Published online Dec 28, 2012. doi: 10.3748/wjg.v18.i48.7225
Revised: November 11, 2012
Accepted: December 5, 2012
Published online: December 28, 2012
Processing time: 154 Days and 20.4 Hours
AIM: To determine whether and how magnetic resonance imaging (MRI)-based total liver volume (TLV) and diffusion weighted imaging (DWI) could predict liver fibrosis.
METHODS: Sixteen experimental mature mini-pigs (6 males, 10 females), weighing between 20.0 and 24.0 kg were prospectively used to model liver fibrosis induced by intraperitoneal injection of 40% CCl4 dissolved in fat emulsion twice a week for 16 wk, and by feeding 40% CCl4 mixed with maize flour twice daily for the subsequent 5 wk. All the survival animals underwent percutaneous liver biopsy and DWI using b = 300, 500 and 800 s/mm2 followed by abdominal gadolinium-enhanced MRI at the 0, 5th, 9th, 16th and 21st weekend after beginning of the modeling. TLV was obtained on enhanced MRI, and apparent diffusion coefficient (ADC) was obtained on DWI. Hepatic tissue specimens were stained with hematoxylin and Masson’s trichrome staining for staging liver fibrosis. Pathological specimens were scored using the human METAVIR classification system. Statistical analyses were performed to determine whether and how the TLV and ADC could be used to predict the stage of liver fibrosis.
RESULTS: TLV increased from stage 0 to 2 and decreased from stage 3 (r = 0.211; P < 0.001). There was a difference in TLV between stage 0-1 and 2-4 (P = 0.03) whereas no difference between stage 0-2 and 3-4 (P = 0.71). TLV could predict stage ≥ 2 [area under receiver operating characteristic curve (AUC) = 0.682]. There was a decrease in ADC values with increasing stage of fibrosis for b = 300, 500 and 800 s/mm2 (r = -0.418, -0.535 and -0.622, respectively; all P < 0.001). Differences were found between stage 0-1 and 2-4 in ADC values for b = 300, 500 and 800 s/mm2, and between stage 0-2 and 3-4 for b = 500 or 800 s/mm2 (all P < 0.05). For predicting stage ≥ 2 and ≥ 3, AUC was 0.803 and 0.847 for b = 500 s/mm2, and 0.848 and 0.887 for b = 800 s/mm2, respectively.
CONCLUSION: ADC for b = 500 or 800 s/mm2 could be better than TLV and ADC for b = 300 s/mm2 to predict fibrosis stage ≥ 2 or ≥ 3.
- Citation: Li H, Chen TW, Chen XL, Zhang XM, Li ZL, Zeng NL, Zhou L, Wang LY, Tang HJ, Li CP, Li L, Xie XY. Magnetic resonance-based total liver volume and magnetic resonance-diffusion weighted imaging for staging liver fibrosis in mini-pigs. World J Gastroenterol 2012; 18(48): 7225-7233
- URL: https://www.wjgnet.com/1007-9327/full/v18/i48/7225.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i48.7225
Liver fibrosis can be prevented or reversed at early stage by antifibrotic treatment or by eliminating the cause[1,2]. If liver fibrosis could not be prevented at early stage, it would lead to end-stage liver diseases[3]. Therefore, accurately staging liver fibrosis is important in choosing appropriate therapy to improve the prognosis of this disease. Percutaneous liver biopsy is currently considered a gold standard for assessing stage of fibrosis, but this invasive method is limited by inter- and intra-observer variability and sampling error[4,5]. To overcome the limitations of liver biopsy, noninvasive, reproducible and reliable methods are greatly needed. Transient elastography or FibroTest (Bio-Predictive) have been proposed for the diagnosis of liver fibrosis, but their use in clinical practice is being investigated[6].
Diffusion-weighted imaging (DWI), a type of functional magnetic resonance (MR) technique, has been developed to characterize diseased tissues. This technique allows measurement of the combined effects of microcirculation of blood (perfusion) and molecular brownian motion of water within liver parenchyma, expressed as a whole by apparent diffusion coefficient (ADC)[7]. Previous studies have shown that ADC values of the liver are lower in patients with cirrhosis compared with control subjects, which is thought to reflect a restriction of the motion of water molecules in fibrotic tissues[8,9]. Nevertheless, ADC values obtained in the previous experiences for studying liver fibrosis widely varied because of the employed settings of so-called b-values[8,10]. In addition, a previous study also reported that total liver volume (TLV) would change with the progress of liver fibrosis[11]. To our knowledge, there have been no data focusing on the correlation of liver ADC value obtained by different b values with the stage of liver fibrosis, and the correlation between magnetic resonance imaging (MRI)-based TLV and stage of liver fibrosis[11-14]. Thus, the purpose of this study was to prospectively investigate these correlations and determine whether and how TLV and DWI could predict the stage of liver fibrosis.
Animals were used in full compliance with the National Council of Animal Care guidelines. The protocol was approved by the Committee of the Ethics of Animal Experiments of our institute.
Sixteen experimental mature mini-pigs (6 males, 10 females), weighing between 20.0 and 24.0 kg, were used in our study. Previous studies have established a standardized experimental model of liver cirrhosis in swine using CCl4 and ethanol[15]. According to the above-mentioned modeling method, liver fibrosis was induced by intraperitoneal injection of 40% CCl4 dissolved in fat emulsion (0.25 mL/kg body weight) twice a week for 16 wk, and by feeding 40% CCl4 mixed with maize flour (0.75 mL/kg body weight) twice daily for the subsequent 5 wk because of the peritoneal adhesions resulting from the intraperitoneal injection. To minimize the chemical peritonitis involving liver, we chose the left hypogastrium as the intraperitoneal injection position and the injections were stopped two days before each MR examination. In addition, because the administration of alcohol in conjunction with CCl4 results in more stable and accelerated liver fibrogenesis in a large animal model, we used 5% alcohol-water mixture as the sole drinking water, and maize flour was taken as the staple food in the 21 wk[16].
On the 0, 5th, 9th, 16th and 21st weekend after the beginning of modeling fibrosis, all mini-pigs were given general anesthesia with an injection of ketamine (15 mL/kg weight) and diazepam (0.8 mg/kg per hour) through the ear vein before the MR examination. The anterior surface of the thorax and abdomen of the animals were shaved to obtain good contact between the skin and the cardiac electrodes or respiratory triggering. We also used a belt around the abdomen to reduce the effect of respiratory motion.
The mini-pigs were examined on a 1.5 T whole body MR scanner (Signa Excite; GE Medical Systems, Milwaukee, WI). When the cardiac and respiratory signals were satisfied, each animal was positioned supinely in an 8-channel phased array body coil. After the pilot scan with axial, coronal, and sagittal images for localization, the MR protocols including SPGR T1-weighted imaging (T1WI), fast recovery fast spin echo (FRFSE) T2-weighted imaging (T2WI), and single-shot echo-planar imaging (EPI) DWI were performed. The parameters for DWI were as follows: TR = 4000 ms, TE = 49.2 ms, field of view (FOV) = 34 × 34 cm, slice thickness = 5.0 mm, slice space = 2.0 mm, matrix of 192 × 256 mm, number of excitation = 2, b values of 0-300, 0-500 and 0-800 s/mm2, and tridirectional diffusion gradients. After DWI acquisition, 20 mL gadodiamide (Magnevist, Bayer Healthcare, Germany) was intravenously injected (0.2 mmol/L per kg body weight) with a pressure injector (Spectris MR Injection System; Medrad, Inc, Warrendale, PA) at a dose of 3 mL/s followed by a 20-mL saline solution flush for contrast-enhanced three-dimensional liver acquisition with volume acceleration (3D LAVA). The parameters for 3D LAVA were: TR = 3.9 ms, TE = 1.8 ms, FOV = 34 × 34 cm, slice thickness = 5.0 mm, and matrix 256 × 224 mm.
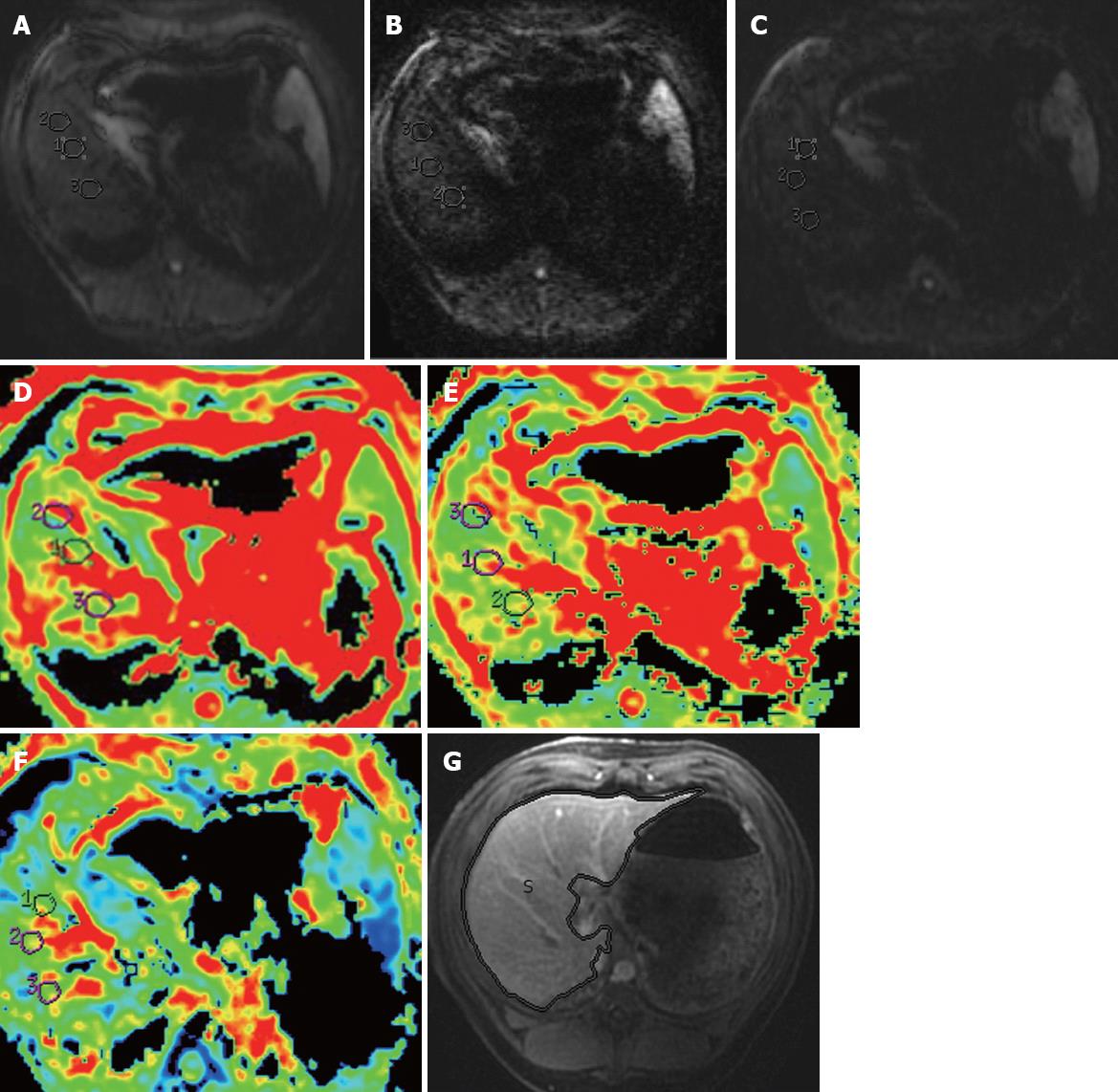
The original MRI data were directly interfaced and forwarded to the workstation (GE, AW4.1, Sun Microsystems, Palo Alto, CA, United States) to obtain ADC maps at each b values. An experienced radiologist (the first author with 3 years of experience in thoracoabdominal radiology) who was blinded to the pathologic results placed three circular regions of interest (ROIs) each with approximately 1-2 cm in diameter in the consecutive three maximal slices of the right lobe of the liver (3 ROIs per slice, 9 ROIs per mini-pig) for each b value (Figure 1A-C), avoiding areas of artifact, diaphragm and intrahepatic vasculature. The ADC value of each ROI as well as the ADC map (Figure 1D-F) was automatically generated. The ADC values of the three ROIs in each slice were then averaged to give an estimate of ADC value for this slice. Representative ADC values of the three slices were then averaged to obtain a final estimate of the liver ADC value to be used for data analysis.
In addition, TLV measurements were performed employing planimetry in all animals by the above-mentioned radiologist. Initially, liver profile was manually traced on each transverse image using a trackball. The software automatically calculated the number of pixels enclosed by the traced liver contours on each image, and provided the cross-sectional area of the liver on a slice-by-slice basis. The sum of the above areas multiplied by the section thickness provided the TLV (Figure 1G)[17].
To test the interobserver variability of TLV and ADC values measurement, we randomly chose the MR data of all animals on the 9th weekend for the repeated MR measurements, which were performed two weeks after the first measurement by an experienced radiological professor (the corresponding author with 14 years of experience in abdominal radiology). The precision of the two measurements on the 9th weekend was determined as the coefficient of variance (CV) on the basis of the formula: CV (%) = (s/X) × 100, where s is the SD, and X is the mean of TLV or ADC values. The resultant precision was expressed as an average %CV. When %CV was less than 10%, interobserver variability of TLV and ADC values measurements were regarded as small, and the results were reliable.
After the MR examination in each mini-pig, an 18-gauge ultrasound-guided core percutaneous biopsy was performed in the right liver lobe because the biopsy in the right lobe of the liver was used as the standard for staging liver fibrosis[18]. When the mini-pigs died during the follow-up period before the 21st weekend, the dead mini-pigs underwent immediate laparotomy, and the entire liver was resected. When the mini-pigs were living on the 21st weekend, 1/3 animals were randomly sacrificed by air injection into the auricular vein shortly after the last percutaneous biopsy and underwent the laparotomy, and the entire liver was also resected. Subsequently, three thin slices were randomly cut from any lobe of the liver and diced into the usual tissue block of about 2 mm thickness for further staging liver fibrosis to confirm the stage of the fibrosis determined by percutaneous biopsy immediately prior to death. Furthermore, the final fibrosis stage of resected liver was determined by the average of the stages across the three thin slices.
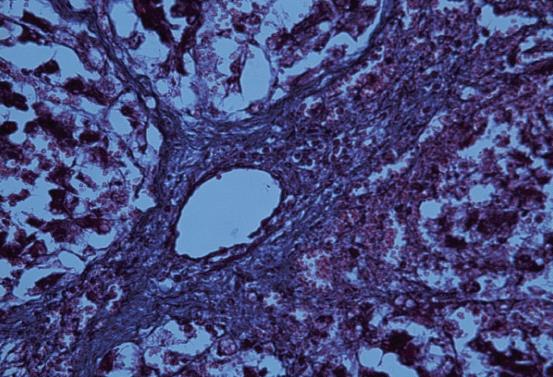
Hepatic tissue specimens obtained by biopsy and laparotomy were stained with hematoxylin and eosin (H and E) and Masson’s trichrome staining for pathologic examination. Because the morphology and histology of experimental mini-pig liver are similar to that of human liver[15], two experienced hepatopathologists (the 11th and 12th authors with 10 and 36 years of experience in hepatopathology, respectively) scored pathological specimens using the human METAVIR classification system[19]. Any discrepancies between the two observers were settled by consensus. This scoring system has a five-point scale: stage 0, no fibrosis; stage 1, portal fibrosis; stage 2, periportal fibrosis (Figure 2); stage 3, septal fibrosis; and stage 4, cirrhosis. Additionally, we considered the weekend on which liver fibrosis was initially confirmed by pathologic examination as that the fibrosis occurred during the follow-up.
All statistical analyses were carried out with SPSS (version 17.0, SPSS, Chicago IL, United States). A P < 0.05 was considered to represent a significant difference. The interobserver agreement for the two pathologists with regard to the fibrosis stage, or for the percutaneous biopsy and the laparotomy was expressed by means of k statistics. When k values were 0-0.40, 0.41-0.60, 0.61-0.80, and 0.81-1.00, the concordance was considered as poor, moderate, good, and excellent agreement, respectively. The TLV and ADC values were of skewness. Spearman’s rank correlation analyses were used to assess the correlation between TLV and fibrosis stage, or between the ADC values and fibrosis stage. TLV or ADC values were compared between patients stratified by fibrosis stages using Mann-Whitney tests together with Bonferroni correction for multiple comparisons. The cutoff value of TLV or ADC values were then determined with receiver-operating characteristic (ROC) analysis for predicting moderate liver fibrosis (stage ≥ 2) and advanced liver fibrosis (stage ≥ 3).
During the follow-up period, one, two and four animals died between week 5 and 9, between week 9 and 16, and between week 16 and 21, respectively. On the 0, 5th, 9th, 16th and 21st weekend, mean body weight of the survived animals was 22 ± 1.31 kg, 24.1 ± 2.41 kg, 24 ± 2.45 kg, 25.3 ± 2.81 kg, and 23.5 ± 1.78 kg, respectively. There was no significant difference in body weight between any two weekends during the follow-up period (all P > 0.05), mini-pigs treated with CCl4 did not put on weight during the whole progress of experiment. In addition, there was good agreement between fibrosis stages determined by the percutaneous biopsy and by the laparotomy (k, 0.80; 95%CI, 0.75-0.84). Therefore, according to the human METAVIR classification system, survived mini-pigs with liver fibrosis at different stages confirmed on the follow-up weekends are illustrated in Table 1.
In all animals on the 9th weekend, CV was 8.2% (range, 2.7%-15.6%) for TLV. CV was 9.2% (range, 5.2%-17.3%), 8.4% (range, 5.3%-15.5%) and 7.6% (range, 4.5%-13.2%) for ADC values with b = 300, 500 and 800 s/mm2, respectively. Therefore, interobserver variability of TLV and ADC values were small, and the first measurement was used as the final results.
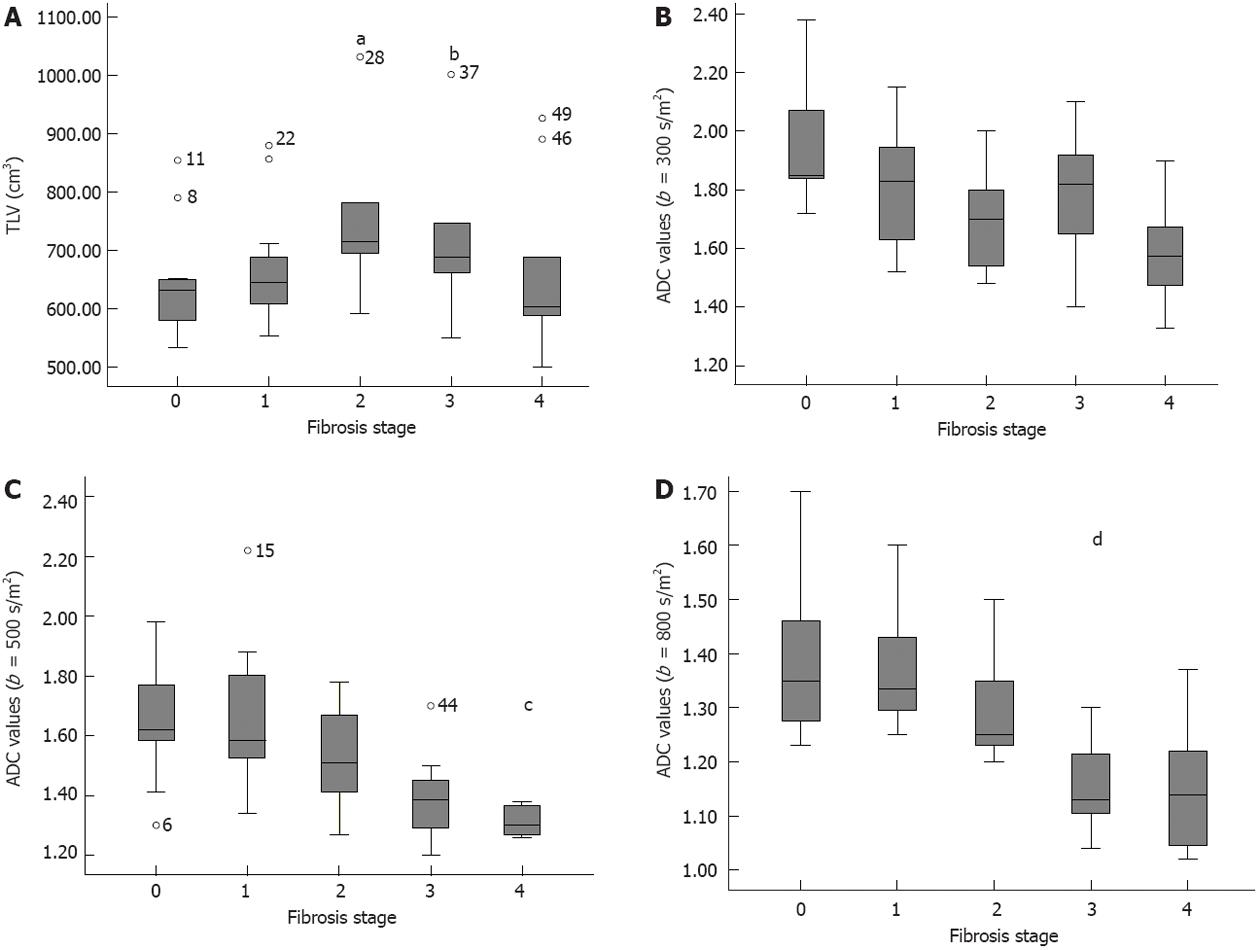
TLV corresponding to stage of liver fibrosis is shown in Table 2. TLV tended to increase from stage 0 to 2, but decrease from stage 3 (Figure 3A) (r = 0.211, P < 0.001). Furthermore, significant difference could be found between stage 0 and 2 (P < 0.001) while TLV could not differentiate other individual stages of fibrosis (all P > 0.05). Additionally, there was significant difference between stage 0-1 and 2-4 (P = 0.03), whereas there was no difference between stage 0-2 and 3-4 (P = 0.71).
| Fibrosis stage (n) | ADC value (×10-3 mm2/s), mean (SD), 95%CI | TLV (cm3), mean (SD), 95%CI | ||
| b = 300 s/mm2 | b = 500 s/mm2 | b = 800 s/mm2 | ||
| 0 (16) | 1.96 (0.19), 1.83-2.09 | 1.67 (0.21), 1.53-1.81 | 1.39 (0.15), 1.29-1.50 | 641.08 (99.22), 574.42-707.74 |
| 1 (11) | 1.81 (0.19), 1.69-1.93 | 1.66 (0.23), 1.51-1.81 | 1.37 (0.11), 1.30-1.44 | 671.62 (106.12), 600.33-742.91 |
| 2 (13) | 1.69 (0.17)1, 1.57-1.81 | 1.52 (0.17), 1.40-1.64 | 1.29 (0.11), 1.22-1.37 | 776.57 (156.91)1, 655.95-897.18 |
| 3 (13) | 1.78 (0.21), 1.64-1.91 | 1.39 (0.13)2, 1.30-1.47 | 1.18 (0.15)2, 1.09-1.28 | 745.04 (155.51), 625.51-864.58 |
| 4 (10) | 1.58 (0.17)1, 1.44-1.73 | 1.35 (0.15)2, 1.23-1.48 | 1.15 (0.12)3, 1.05-1.25 | 672.03 (145.33), 560.32-783.75 |
| Grouped stages | ||||
| 0-1 (27) | 1.88 (0.20), 1.79-1.97 | 1.66 (0.22), 1.57-1.76 | 1.38 (0.13), 1.32-1.44 | 656.35 (101.46), 611.36-701.34 |
| 0-2 (40) | 1.82 (0.21), 1.75-1.90 | 1.62 (0.21), 1.54-1.70 | 1.35 (0.13), 1.31-1.40 | 691.25 (129.80), 643.64-738.86 |
| 2-4 (36) | 1.69 (0.19)4, 1.62-1.77 | 1.42 (0.16)4, 1.36-1.48 | 1.21 (0.14)4, 1.16-1.26 | 731.21 (153.32)4, 670.56-791.81 |
| 3-4 (23) | 1.70 (0.21), 1.60-1.80 | 1.37 (0.14)5, 1.31-1.44 | 1.17 (0.13)5, 1.10-1.23 | 708.54 (150.77), 633.56-783.51 |
ADC values corresponding to stage of liver fibrosis are illustrated in Table 2. There was a decrease in liver ADC values with the increasing degree of fibrosis for b = 300, 500 and 800 s/mm2 (Figure 3B-D, respectively) (r = -0.418, -0.535 and -0.622, respectively; P < 0.001). ADC values could differentiate fibrosis stage between stage 0 and 2 for b = 300 s/mm2; between stage 0 and 4 for b = 300, 500 and 800 s/mm2; between stage 1 and 4, stage 0 and 3, or stage 1 and 3 for b = 500 or 800 s/mm2; and between stage 2 and 4 for b = 800 s/mm2 (all P < 0.05). There were no differences between stage 0 and 1, between stage 2 and 3, and between stage 3 and 4 for b = 300, 500 and 800 s/mm2 (all P > 0.05). Additionally, there were significant differences between stage 0-1 and 2-4 for b = 300, 500 and 800 s/mm2, and between stage 0-2 and 3-4 for b = 500 or 800 s/mm2 (all P < 0.05).
ROC analyses of TLV and liver ADC for predicting stage≥2 and≥3
According to the generally accepted guidelines in Turkey and elsewhere[20], the patients who had liver fibrosis stage ≥ 2 should receive treatment whereas those with liver fibrosis stage ≤ 1 should not. Therefore, we did not perform ROC analyses of TLV and liver ADC values for predicting fibrosis stage ≥ 1. There was relatively more overlap for the TLV predicting fibrosis stage ≥ 2 and stage ≥ 3. ROC analyses showed that TLV could predict fibrosis stage ≥ 2 with a cut-off value of 656.25 cm3, representing a small AUC. However, liver ADC values had a better sensitivity and a specificity for predicting fibrosis stage ≥ 2 and ≥ 3 (Table 3). We also found that there was a higher diagnostic performance for b = 500 or 800 s/mm2 compared with b = 300 s/mm2 for prediction of fibrosis stage ≥ 2 and ≥ 3.
| Cut-off | Stage differentiations | AUC | Sensitivity | Specificity |
| TLV 656.25 cm3 | Prediction of stage ≥ 2 | 0.682 | 74.1 | 73.7 |
| ADC (b = 800 s/mm2) | ||||
| 1.82 × 10-3 mm2/s | Prediction of stage ≥ 2 | 0.743 | 69.6 | 73.3 |
| 1.75 × 10-3 mm2/s | Prediction of stage ≥ 3 | 0.646 | 63.6 | 60.0 |
| ADC (b = 800 s/mm2) | ||||
| 1.51 × 10-3 mm2/s | Prediction of stage ≥ 2 | 0.803 | 82.6 | 76.7 |
| 1.44 × 10-3 mm2/s | Prediction of stage ≥ 3 | 0.847 | 78.8 | 80.0 |
| ADC (b = 800 s/mm2) | ||||
| 1.29 × 10-3 mm2/s | Prediction of stage ≥ 2 | 0.848 | 78.3 | 80.0 |
| 1.23 × 10-3 mm2/s | Prediction of stage ≥ 3 | 0.887 | 84.8 | 81.2 |
Activation of hepatic stellate cells (the main collagen-producing cells) by fibrogenic cytokines is a central event in fibrosis[21]. Other cells including portal fibroblasts and bone marrow - derived cells may also be involved in the fibrogenic process[22-26]. In the past, liver fibrosis was considered to be irreversible, however, hepatic fibrosis is now regarded as a dynamic process with potential for regression[27]. The accumulation of proteins in the extracellular matrix promotes the formation of scars that bridge together adjacent portal triads and central veins. Ultimately, hepatic fibrosis leads to cirrhosis, associated with nodule formation and organ contraction[27].
To prevent progression of this disease resulting in cirrhosis, accurately staging fibrosis is of clinical importance. Some studies indicated that DWI could be a useful technique for staging liver fibrosis[12,28], and that TLV would change with the progress of liver fibrosis[11,14]. We initially performed a follow-up study focusing on the correlation between stage of liver fibrosis and TLV or liver ADC values by comparing the performance of different b values, and determining how TLV and DWI could predict the stage of liver fibrosis. This study was performed with a large animal model (experimental mini-pigs) because the morphology and histology of its liver were similar to that of human liver, and the liver fibrosis was induced mainly by a matured method of inferior intraperitoneal injection of 40% CCl4 according to a previous study[15].
In this study, we found that TLV tended to increase from stage 0 to 2, but decrease from stage 3 of liver fibrosis. Our results were almost consistent with a published study[29]. According to Liu et al[29], liver volume (LV) in patients with liver fibrosis tended to increase with the severity of fibrosis from stage 0 to 3, but decrease in stage 4, indicating that TLV tended to increase gradually with the severity of fibrosis. The presumed pathologic mechanism for LV increase in early stage would be the ballooning of hepatocytes along with increased fibrotic component[14]. In advanced fibrosis, LV gradually decreased because of the segmental or global liver atrophy[30]. However, our findings were inconsistent with other published reports[11,14]. According to Tarao et al[14], LV in patients with alcoholic liver fibrosis increased gradually with the severity of fibrosis but did not decrease in the stage of alcoholic cirrhosis. The inconsistency of our findings with this published article could be explained by the reason that liver fibrosis was induced by alcoholics. As demonstrated by Li et al[11], TLV tended to decrease gradually with the increasing degree of virus-induced liver fibrosis staged with the pathologic scoring system (Ishak scale). It is the difference in pathologic scoring system that could be used to explain the inconsistency of our findings with this published article.
In addition to TLV, liver ADC value would be another indicator to quantitatively evaluate liver fibrosis. We found negative correlations between ADC and stage of fibrosis for b = 300, 500 and 800 s/mm2, which were consistent with these previous studies[12,31]. According to these articles[12,31], there was a decrease in liver ADC with increasing degree of fibrosis, and moderate negative correlations could be found between ADC values and fibrosis stages. However, Boulanger et al[32] found that there was no correlation between the histological grade of fibrosis and ADC using small b values of 50-250 s/mm2, and we could presume that the difference in ADC value between normal and fibrotic liver could not be found by using the small b values because the ADC value obtained by the small b values could be prone to be influenced by liver perfusion[33].
For prediction of fibrosis stage ≥ 2, ROC analyses in our study showed that TLV could be a predictor with an AUC of 0.682 and the sensitivity and specificity of more than 70% by using a cut-off value of 656.25 cm3. TLV could not be a predictor of stage ≥ 3 because there was no difference between stage 0-2 and 3-4.
In comparison with TLV, we found that ADC values obtained with b = 500 s/mm2 and 800 s/mm2 could be better predictors to predict liver fibrosis stage ≥ 2 because of higher AUC of 0.803 and 0.848 using cut-off ADC of 1.51 × 10-3 mm2/s and 1.29 × 10-3 mm2/s, respectively. Moreover, ADC values obtained with b = 500 s/mm2 and 800 s/mm2 could also predict liver fibrosis stage ≥ 3 with AUC of 0.847 and 0.887 using cut-off ADC of 1.44 × 10-3 mm2/s and 1.23 × 10-3 mm2/s, respectively. Comparing with b = 500 s/mm2 and 800 s/mm2, we found a relatively lower AUC of 0.743 for a liver ADC value of 1.82 × 10-3 mm2/s obtained using b = 300 s/mm2 for prediction of fibrosis stage ≥ 2. Because there was no difference in ADC value for b = 300 s/mm2 between liver fibrosis stage 0-2 and 3-4, ADC value obtained in the current setting could not be used to predict liver fibrosis stage ≥ 3. Generally, we found that ADC values with b = 500 or 800 s/mm2 could be better than b = 300 s/mm2 for predicting stage of liver fibrosis, which were consistent with the study by Taouli et al[12]. They suggested that ADC measured on DWI could be used to best quantify liver fibrosis when the b value is 500 s/mm2 or greater in the comparisons among b values of 0, 50, 300, 500, 700 and 1000 s/mm2. This may be because ADC maps acquired with longer b values are less contaminated by perfusion effects. That is, they are more truly diffusion weighted[33]. Taken together, we concluded that liver ADC measured on DWI with b = 500 or 800 s/mm2 could be recommended for predicting stage of liver fibrosis.
Compared with previous studies, the advantages of our study might be more persuasive to confirm that DWI obtained with several b values could be used to predict stage of liver fibrosis because the normal animals used in this study had been confirmed to have no fibrosis, hepatic steatosis and inflammation by biopsy. A previous clinical study suggested that healthy patients without any symptoms of liver disease could be defined as nonfibrotic, free of steatosis and inflammation, which might influence the ADC value[34]. According to Angulo et al[35], most patients with acute liver disease and even with chronic liver cirrhosis remained asymptomatic until decompensation occurred. We could presume that the authors of the previous study might consider the patients with asymptomatic acute or chronic liver disease as healthy patients, which resulted in selection bias. Furthermore, there might be a long interval between patients undergoing liver biopsy and undergoing MRI, and the stage of liver fibrosis might change during the interval, which would potentially affect the results[36]. Another advantage of this study was that we determined how to use TLV and ADC values to predict stage of liver fibrosis.
There were some limitations in our study. Firstly, our sample size was relatively small. Therefore, further studies involving a larger number of samples are needed to evaluate TLV and ADC values for predicting liver fibrosis stage. Secondly, our study was based on an animal experiment, but our findings could provide some useful information that TLV and ADC values could predict the stage of fibrosis, and we will conduct further studies to confirm the results.
In conclusion, TLV and ADC values might be used to predict stage of liver fibrosis. TLV could predict stage ≥ 2 with a lower diagnostic accuracy, but ADC values with b = 500 or 800 s/mm2 might be good predictors for stage ≥ 2 and ≥ 3 with a higher diagnostic accuracy. This study might provide a noninvasive method for predicting stage of liver fibrosis.
As a noninvasive method, magnetic resonance imaging has been developed to characterize liver fibrosis. Apparent diffusion coefficient (ADC) obtained by diffusion-weighted imaging (DWI) for studying liver fibrosis widely varied because of the employed settings of so-called b-values. In addition, total liver volume (TLV) obtained by computed tomography would change with the progress of liver fibrosis. However, correlations of liver ADC value obtained by different b values or magnetic resonance imaging (MRI)-based TLV with the stage of liver fibrosis, and whether and how TLV and DWI could predict the stage of liver fibrosis remained unclear.
ADC obtained by magnetic resonance (MR) DWI for multiple b values and TLV obtained by enhanced MR imaging are the hotspots for the research on assessing the stages of liver fibrosis. How the ADC and TLV would change with the increasing stage of liver fibrosis, and whether TLV and DWI could predict the stage of liver fibrosis have not been determined.
The authors utilized magnetic resonance imaging to assess the changes of apparent diffusion coefficient obtained by DWI and TLV obtained by enhanced scans with the stage of liver fibrosis, and utilized receiver-operating characteristic (ROC) curve analysis to determine whether TLV and DWI could predict the stage of liver fibrosis.
The authors found that TLV and ADC values might predict stage of liver fibrosis. ADC values with b = 500 or 800 s/mm2 might be good predictors for stage ≥ 2 and ≥ 3 with higher diagnostic accuracy, but TLV could predict stage ≥ 2 with a lower diagnostic accuracy. Although this is an experimental study, the findings could be helpful for the relevant clinical studies.
DWI, a method that assesses the diffusion of protons within tissue by applying motion sensitizing gradients that causes diffusing protons to lose signal. The amount of signal loss is influenced by the strength of the diffusion weighting (the diffusion sensitivity parameter or b value of the sequence) and the ability of protons to diffuse through tissues (the ADC).
This interesting study determined whether MRI based liver volume and DWI are useful to stage liver fibrosis in mini-pigs. Results presented in this paper showed that TLV increased during stage 0-2, whereas it decreased from stage 3 liver fibrosis. The ADC values were decreased with increasing stage of fibrosis. Authors conducted histopathological studies in liver biopsy specimens to analyze the damage during fibrosis. Overall, these findings show that both TLV and ADC values provide an important tool to predict stage of liver fibrosis.
Peer reviewer: JY Wang, Reprint Author, Vet Adm Med Ctr, Dept Surg, 10 N Greene St, Baltimore, MD 21201, United States
S- Editor Song XX L- Editor Ma JY E- Editor Li JY
| 1. | Malekzadeh R, Mohamadnejad M, Nasseri-Moghaddam S, Rakhshani N, Tavangar SM, Sohrabpour AA, Tahaghoghi S. Reversibility of cirrhosis in autoimmune hepatitis. Am J Med. 2004;117:125-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Safadi R, Zigmond E, Pappo O, Shalev Z, Ilan Y. Amelioration of hepatic fibrosis via beta-glucosylceramide-mediated immune modulation is associated with altered CD8 and NKT lymphocyte distribution. Int Immunol. 2007;19:1021-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Afdhal NH, Nunes D. Evaluation of liver fibrosis: a concise review. Am J Gastroenterol. 2004;99:1160-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 422] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 4. | Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF). Hepatology. 2000;32:477-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 731] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 5. | Goldin RD, Goldin JG, Burt AD, Dhillon PA, Hubscher S, Wyatt J, Patel N. Intra-observer and inter-observer variation in the histopathological assessment of chronic viral hepatitis. J Hepatol. 1996;25:649-654. [PubMed] |
| 6. | Foucher J, Chanteloup E, Vergniol J, Castéra L, Le Bail B, Adhoute X, Bertet J, Couzigou P, de Lédinghen V. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55:403-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 955] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 7. | Colagrande S, Pallotta S, Vanzulli A, Napolitano M, Villari N. The diffusion parameter in magnetic resonance: physics, techniques, and semeiotics. Radiol Med. 2005;109:1-16. [PubMed] |
| 8. | Taouli B, Vilgrain V, Dumont E, Daire JL, Fan B, Menu Y. Evaluation of liver diffusion isotropy and characterization of focal hepatic lesions with two single-shot echo-planar MR imaging sequences: prospective study in 66 patients. Radiology. 2003;226:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 446] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 9. | Amano Y, Kumazaki T, Ishihara M. Single-shot diffusion-weighted echo-planar imaging of normal and cirrhotic livers using a phased-array multicoil. Acta Radiol. 1998;39:440-442. [PubMed] |
| 10. | Kim T, Murakami T, Takahashi S, Hori M, Tsuda K, Nakamura H. Diffusion-weighted single-shot echoplanar MR imaging for liver disease. AJR Am J Roentgenol. 1999;173:393-398. [PubMed] |
| 11. | Li WX, Zhao XT, Chai WM, Zhu NY, DU LJ, Huang W, Ling HW, Chen KM, Xie Q. Hepatitis B virus-induced liver fibrosis and cirrhosis: the value of liver and spleen volumetry with multi-detector spiral computed tomography. J Dig Dis. 2010;11:215-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Taouli B, Tolia AJ, Losada M, Babb JS, Chan ES, Bannan MA, Tobias H. Diffusion-weighted MRI for quantification of liver fibrosis: preliminary experience. AJR Am J Roentgenol. 2007;189:799-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 279] [Article Influence: 15.5] [Reference Citation Analysis (1)] |
| 13. | Vaziri-Bozorg SM, Ghasemi-Esfe AR, Khalilzadeh O, Mazloumi M, Nassiri-Toosi M, Ghanaati H, Rokni-Yazdi H. Diffusion-weighted magnetic resonance imaging for diagnosis of liver fibrosis and inflammation in chronic viral hepatitis: the performance of low or high B values and small or large regions of interest. Can Assoc Radiol J. 2012;63:304-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Tarao K, Hoshino H, Motohashi I, Iimori K, Tamai S, Ito Y, Takagi S, Oikawa Y, Unayama S, Fujiwara T. Changes in liver and spleen volume in alcoholic liver fibrosis of man. Hepatology. 1989;9:589-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Zhang JJ, Meng XK, Dong C, Qiao JL, Zhang RF, Yue GQ, Zhong HY. Development of a new animal model of liver cirrhosis in swine. Eur Surg Res. 2009;42:35-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Bosma A, Brouwer A, Seifert WF, Knook DL. Synergism between ethanol and carbon tetrachloride in the generation of liver fibrosis. J Pathol. 1988;156:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Mazonakis M, Damilakis J, Maris T, Prassopoulos P, Gourtsoyiannis N. Comparison of two volumetric techniques for estimating liver volume using magnetic resonance imaging. J Magn Reson Imaging. 2002;15:557-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology. 2009;49:1017-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1449] [Cited by in RCA: 1581] [Article Influence: 98.8] [Reference Citation Analysis (1)] |
| 19. | Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology. 1994;20:15-20. [PubMed] |
| 20. | Heintges T, Mohr L, Hensel F, Petry W, Borchard F, Häussinger D, Niederau C. Value of liver biopsy prior to interferon therapy for chronic viral hepatitis. Dig Dis Sci. 1998;43:1562-1565. [PubMed] |
| 21. | Friedman SL. Hepatic fibrosis -- overview. Toxicology. 2008;254:120-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 275] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 22. | Wells RG, Kruglov E, Dranoff JA. Autocrine release of TGF-beta by portal fibroblasts regulates cell growth. FEBS Lett. 2004;559:107-110. [PubMed] |
| 23. | Kruglov EA, Jain D, Dranoff JA. Isolation of primary rat liver fibroblasts. J Investig Med. 2002;50:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Kinnman N, Housset C. Peribiliary myofibroblasts in biliary type liver fibrosis. Front Biosci. 2002;7:d496-d503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Forbes SJ, Russo FP, Rey V, Burra P, Rugge M, Wright NA, Alison MR. A significant proportion of myofibroblasts are of bone marrow origin in human liver fibrosis. Gastroenterology. 2004;126:955-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 310] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 26. | Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776-1784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1760] [Cited by in RCA: 1821] [Article Influence: 86.7] [Reference Citation Analysis (0)] |
| 28. | Bakan AA, Inci E, Bakan S, Gokturk S, Cimilli T. Utility of diffusion-weighted imaging in the evaluation of liver fibrosis. Eur Radiol. 2012;22:682-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Liu P, Li P, He W, Zhao LQ. Liver and spleen volume variations in patients with hepatic fibrosis. World J Gastroenterol. 2009;15:3298-3302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Blachar A, Federle MP, Brancatelli G. Primary biliary cirrhosis: clinical, pathologic, and helical CT findings in 53 patients. Radiology. 2001;220:329-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Lewin M, Poujol-Robert A, Boëlle PY, Wendum D, Lasnier E, Viallon M, Guéchot J, Hoeffel C, Arrivé L, Tubiana JM. Diffusion-weighted magnetic resonance imaging for the assessment of fibrosis in chronic hepatitis C. Hepatology. 2007;46:658-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 200] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 32. | Boulanger Y, Amara M, Lepanto L, Beaudoin G, Nguyen BN, Allaire G, Poliquin M, Nicolet V. Diffusion-weighted MR imaging of the liver of hepatitis C patients. NMR Biomed. 2003;16:132-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 33. | Hollingsworth KG, Lomas DJ. Influence of perfusion on hepatic MR diffusion measurement. NMR Biomed. 2006;19:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | Bonekamp S, Torbenson MS, Kamel IR. Diffusion-weighted magnetic resonance imaging for the staging of liver fibrosis. J Clin Gastroenterol. 2011;45:885-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 35. | Angulo P. GI epidemiology: nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2007;25:883-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 261] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 36. | Wang Y, Ganger DR, Levitsky J, Sternick LA, McCarthy RJ, Chen ZE, Fasanati CW, Bolster B, Shah S, Zuehlsdorff S. Assessment of chronic hepatitis and fibrosis: comparison of MR elastography and diffusion-weighted imaging. AJR Am J Roentgenol. 2011;196:553-561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 183] [Article Influence: 13.1] [Reference Citation Analysis (0)] |