Published online Dec 28, 2012. doi: 10.3748/wjg.v18.i48.7397
Revised: September 13, 2012
Accepted: October 30, 2012
Published online: December 28, 2012
Processing time: 155 Days and 11.8 Hours
Gastric schwannomas are rare mesenchymal tumors of the gastrointestinal tract. They are usually misdiagnosed as other submucosal tumors preoperatively. Experience of the imaging features of gastric schwannomas is extremely limited. In this report, we summarize the features of a series of endoscopic ultrasound (EUS) images of gastric schwannomas in an effort to improve the diagnosis and differential diagnosis rate. We retrospectively reviewed the endosonographic features of four patients with gastric schwannomas and their computed tomography imaging results. Gastric schwannomas had heterogeneous hypoechogenicity or isoechogenicity, and a well-demarcated margin. The tumors originated from the fourth layer. Cystic changes and calcification were uncommon. Marginal hypoechoic haloes were observed in two patients. The results described here were different from those of previous studies. In the EUS evaluation, the internal echogenicity of gastric schwannomas was heterogeneous and low, but slightly higher than that of muscularis propria. These features might help us differentiate gastric schwannomas from other submucosal tumors. Further investigation is needed to differentiate these mesenchymal tumors.
- Citation: Zhong DD, Wang CH, Xu JH, Chen MY, Cai JT. Endoscopic ultrasound features of gastric schwannomas with radiological correlation: A case series report. World J Gastroenterol 2012; 18(48): 7397-7401
- URL: https://www.wjgnet.com/1007-9327/full/v18/i48/7397.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i48.7397
Schwannomas of the gastrointestinal (GI) tract are relatively rare, representing about 3% of all mesenchymal tumors of the GI tract[1]. They are usually encapsulated benign tumors of the peripheral nerve sheath composed of differentiated schwann cells, with an excellent prognosis after surgical resection. Malignant transformation of gastric schwannomas is extremely rare[2,3]. It is difficult to differentiate a gastric schwannoma from gastrointestinal stromal tumors (GISTs). Schwannomas are often misdiagnosed as GISTs on radiological examination[4]. However, GISTs have greater malignant potential and require surgical resection and imatinib-based adjuvant therapy. Therefore, it is important to differentiate between gastric schwannomas and other potentially malignant gastrointestinal tumors, especially GISTs. To date, there is only one report describing the endoscopic ultrasonography (EUS) features of gastric schwannomas[5]. In this study, we analyzed 4 gastric schwannomas using EUS imaging. The results we observed were unlike those of previous studies.
From October 21, 2008 to December 27, 2011, four tumors were histologically identified as gastric schwannomas after surgery at the Second Affiliated Hospital of Zhejiang University School of Medicine. Clinical data and tumor size were recorded. This retrospective review was approved by the Ethics Committee of the Second Affiliated Hospital of Zhejiang University School of Medicine. All patients provided written informed consent before performing EUS and surgery.
Endosonography was applied using ultrasound endoscopes [the Olympus GF-UM200, GF-UCT240-AL5 and GF-UE260-AL5 (7.5 MHz; Olympus Optical Co. Japan)]. EUS scanning showed many features that were evaluated, including the following: presence or absence of mucosal ulceration; the regularity of the marginal border (regular or irregular); the presence of lobulation of the surface; the shape of the tumor (oval to round or non-oval distorted); the presence of a marginal halo, echogenicity (hypoechoic or hyperechoic); homogeneity (homogeneous or heterogeneous); presence of internal echogenic or cystic foci; and presence of exophytic development (tumor development outside the gastric wall). Computed tomography (CT) images and radiological reports were retrospectively reviewed. The CT features were analyzed with the pattern of contrast enhancement.
Two patients underwent laparotomy and resection of tumor and two other patients underwent subtotal gastrectomy. Three of 4 patients underwent regional lymphadenectomy. Immunohistochemical studies were performed on the resected tissue to evaluate the expression of S100 protein, α-smooth muscle actin (α-SMA), desmin, CD117, CD34 and Ki67. All four patients underwent EUS and CT examinations before surgery.
Follow-up data were obtained from clinical records and from the treating doctors.
The clinicopathological characteristics of the patients and the surgical data and outcomes are summarized in Table 1. All 4 cases were female (age ranged from 32 to 50 years). One patient was asymptomatic, and the schwannoma was accidentally discovered in this patient by ultrasonic examination in the follow-up of a hysteromyoma. Three patients had symptoms including ructus, bloating and epigastric pain, weight loss, melena and hematemesis.
| Case | Age (yr) | Sex | Size (cm) | Clinical Presentation | Site (gastric body) | Management | Outcome (mo) |
| 1 | 32 | Female | 3.3 × 4.0 | Ructus, bloating, epigastric pain | Greater curvature | Laparoscopic wedge resection | 14 |
| 2 | 39 | Female | 4.9 × 4.0 | Weight loss | Lesser curvature | Laparoscopic wedge resection | 4 |
| 3 | 50 | Female | 8.1 × 5.3 | Melena, hematemesis (GI bleeding) | Great curvature | Subtotal gastrectomy | 5 |
| 4 | 49 | Female | 5.0 × 3.5 | Incidental on ultrasonic examination for hysteromyoma follow-up | Lesser curvature | Subtotal gastrectomy | 39 |
The patients underwent multiple examinations before surgery, but none had a correct preoperative diagnosis of schwannoma. They were misdiagnosed as having GISTs. Upper endoscopy revealed that one patient had a submucosal tumor with a central ulcer and that the other patients did not have ulceration. All the tumors were located in the gastric body (2 in the greater curvature and 2 in the lesser curvature).
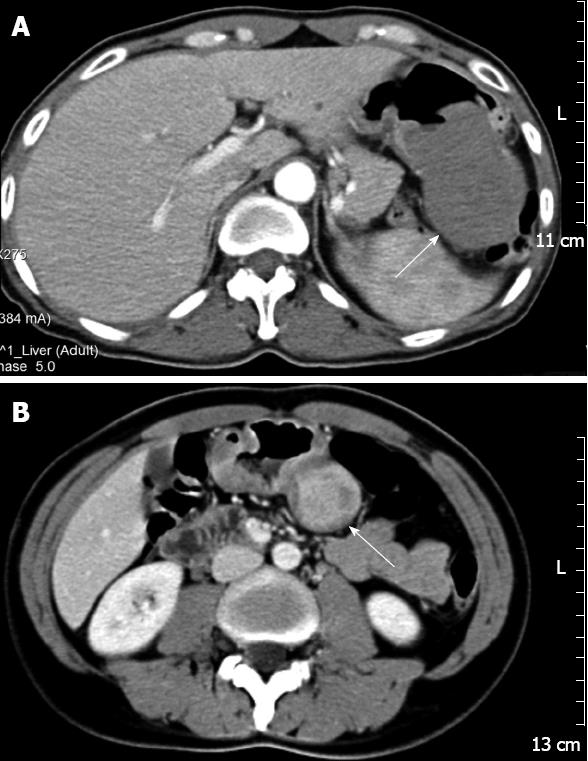
The CT images showed rounded masses in the stomachs, with homogeneous (n = 3) (Figure 1A) or heterogeneous (n = 1) (Figure 1B) internal contrast enhancement. One of the masses was accompanied by enlargement of the lymph nodes.
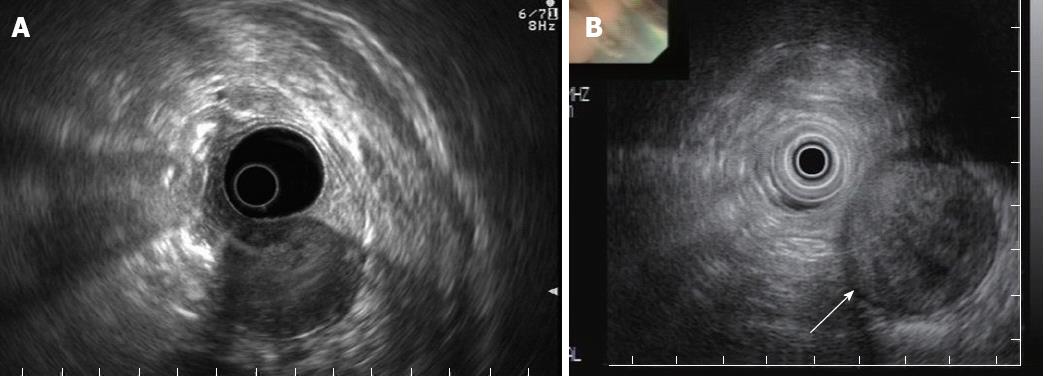
The EUS evaluation revealed that all the tumors were hypoechoic, with a connection between the tumor and the muscularis propria. The echogenicities of the tumors were heterogeneous with an internal high-echo region (Figure 2A). Two of the patients had tumors with marginal hypoechoic regions (Figure 2B). The shapes of all tumors were oval, with well-demarcated margins, and the growth patterns of the tumors were exogastric. The tumors had smooth surfaces, except one which had an ulcer. None had internal cystic lesions, lobulations, or calcification (Table 2). Endoscopic ultrasound-guided fine needle aspiration was performed in one patient, but failed to confirm the diagnosis because of insufficient tissue biopsy for immunohistochemistry.
| Case | Echogenicity | Ulcer | Shape | Margin | Lob | Halo | Cyst | Spots | Cal | Growth |
| 1 | Low | - | Oval | Regular | - | + | - | + | - | In < out |
| 2 | Low | - | Oval | Regular | - | + | - | + | - | In < out |
| 3 | Low | 2 | Oval | Regular | - | - | - | + | - | In < out |
| 4 | Low | - | Oval | Regular | - | - | - | + | - | In < out |
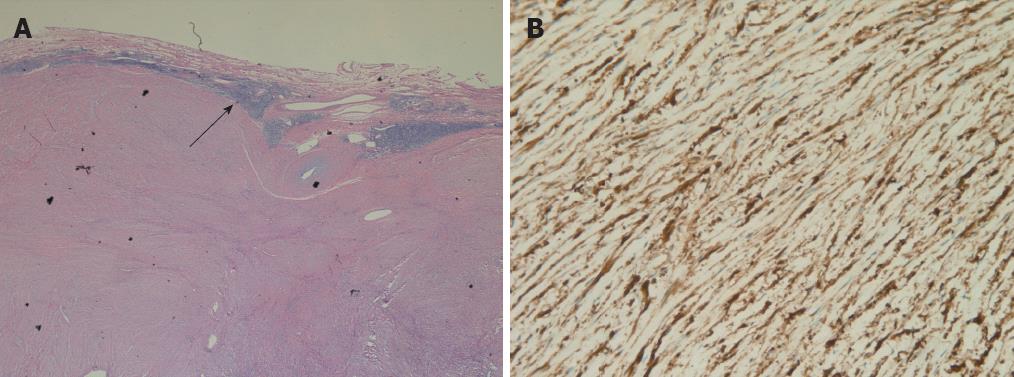
Histological examination showed that the tumors were composed of spindle cells. There were lymphoid cuffs surrounding the tumors (Figure 3A). Immunohistochemical evaluation revealed strong expression of S-100 protein in all tumors (Figure 3B). None of the tumors showed expression of CD117, CD34 or desmin. All tumors revealed low proliferation, as estimated from the low proportion of Ki67-positive cells (Ki67 < 5%).
GI schwannomas are benign, slow growing tumors regarded as tumors distinct from conventional schwannomas. These tumors arise from the nerve sheath of the gut wall, rather than from the central nervous system and from soft tissues[6]. The stomach is the most common site of origin of GI schwannomas[2]. The tumors are most commonly located in the body of the stomach[3]. The tumors predominantly occur in older adults (mean age is 58 years) with a marked female predominance[6,7]. Gastric schwannomas are usually asymptomatic or associated with non-specific abdominal discomfort. They are accidentally discovered or when complications, such as GI bleeding, arise. Endoscopically, gastric schwannomas appear as elevated submucosal masses, with or without central ulcers. Endoscopic biopsies always yield false-negative results. The definitive diagnosis of gastric schwannomas is determined by pathological and immunohistochemical examination of surgical specimens. The tumors are typically negative for CD117, desmin, α-SMA and positive for S100. They stain variably with CD34[2].
It may be helpful to gain limited information through EUS, CT, magnetic resonance imaging (MRI), and positron emission tomography (PET) to differentiate gastric schwannomas from other gastric submuscosal tumors. In previous studies, gastric schwannomas displayed well-circumscribed masses with heterogeneous or homogeneous contrast enhancement on CT[7,8]. On MRI examination, gastric schwannomas are sharply demarcated, strongly enhanced tumors, having low to medium signal intensity on T1 weighted images, and high signal intensity on T2 weighted images[9]. However, the radiological imaging features of gastric schwannomas are not specific. They are quite similar to those of gastric stromal tumors. Recent reports described several cases of gastric schwannoma with increased fluorodeoxyglucose (FDG) uptake on PET. It is therefore necessary to distinguish schwannomas from low risk GISTs when assessing submucosal tumors of the stomach that show a high FDG accumulation[4,10].
Presently, reports on the EUS features of gastric schwannomas are extremely limited, due to the rarity of these tumors. The EUS features of gastric schwannomas have been described as round submusosal masses with marginal haloes, and homogeneous internal echogenicity without internal echogenic foci[5,11]. One study indicated that the echogenicities of gastric schwannomas were much lower than the normal surrounding muscle layers. Therefore, the authors considered that these findings may be useful for differentiating schwannomas from GISTs[5]. Nevertheless, EUS findings of gastric schwannomas revealed a hypoechoic mass, with some hyperechoic foci, in some other case reports[12,13]. According to our results, the internal echogenicity of gastric schwannomas was heterogeneous and low, but slightly higher than that of muscularis propria, with internal patch high echo. They were not homogeneous and extremely low. CT imaging showed that the tumors showed homogeneous or heterogeneous contrast enhancement. This meant that these tumors were hypervascular. The blood within the schwannomas was very slow-flowing. It is usually not possible to demonstrate flow with color or power Doppler sonography. The slow flow of blood, in spite of the hypervascular nature, can be explained by the observation that the internal echoes of the four tumors appeared similar to or slightly higher than those of muscularis propria, with some patchy hyperechoic areas. Although a lymphoid cuff was observed in each patient, it was not always continuous, which may explain why the marginal hypoechoic halo was only found in two cases. All 4 gastric schwannomas in our study lacked a cystic change, indicating that cystic changes are uncommon in this type of tumor. Only 2 cases with cystic changes on CT examinations have been reported in the literature[8]. Another clinicopathogical study found no cases of cystic change or gross necrosis in 51 cases[3]. No calcification within gastric schwannomas was reported in previous reports. However, cysts, hemorrhagia, and necrosis were common in GISTs, and calcification was seen in 6% of GISTs[14]. The differential diagnosis of gastric schwannomas and GISTs in EUS images are summarized in Table 3.
| Gastric schwannomas | Low-risk GIST | High-risk GIST | |
| Echogenicity | Heterogeneous and hypoechoic, but slightly higher than that of muscularis propria | Homogeneous and hypoechoic | Heterogeneous and hypoechoic |
| Halo | Frequent | Uncertain | Uncertain |
| Growth | In < out (mostly) | In ≥ out (mostly) | Variety |
| Margin | Regular | Regular | Irregular |
| Lobulation | Rare | Uncommon | Common |
| High echo spot | Common | Occasional | Common |
| Cyst | Very rare | Frequent | Very frequent |
| Calcification | Scarce | Occasional | Occasional |
In conclusion, gastric schwannomas are rare benign mesenchymal tumors of the stomach. However, it is necessary to differentiate these tumors from other submucosal tumors of the stomach, particularly GISTs. The EUS features of gastric schwannomas are varied. On EUS evaluation, heterogeneous hypoechogenicity or isoechogenicity, a well-demarcated margin, fourth-layer origination, and lack of cystic change and calcification may be considered to be helpful findings for the diagnosis of gastric schwannoma. A marginal halo was perhaps the characteristic feature, but not an essential one. Ulceration may be seen in large tumors. More EUS studies should be conducted to delineate the characteristic features that can help differentiate these mesenchymal tumors.
Peer reviewers: Dr. Fabiano Maluf, UNESCO de Bioética, Faculdade de Ciências da Saúde, Universidade de Brasília, Brasília, DF 70910-900, Brazil; Fernando de la Portilla, Coloproctology Unit, Gastrointestinal Surgery Department, Virgen del Rocio University Hospital, Avda. Manuel Siurot s/n, 41013 Seville, Spain
S- Editor Jiang L L- Editor Webster JR E- Editor Xiong L
| 1. | Hou YY, Tan YS, Xu JF, Wang XN, Lu SH, Ji Y, Wang J, Zhu XZ. Schwannoma of the gastrointestinal tract: a clinicopathological, immunohistochemical and ultrastructural study of 33 cases. Histopathology. 2006;48:536-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 110] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 2. | Agaimy A, Märkl B, Kitz J, Wünsch PH, Arnholdt H, Füzesi L, Hartmann A, Chetty R. Peripheral nerve sheath tumors of the gastrointestinal tract: a multicenter study of 58 patients including NF1-associated gastric schwannoma and unusual morphologic variants. Virchows Arch. 2010;456:411-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | Voltaggio L, Murray R, Lasota J, Miettinen M. Gastric schwannoma: a clinicopathologic study of 51 cases and critical review of the literature. Hum Pathol. 2012;43:650-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 4. | Goh BK, Chow PK, Kesavan S, Yap WM, Ong HS, Song IC, Eu KW, Wong WK. Intraabdominal schwannomas: a single institution experience. J Gastrointest Surg. 2008;12:756-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Jung MK, Jeon SW, Cho CM, Tak WY, Kweon YO, Kim SK, Choi YH, Bae HI. Gastric schwannomas: endosonographic characteristics. Abdom Imaging. 2008;33:388-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Daimaru Y, Kido H, Hashimoto H, Enjoji M. Benign schwannoma of the gastrointestinal tract: a clinicopathologic and immunohistochemical study. Hum Pathol. 1988;19:257-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 211] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Levy AD, Quiles AM, Miettinen M, Sobin LH. Gastrointestinal schwannomas: CT features with clinicopathologic correlation. AJR Am J Roentgenol. 2005;184:797-802. [PubMed] |
| 8. | Hong HS, Ha HK, Won HJ, Byun JH, Shin YM, Kim AY, Kim PN, Lee MG, Lee GH, Kim MJ. Gastric schwannomas: radiological features with endoscopic and pathological correlation. Clin Radiol. 2008;63:536-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Karabulut N, Martin DR, Yang M. Case report: gastric schwannoma: MRI findings. Br J Radiol. 2002;75:624-626. [PubMed] |
| 10. | Ohno T, Ogata K, Kogure N, Ando H, Aihara R, Mochiki E, Zai H, Sano A, Kato T, Sakurai S. Gastric schwannomas show an obviously increased fluorodeoxyglucose uptake in positron emission tomography: report of two cases. Surg Today. 2011;41:1133-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Okai T, Minamoto T, Ohtsubo K, Minato H, Kurumaya H, Oda Y, Mai M, Sawabu N. Endosonographic evaluation of c-kit-positive gastrointestinal stromal tumor. Abdom Imaging. 2003;28:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Froutan H, Shafaghi A, Hashemi M, Shariat-Torbaghan S. Gastric Schwannoma: a case report. Med J Islam Repub Iran. 2008;21:227-230. |
| 13. | Xu M. Gastric Schwannoma: a rare Schwann cell tumour of the GI tract. Univ West Ont Med J. 2011;80:14-16. |
| 14. | Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 852] [Cited by in RCA: 866] [Article Influence: 43.3] [Reference Citation Analysis (0)] |