is considered a potentially new treatment for colon cancer[17].
However, whether the antitumor effect of oxaliplatin in colon cancer has a linear relationship with temperature is not known. Therefore, we explored the effect of thermal treatment in colon cancer cells and investigated the optimal temperature for the inhibitory effect of oxaliplatin in colon cancer cell lines.
Newborn calf serum and Dulbecco’s modified Eagle’s medium (DMEM, low glucose) were purchased from GIBCO Corporation, penicillin/streptomycin was from Gibco-BRL (Germany), while 0.25% trypsin digest and trizol reagent were purchased from Invitrogen Corporation. 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and dimethyl sulfoxide (DMSO) were obtained from Sigma Corporation. Primary antibodies (applied for 1 h at room temperature or overnight at 4 °C) were: anti-Bcl-2, anti-Bax and anti-p53 monoclonal antibody all purchased from Santa Cruz Biotechnology (Heidelberg, Germany), goat anti-rabbit IgG (secondary) and prestained protein molecular weight marker were purchased from Cell Signaling Technology (United States).
The human colon adenocarcinoma cell line Lovo was obtained from the Cell Bank of Sun Yat-Sen University. All cell culture operations were carried out in a sterile class II biological safety cabinet (Thermo Fisher, United States). The Lovo cell lines were cultured in DMEM containing 10% fetal bovine serum (GIBCO Corporation, Carlsbad, CA, United States), 50 U/mL of penicillin and 50 mg/mL of streptomycin (Invitrogen, Carlsbad, California, United States). The cells were grown in 25-mL and 75-mL flasks (Corning, New York, United States) in a humidified incubator at 37 °C with 5% CO2 atmosphere (Thermo Fisher, United States). Cells were stained with Trypan blue (Sigma-Aldrich, St Louis, Missouri, United States) and then counted on a hemacytometer.
Cells cultured in 60-mm dishes were sealed with parafilm and placed in a circulating water bath (Yiheng Corporation, Shanghai, China), and was maintained within 0.01 °C of the desired temperatures (37 °C, 39 °C, 41 °C, 43 °C, 45 °C).Thermal therapy was given alone to the control group while oxaliplatin was administered to the treatment group at doses of 12.5 μg/mL and 50 μg/mL. The duration of thermal therapy and chemotherapy was 1 h, and the cells were cultured in a humidified incubator at 37 °C with 5% CO2 atmosphere for 24 h.
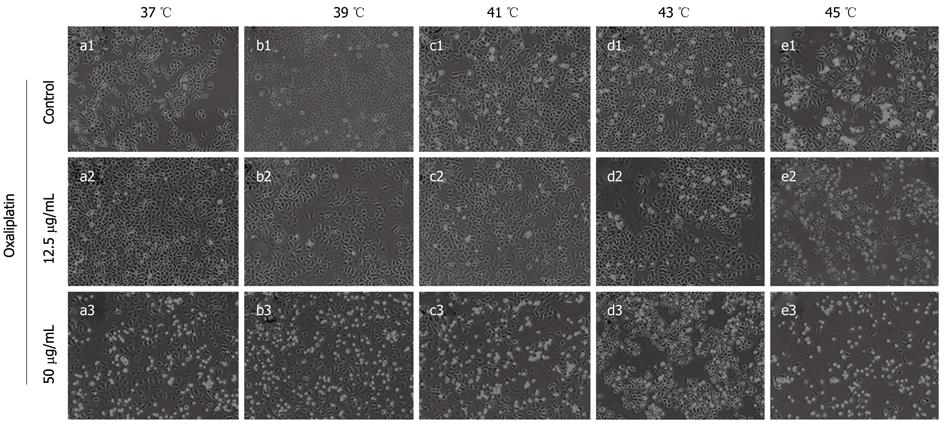
The cells were observed under inverted phase contrast microscope (Olympus, Japan) after completion of thermal therapy or thermochemotherapy.
Measurement of cell growth inhibition by MTT assay was described previously[18]. Lovo cells were seeded in 96-well plates (5 × 103 cells/well) and were treated with 12.5 μg/mL or 50 μg/mL oxaliplatin at 37 °C, 39 °C, 41 °C, 43 °C or 45 °C. Negative control wells contained only CRC cells but not oxaliplatin. The experiment was repeated 3 times. Untreated and treated cells were cultured at 37 °C with 5% CO2 for 24 h. MTT was added to 50 μL of cell suspension for 4 h. After supernatant was removed, DMSO (150 μL) was added to each well and mixed at a low speed for 10 min to fully dissolve the blue crystals. Absorbance was measured at 570 nm (A570) and the percentage of growth inhibition of Lovo cells was calculated at each time point and for each concentration of oxaliplatin according to the following formula: % cell survival = (Lovo oxaliplatin group - Lovo blank)/(Lovo negative - Lovo blank) × 100% and % cell growth inhibition = 1 - % cell survival.
The fraction for each concentration of oxaliplatin was calculated. Model parameters included the concentration to inhibit 50% of cell growth (IC50). At least three independent experiments were carried out to test the relationship between temperature of drug exposure and the concentration of oxaliplatin.
Cells were plated at 1 × 106 cells/well in 6-well plates, incubated for 24 h, and then treated with different concentrations of oxaliplatin for 1 h. Trypsinized cells were washed with PBS and fixed in 70% ethanol. After fixation, the cells were incubated for 30 min with 200 mg/mL of RNase A and stained with 25 μg/mL propidium iodide (PI). The stained cells were analyzed using a flow cytometry cell sorter (Becton Dickinson, NJ, United States). Samples were analyzed using a FACScan flow cytometer (Becton Dickinson) according to the manufacturer’s protocol. Experiments were performed in triplicate.
Apoptosis was measured according to the manufacturer’s instructions, using an annexin V-FITC kit (BD Biosciences, San Jose, CA, United States). The cells were collected after drug delivery, washed twice with PBS and then centrifuged. The cell pellet was resuspended in ice-cold binding buffer. The annexin V-FITC and PI solutions were added to the cell suspension and mixed gently. The samples were then incubated for 15 min in the dark before flow cytometric analysis[19]. The analysis of the apoptotic cells was performed by flow cytometry (FACScan, Becton Dickinson, NJ, United States).
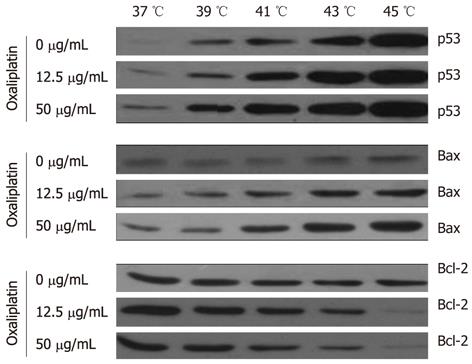
Lovo cells (cultured in 6-well plate at 1.5 × 105 cells/well) were treated with 12.5 μg/mL or 50 μg/mL oxaliplatin at 37 °C, 39 °C, 41 °C, 43 °C or 45 °C and total proteins were extracted. Protein samples were separated by SDS-PAGE and electrophoretically transferred onto a polyvinylidene difluoride membrane (Millipore, United States). The membrane was blocked overnight at 4 °C in TBS-Tween 20 (TBST) buffer containing 5% skimmed milk powder. The membrane was washed with TBST (3 × 8 min). Membranes were then incubated overnight at 4 °C in primary antibody (125 μL/cm3; diluted 1:1000) with gentle shaking. The membranes were washed with TBST (3 × 8 min) and incubated for 1 h at room temperature in horse radish peroxidase (HRP)-conjugated secondary antibody (125 μL/cm3; diluted 1:2500). The membranes were washed with TBST (3 × 8 min) and protein signals were detected by chemiluminescence kit (Cell signaling Technology, United States). Primary antibodies (applied for 1 h at room temperature, or overnight at 4 °C) were: anti-Bcl-2, anti-Bax and anti-p53 monoclonal antibody all purchased from Santa Cruz Biotechnology (Heidelberg, Germany).
Normally distributed continuous variables were compared by one-way analysis of variance (ANOVA). Statistical analyses were performed using SPSS 13.0 statistical software (SPSS Inc, Chicago, IL). When a significant difference between groups was apparent, multiple comparisons of means were performed using the Holm-Bonferroni procedure with type-I error adjustment. Data were presented as means ± SD. All statistical assessments were two-sided and evaluated at the 0.05 level of significant difference.
Lovo cells were treated with oxaliplatin (12.5 μg/mL or 50 μg/mL) at different temperatures for 1 h and then cultured under normal conditions for 24 h. There were disparities after treatment between the control group and the thermochemotherapy group (Figure 1). Thermotherapy alone caused the inhibition of cell growth by inducing cell apoptosis and cell cycle arrest. However, thermochemotherapy caused a greater decrease of Lovo cells as compared with the control group, especially in the 50 μg/mL group. The effect of thermochemotherapy on tumor growth was observed as early as 24 h after treatment.
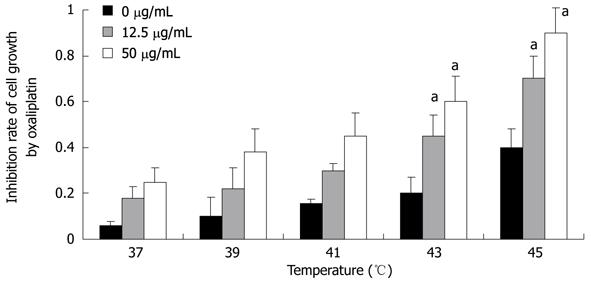
First, we used the MTT assay to detect the effect of temperature on Lovo cell proliferation. With increasing temperature, the inhibition rate of cell growth became higher and higher. The cytotoxicity of Lovo cells exposed to 50 μg/mL oxaliplatin for 1 h was higher than those exposed to 12.5 μg/mL oxaliplatin at the same temperature (P < 0.05). And 43 °C was the optimal temperature to inhibit cell proliferation when the cells were exposed to 50 μg/mL oxaliplatin (P < 0.05, Figure 2).
To elucidate the mechanism of action of thermotherapy and oxaliplatin, we used flow cytometry to determine cell cycle distribution and apoptosis in Lovo cells exposed to different temperatures.
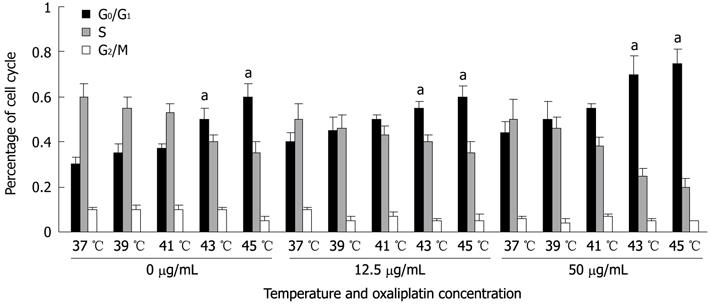
A significant increase in the number of G0/G1 phase cells and a decrease in the number of S and G2/M phase cells after 1 h of oxaliplatin treatment were observed. There was a linear relationship between the cell cycle and the temperature (P < 0.05). The proliferation and proportions of cells in different phases of the cell cycle were analyzed at 24 h by the incorporation of PI. DNA histogram analysis revealed that thermal therapy induced a temperature-dependent increase in the number of cells within the G0/G1 phase. This increase was accompanied by a decrease in the percentage of proliferating cells (16.7% ± 4.8% in phase S and 3.7% ± 2.4% in phase G2/M) (P < 0.05, Figure 3). Accumulation of 12.5 μg/mL oxaliplatin-treated cells at any phase was less remarkable than that of the 50 μg/mL-treated cells (P < 0.05).
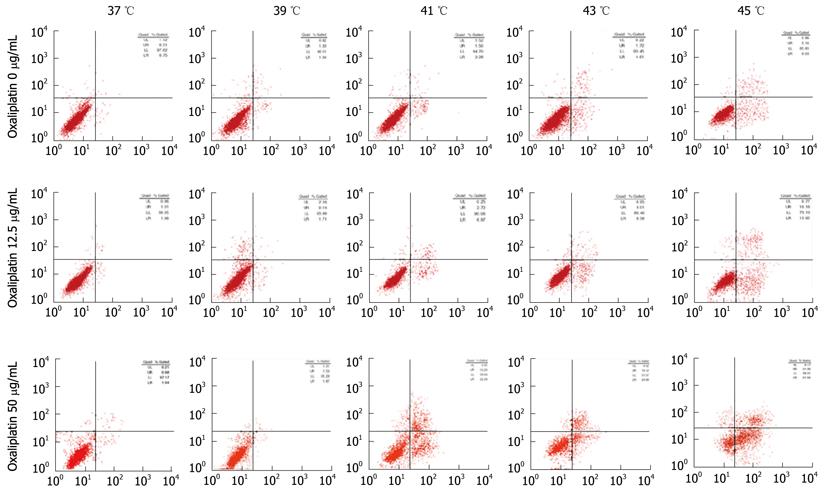
We also used PI staining to show that thermotherapy induced apoptosis of Lovo cells in a temperature-dependent manner (Figure 4).
It is well known that reduction of intra-cellular apoptotic molecules, such as p53 and Bax/Bcl-2, sensitizes Lovo cells to thermotherapy. We therefore investigated whether changes in the amounts of apoptotic proteins were associated with the promotion of hyperthermia and oxaliplatin.
p53 stimulated the mitochondrial apoptotic pathway, thus enabling direct protein interaction or inhibition of the Bcl-2 protein family. p53 can also induce the pro-apoptotic Bcl-2 proteins by transcripting or inhibiting the transcription of anti-apoptotic Bcl-2 proteins. We examined the effect of thermal therapy on the expression of the Bcl-2 protein group.
The results showed a thermal-dependent increase in Bax expression over temperature and a concomitant decrease in the expression of Bcl-2. The maximal levels of Bax reached a peak at 43 °C. As for Bcl-2, there was a marked decrease when compared 43 °C and 45 °C with 37 °C. These levels later increased somewhat but always remained lower than that in the control group (P < 0.05). Both Bax activation and Bcl-2 inhibition were required for the release of mitochondrial apoptotic factors and the activation of the intrinsic apoptotic route.
Finally, we detected the possible signal pathway involved in the effects of oxaliplatin on Lovo cells. There was an increase in the expression of p53 and Bax protein in cells treated with oxaliplatin for 1 h. Compared with the cells of the control group, a gradual decrease in Bcl-2 levels was found at an increasing temperature, with the most significant reduction at 43 °C (Figure 5).
Thermotherapy combined with immediate intraperitoneal delivery of adjuvant anti-cancer drugs is a novel cancer treatment strategy to improve prognosis and prolong the overall survival of the patients with advanced CRC. The aim of this study was to assess the role of hyperthermia in oxaliplatin-induced cytotoxicity, to find an appropriate temperature using in vitro studies in maximizing anti-tumor activities and discuss the possible mechanism of thermochemotherapy. Preclinical studies have suggested that oxaliplatin may offer therapeutic advantages in a variety of malignancies with either intrinsic or acquired cisplatin resistance[20]. The primary mechanism of oxaliplatin has been shown to be mediated by the formation of intrastrand DNA cross-links[21]. Rietbroek et al[22] reported that thermotherapy at 43 °C enhanced the formation of DNA cross-links and concluded that a large portion of enhanced cytotoxicity may be attributed to the increased cross-links. Other factors such as an increased drug uptake at the elevated temperature are also suggested[23]. Evidence that oxaliplatin exerts a specific anti-tumor effect strongly suggests that oxaliplatin may be a promising new compound for the treatment of gastrointestinal tumors[24-26].
The in vitro exposure of human Lovo cells at clinical concentration of oxaliplatin with thermotherapy exerted a strong anti-proliferative effect and induced apoptosis of Lovo cells in this study. Our results highlight the potential clinical value of thermochemotherapy in the treatment of colon cancer. The exposure to 43 °C or above augmented the cytotoxicity of the oxaliplatin-treated Lovo cells in the thermochemotherapy group in a temperature-dependent fashion. This effect was observed by the MTT assay which demonstrated a linear increase in cytotoxicity with thermotherapy. After exposure to 43 °C, the activity of oxaliplatin markedly and rapidly increased, indicating its potential inhibition ability in Lovo cells. Consistently, previous studies have demonstrated that oxaliplatin treatment with different concentrations (7.5-39.7 μg/mL) for 5-150 min can enhance cell death in various cell lines[27,28].
The exact mechanisms of thermochemotherapy remain unclear. Exposure to high temperatures can alter the fluidity of cell membranes, inhibit protein synthesis, and destroy DNA synthesis enzymes. Regulation of the cell cycle and apoptosis is a major strategy to inhibit the progression of a number of cancers. A critical role of p53, a sequence-specific DNA-binding protein, has been demonstrated to execute apoptosis. Either thermotherapy or oxaliplatin can cause cell cycle arrest at the G0/G1 phase and can induce apoptosis of human colon cancer cells.
Some studies have reported that p53 changes cell-cycle arrest and apoptosis induction by regulating the expression of different proteins such as p21, Bax and Bcl-2[29,30]. The Bcl-2 gene product functions as an anti-apoptotic signal, suppressing apoptosis induced by chemotherapeutic drugs. The exact mechanism of Bcl-2 in preventing apoptosis is still not clear. Others have described p53 expression in response to genotoxicity. It has been proposed that p53 may be involved in the cellular response to DNA damage, producing arrest in the G0/G1 phase of the cell cycle to allow efficient repair of DNA before entry into S phase[31,32]. For these reasons, we inferred that the effects of thermotherapy in Lovo cells were mediated partly by the expression of p53.
Our results showed that thermotherapy could induce apoptosis and cell cycle arrest in Lovo cell lines in a temperature-dependent manner. Thermochemotherapy significantly induced cell cycle of G0/G1 phase arrest by upregulating p53 and Bax expression and downregulating Bcl-2 in Lovo cells. We also detected the expression of Bcl-2 and Bax in Lovo cells to understand the impact of thermotherapy on the mitochondrial pathway of apoptosis. Down-regulation of Bcl-2 and up-regulation of p53 and Bax synthesis showed that apoptosis induced by thermochemotherapy may be mediated by the mitochondrial pathway[33,34]. Our findings were consistent with other studies[35,36] that thermotherapy caused G0/G1 cell cycle arrest and promoted apoptosis of Lovo cells by upregulating p53, Bax expression and downregulating Bcl-2[37,38].
In conclusion, our findings indicate that oxaliplatin is a promising agent for the treatment of human Lovo cells. Thermotherapy exerted synergistic interaction with oxaliplatin especially at 43 °C or above, inhibiting the survival of Lovo cells in vitro. Apparently, the observed synergism between thermotherapy and oxaliplatin results in mutual completion and enhancement of anticancer activity that may be extrapolated to animal models of colon cancer and to clinical use. The results of the present study suggest that thermochemotherapy might be a useful future strategy for treating colon cancers.
We thank Dr. Shi-Hui Xing for his careful revision of this manuscript and the assistance in the figure and data analyses.
Oxaliplatin is reported to be active in a broad range of cancer cell lines and hyperthermia is a strong factor that increases tumor sensitivity to chemotherapy. However, whether the antitumor effect of oxaliplatin in colon cancers has a linear relationship with temperature and the optimal temperature for the inhibitory effect of oxaliplatin in colon cancer cell lines remains elusive.
Thermochemotherapy may play an important role in the inhibition of the Lovo cells and the optimal temperature is 43 °C. In addition, down-regulation of Bcl-2 and up-regulation of p53 and Bax may be essential in inducing apoptosis in human colon cancer cell lines.
Thermal therapy and oxaliplatin can inhibit colon cancer cells through inducing apoptosis and regulating the cell cycle. Suppression of Bcl-2 and up-regulation of p53 and Bax might contribute to the regulation of human Lovo cell lines.
This study may help clarify the mechanism of thermochemotherapy for colon cancer and choose the appropriate therapeutic strategy in clinical practice.
Thermochemotherapy is the combination of thermal therapy and chemotherapy.
This is a study using a single cell line. The experiments are straightforward and the data are short and succinct.
Peer reviewers: Luis Bujanda, PhD, Professor, Departament of Gastroenterology, CIBEREHD, University of Country Basque, Donostia Hospital, Paseo Dr. Beguiristain s/n, 20014 San Sebastián, Spain; Haruhiko Sugimura, MD, PhD, Professor, Department of Pathology, Hamamatsu University School of Medicine, 1-20-1 Handayama, Higashi-ku, Hamamatsu 431-3192, Japan
S- Editor Tian L L- Editor Ma JY E- Editor Zhang DN
| 1. | Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449-2460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1274] [Cited by in RCA: 1392] [Article Influence: 87.0] [Reference Citation Analysis (2)] |
| 2. | Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA Cancer J Clin. 2009;59:366-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 786] [Cited by in RCA: 865] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 3. | Wang YX, Zhang XY, Zhang BF, Yang CQ, Chen XM, Gao HJ. Initial study of microRNA expression profiles of colonic cancer without lymph node metastasis. J Dig Dis. 2010;11:50-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 180] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 4. | Punt CJ, Tol J. More is less -- combining targeted therapies in metastatic colorectal cancer. Nat Rev Clin Oncol. 2009;6:731-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Hong Y, Downey T, Eu KW, Koh PK, Cheah PY. A 'metastasis-prone' signature for early-stage mismatch-repair proficient sporadic colorectal cancer patients and its implications for possible therapeutics. Clin Exp Metastasis. 2010;27:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 259] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 6. | Obeidat NA, Pradel FG, Zuckerman IH, DeLisle S, Mullins CD. Outcomes of irinotecan-based chemotherapy regimens in elderly Medicare patients with metastatic colorectal cancer. Am J Geriatr Pharmacother. 2009;7:343-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Liu HF, Hu HC, Chao JI. Oxaliplatin down-regulates survivin by p38 MAP kinase and proteasome in human colon cancer cells. Chem Biol Interact. 2010;188:535-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Nannizzi S, Veal GJ, Giovannetti E, Mey V, Ricciardi S, Ottley CJ, Del Tacca M, Danesi R. Cellular and molecular mechanisms for the synergistic cytotoxicity elicited by oxaliplatin and pemetrexed in colon cancer cell lines. Cancer Chemother Pharmacol. 2010;66:547-558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Rixe O, Ortuzar W, Alvarez M, Parker R, Reed E, Paull K, Fojo T. Oxaliplatin, tetraplatin, cisplatin, and carboplatin: spectrum of activity in drug-resistant cell lines and in the cell lines of the National Cancer Institute's Anticancer Drug Screen panel. Biochem Pharmacol. 1996;52:1855-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 444] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 10. | Fisher RS, Parkman HP. Management of nonulcer dyspepsia. N Engl J Med. 1998;339:1376-1381. [PubMed] |
| 11. | Krause C, Klüttermann K, Mauz-Körholz C. Molecular mechanisms and gene regulation of melphalan- and hyperthermia-induced apoptosis in Ewing sarcoma cells. Anticancer Res. 2008;28:2585-2593. [PubMed] |
| 12. | Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, Felix R, Schlag PM. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002;3:487-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1439] [Cited by in RCA: 1161] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 13. | Hettinga JV, Lemstra W, Konings AW, Kampinga HH. Cisplatin sensitivity and thermochemosensitisation in thermotolerant cDDP-sensitive and -resistant cell lines. Br J Cancer. 1995;71:498-504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Kirstein MN, Root SA, Moore MM, Wieman KM, Williams BW, Jacobson PA, Marker PH, Tuttle TM. Exposure-response relationships for oxaliplatin-treated colon cancer cells. Anticancer Drugs. 2008;19:37-44. [PubMed] |
| 15. | van Geelen CM, Pennarun B, Le PT, de Vries EG, de Jong S. Modulation of TRAIL resistance in colon carcinoma cells: different contributions of DR4 and DR5. BMC Cancer. 2011;11:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Miura K, Fujibuchi W, Ishida K, Naitoh T, Ogawa H, Ando T, Yazaki N, Watanabe K, Haneda S, Shibata C. Inhibitor of apoptosis protein family as diagnostic markers and therapeutic targets of colorectal cancer. Surg Today. 2011;41:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Bernas T, Dobrucki J. Mitochondrial and nonmitochondrial reduction of MTT: interaction of MTT with TMRE, JC-1, and NAO mitochondrial fluorescent probes. Cytometry. 2002;47:236-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 206] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 18. | Meyer M, Essack M, Kanyanda S, Rees J. A low-cost flow cytometric assay for the detection and quantification of apoptosis using an anionic halogenated fluorescein dye. Biotechniques. 2008;45:317-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Maruyama W, Irie S, Sato TA. Morphological changes in the nucleus and actin cytoskeleton in the process of Fas-induced apoptosis in Jurkat T cells. Histochem J. 2000;32:495-503. [PubMed] |
| 20. | Yang J, Parsons J, Nicolay NH, Caporali S, Harrington CF, Singh R, Finch D, D'Atri S, Farmer PB, Johnston PG. Cells deficient in the base excision repair protein, DNA polymerase beta, are hypersensitive to oxaliplatin chemotherapy. Oncogene. 2010;29:463-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Hector S, Bolanowska-Higdon W, Zdanowicz J, Hitt S, Pendyala L. In vitro studies on the mechanisms of oxaliplatin resistance. Cancer Chemother Pharmacol. 2001;48:398-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 115] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Rietbroek RC, van de Vaart PJ, Haveman J, Blommaert FA, Geerdink A, Bakker PJ, Veenhof CH. Hyperthermia enhances the cytotoxicity and platinum-DNA adduct formation of lobaplatin and oxaliplatin in cultured SW 1573 cells. J Cancer Res Clin Oncol. 1997;123:6-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Helm CW. The role of hyperthermic intraperitoneal chemotherapy (HIPEC) in ovarian cancer. Oncologist. 2009;14:683-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Tippayamontri T, Kotb R, Paquette B, Sanche L. Cellular uptake and cytoplasm/DNA distribution of cisplatin and oxaliplatin and their liposomal formulation in human colorectal cancer cell HCT116. Invest New Drugs. 2011;29:1321-1327. [PubMed] |
| 25. | Zhang XL, Shi HJ, Cui SZ, Tang YQ, Ba MC. Prospective, randomized trial comparing 5-FU/LV with or without oxaliplatin as adjuvant treatment following curative resection of gastric adenocarcinoma. Eur J Surg Oncol. 2011;37:466-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Ducreux M, Bennouna J, Hebbar M, Ychou M, Lledo G, Conroy T, Adenis A, Faroux R, Rebischung C, Bergougnoux L. Capecitabine plus oxaliplatin (XELOX) versus 5-fluorouracil/leucovorin plus oxaliplatin (FOLFOX-6) as first-line treatment for metastatic colorectal cancer. Int J Cancer. 2011;128:682-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 27. | Urano M, Ling CC. Thermal enhancement of melphalan and oxaliplatin cytotoxicity in vitro. Int J Hyperthermia. 2002;18:307-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Atallah D, Marsaud V, Radanyi C, Kornprobst M, Rouzier R, Elias D, Renoir JM. Thermal enhancement of oxaliplatin-induced inhibition of cell proliferation and cell cycle progression in human carcinoma cell lines. Int J Hyperthermia. 2004;20:405-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1484] [Cited by in RCA: 1575] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 30. | Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, Moll UM. p53 has a direct apoptogenic role at the mitochondria. Mol Cell. 2003;11:577-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1297] [Cited by in RCA: 1313] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 31. | Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8:275-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1617] [Cited by in RCA: 1695] [Article Influence: 94.2] [Reference Citation Analysis (0)] |
| 32. | Gupta S, Sathishkumar S, Ahmed MM. Influence of cell cycle checkpoints and p53 function on the toxicity of temozolomide in human pancreatic cancer cells. Pancreatology. 2010;10:565-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Vanamala J, Reddivari L, Radhakrishnan S, Tarver C. Resveratrol suppresses IGF-1 induced human colon cancer cell proliferation and elevates apoptosis via suppression of IGF-1R/Wnt and activation of p53 signaling pathways. BMC Cancer. 2010;10:238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 176] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 34. | Zeise E, Rensing L. Hyperthermic pre-treatment protects rat IPC-81 leukaemia cells against heat- and hydrogen peroxide-induced apoptosis. Int J Hyperthermia. 2002;18:344-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Hayashi S, Koshiba K, Hatashita M, Sato T, Jujo Y, Suzuki R, Tanaka Y, Shioura H. Thermosensitization and induction of apoptosis or cell-cycle arrest via the MAPK cascade by parthenolide, an NF-κB inhibitor, in human prostate cancer androgen-independent cell lines. Int J Mol Med. 2011;28:1033-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Hayashi S, Sakurai H, Hayashi A, Tanaka Y, Hatashita M, Shioura H. Inhibition of NF-kappaB by combination therapy with parthenolide and hyperthermia and kinetics of apoptosis induction and cell cycle arrest in human lung adenocarcinoma cells. Int J Mol Med. 2010;25:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 37. | Ohnishi T. The role of the p53 molecule in cancer therapies with radiation and/or hyperthermia. J Cancer Res Ther. 2005;1:147-150. [PubMed] |
| 38. | Jung HJ, Hwang JN, Seo YR. Elimination of methyl methanesulfonate (MMS)-induced micronuclei (MNS) under mild hyperthermia via p53-dependent pathway in human lymphoid cells. Res Commun Mol Pathol Pharmacol. 2004;115-116:175-183. [PubMed] |