Published online Dec 28, 2012. doi: 10.3748/wjg.v18.i48.7194
Revised: October 30, 2012
Accepted: November 11, 2012
Published online: December 28, 2012
Processing time: 141 Days and 19.7 Hours
AIM: To investigate the impact of different time points of secondary warm ischemia on bile duct in a rat autologous liver transplantation model with external bile drainage.
METHODS: One hundred and thirty-six male inbred SD rats were randomly assigned to one of four groups (I-IV) according to the secondary warm ischemia time of 0, 10, 20 and 40 min. A rat model of autologous liver transplantation with continuous external biliary drainage under ether anesthesia was established. Ten rats in each group were used to evaluate the one-week survival rate. At 6 h, 24 h, 3 d and 7 d after reperfusion of the hepatic artery, 6 rats were killed in each group to collect the blood sample via the infrahepatic vena cava and the median lobe of liver for assay. Warm ischemia time of liver, cold perfusion time, anhepatic phase, operative duration for biliary external drainage and survival rates in the four groups were analyzed for the establishment of models.
RESULTS: No significant difference was shown in warm ischemia time, anhepatic phase and operative duration for biliary external drainage among the four groups. Five of the 40 rats in this study evaluated for the one-week survival rate died, including three deaths of severe pulmonary infection in group IV. A significant decrease of one-week survival rate in group IV was noted compared with the other three groups. With the prolongation of the biliary warm ischemia time, the indexes of the liver function assessment were significantly elevated, and biliary epithelial cell apoptosis index also increased. Pathological examinations showed significantly aggravated inflammation in the portal area and bile duct epithelial cell injury with the prolonged secondary warm ischemia time. Microthrombi were found in the micrangium around the biliary tract in some sections from groups III and IV.
CONCLUSION: The relationship between secondary warm ischemia time and the bile duct injury degree is time-dependent, and 20 min of secondary warm ischemia time is feasible for the study of bile duct injury.
- Citation: Zhu XH, Pan JP, Wu YF, Ding YT. Establishment of a rat liver transplantation model with prolonged biliary warm ischemia time. World J Gastroenterol 2012; 18(48): 7194-7200
- URL: https://www.wjgnet.com/1007-9327/full/v18/i48/7194.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i48.7194
Liver transplantation has dramatically improved the prognosis, morbidity and mortality of the patients with end-stage liver diseases. Of all liver transplant recipients, 10%-40% develop biliary complications associated with a mortality rate of 8%-15%[1,2]. With the improvement of surgical techniques, the incidence of anastomotic biliary strictures decreased remarkably, whereas the nonanastomotic biliary strictures (NAS) have become the major type of biliary complications of liver allograft. NAS, which is also called ischemic cholangiopathy, appears early during the immediate postoperative period, and it is characterized by biliary strictures and dilatations at any location in the biliary system of the transplanted liver[3].
Knowledge about the pathogenesis of NAS is slowly emerging from clinical and experimental studies performed during the last decade. The cause of NAS is multifactorial, and ischemia/reperfusion injury of the biliary epithelium is considered as one of the major causes[4]. The most commonly used procedure for revascularization of the liver graft in clinical practice is initial portal reperfusion and subsequent reconstruction of the hepatic artery. Compared with liver cells, the bile duct epithelial cells experience an extra ischemia process [the time from portal vein (PV) recanalization to hepatic artery (HA) recanalization], which is “secondary warm ischemia time in the biliary tract (SWIT)” or “relative warm ischemia time in the biliary tract”. This is the special phase of biliary tract warm ischemia in the graft.
During the transplantation process, the warm ischemia of biliary tract includes temporal warm ischemia of liver graft during procurement and secondary warm ischemia in the biliary tract. Because warm ischemic time in the harvesting of donor liver after cardiac death (DCD) is inevitable, more and more studies focus on the effect of secondary warm ischemia time on bile duct injury[5]. In this study, we investigated the impact of different time points of secondary warm ischemia in a rat autologous liver transplantation model with external bile drainage.
One hundred and thirty-six male inbred SD rats weighing 220-250 g were purchased from the Animal Center of Yangzhou University (Yangzhou, China). The rats were housed and fed at the Animal Center of Drum Tower Hospital for least 7 d before transplantation to acclimate them to the environment. All rats were provided with standard laboratory chow and water and housed in accordance with institutional animal care policies. Prior to the study, the rats were fasted for 8 h and were allowed free access to water.
The following experimental protocol was approved by the Animal Care and Use Committee of the Drum Tower Hospital and conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health.
All rats were randomly assigned to four experimental groups according to the secondary warm ischemia time. Group I had no SWIT, in which simultaneous reperfusion was performed through the PV and HA after cold perfusion. The SWIT in groups II-IV was 10, 20 and 40 min, in which blood reperfusion of HA was performed 10, 20 or 40 min after PV reperfusion, respectively. Ten rats in each group were used to evaluate the one-week survival rate.
A rat model of autologous liver transplantation with continuous external biliary drainage under ether anesthesia was established in this study. It was described as follows:
A midline incision was made on the abdomen. The ligaments around the liver were dissociated and severed. The left diaphragm vein, hepatoesophageal ligament vein and right adrenal vein were separated, ligated and severed. The liver was turned left, and the suprahepatic vena cava (SHVC) was anatomized. And the common bile duct, HA, and PV were anatomized over the margin of the duodenal bulb. The infrahepatic vena cava (IHVC) was dissociated downwards about 6-8 mm. The liver was dissociated completely except for the hepatic blood vessels in and out, and the common bile duct.
A clamp was placed on the crossing of the cranial mesenteric vein and splenic vein, and the PV was pricked with a transfixion pin and fixed by another clamp. Then heparin saline (35 U/mL, 3 mL) was injected to make the rat heparinized and allow liver blood to enter the general circulation.
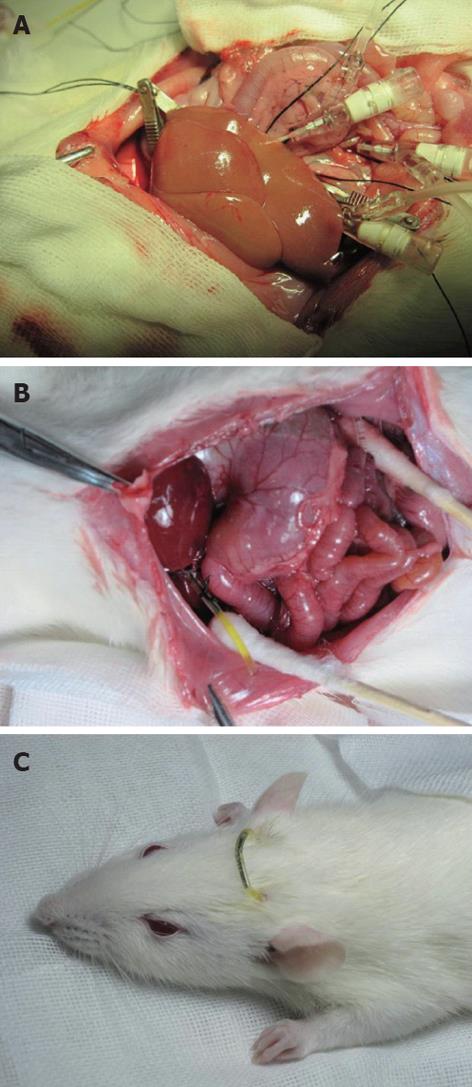
The abdominal aorta was pricked between the common iliac artery and left renal artery with pinhead and fixed by a clamp, and it was blocked from the area above the celiac trunk to the area below the puncture point by a clamp. The clamps were placed on the SHVC and IHVC with IHVC outflow tract left before cold perfusion. Lactated Ringer’s solution of 20 mL (4 °C, containing heparin 12.5 U/mL) was perfused at 2.5 mL/min through the PV by an infusion pump and the abdominal aorta, respectively. The liver surface was covered with 4 °C lactated Ringer’s solution to lower the temperature. The normal anatomical position of liver was maintained to avoid uneven perfusion. Subsequently, the color of the liver faded (Figure 1A).
The transfixion pin was removed after the perfusion, the PV and abdominal aorta were repaired using 9-0 prolene suture, and the IHVC outflow tract was restored with 8-0 prolene suture. The clamp was loosened to check whether the restoration was successful. The HA was clamped for the removal of the pre-remained line in groups II-V, then the PV, abdominal aorta, SHVC and IHVC were unclamped to end the anhepatic phase. The liver surface was covered with 38 °C normal saline (20 mL) for rapid rewarming. Thus the liver was filled with blood and turned red. The intestinal tract was placed back to the abdominal cavity during the secondary warm ischemia-reperfusion. The abdominal cavity was covered with wet gauze and heated by a lamp to maintain normal temperature.
The distal end of the bile duct was ligated and a polyethylene catheter (inner diameter 0.8 mm, outer diameter 1.2 mm) was inserted into the common bile duct for external biliary drainage. A jejunal fistula was established to return the remaining bile into the enterohepatic circulation. A 15-cm portion of polyethylene catheter (inner diameter 1.2 mm, outer diameter 1.5 mm) was inserted approximately 2 cm into jejunum through a stab in the jejunum wall and fixed to the peritoneum (Figure 1B). The free ends of external biliary drainage tube and jejunal fistula tube were brought out of the body through a stab on the back of the rat’s neck and connected with a short hypodermic needle (Figure 1C).
At 6 h, 24 h, 3 d and 7 d after reperfusion of the hepatic artery, six rats were killed in each group to collect blood sample via the infrahepatic vena cava and the median lobe of liver for assay. The serum was separated and stored at -70 °C until analysis. Washed with cold saline solution, the liver samples were stored immediately in liquid nitrogen until analysis.
Analysis of establishment of models included warm ischemia time of liver (the time from abdominal aorta clamping to cold perfusion of liver), cold perfusion time, anhepatic phase (the time from PV clamping to PV reperfusion), operative duration for biliary external drainage and survival rates in the four groups.
The liver function assessment included measurements of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (AKP), total bilirubin (TB) and direct bilirubin (DB). The serum samples were collected at 6 h, 24 h, 3 d and 7 d after reperfusion of HA and liver function test was conducted by automatic biochemistry analyzer (HITACHI 7600).
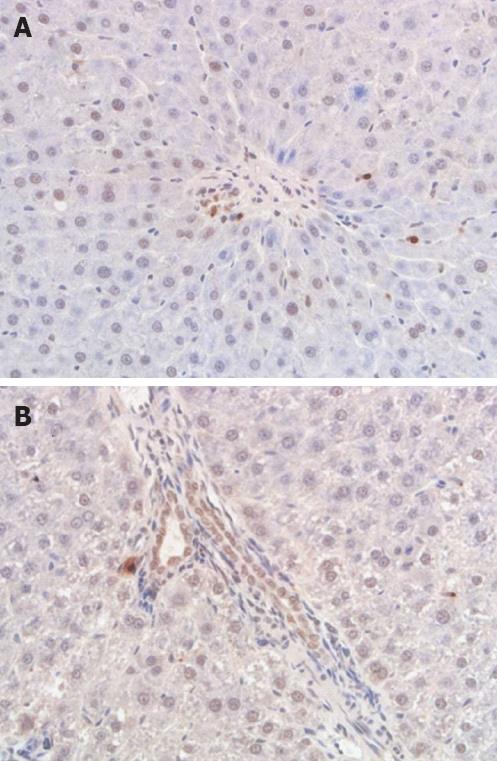
Apoptosis of biliary tract epithelia was identified by detecting DNA fragmentation in situ in serial sections at 6 and 24 h after HA reperfusion. DNA fragmentation was detected by TUNEL staining, which was performed on deparaffinized and dehydrated sections using the In Situ Cell Death Detection kit (Zhongshan Biomedical Technology Co., Beijing, China) according to the manufacturer’s instructions. TUNEL-positive cholangiocytes displayed a characteristic morphology of apoptosis, such as chromatin condensation, cell fragmentation and apoptotic bodies. Apoptotic cells were examined at original magnification × 400 in 10 randomly chosen fields per section. The apoptotic index was calculated as percentage of apoptotic cells related to the total number of cholangiocytes.
Six hepatic specimens were collected at 24 h after reperfusion of HA in each group. The liver specimens for light microscopy were fixed with 10% formalin and then embedded in paraffin. The sections were stained with hematoxylin and eosin for histological examination. Bile duct injury in specimens was semi-quantified by calculating a bile duct injury severity score (BDISS)[6] based on the following three components: bile duct damage (graded as 0, absent; 1, mild; 2, moderate; and 3, severe; modified from the Banff criteria for defining acute rejection); ductular proliferation (graded 0-3, using a similar scale as stated earlier); and cholestasis (graded 0-3, using a similar scale as stated earlier). This resulted in a minimal BDISS of zero and a maximum score of nine points. All examinations were conducted by an experienced pathologist who was unaware of the other study data.
Results were expressed as mean ± SD. Numerical data was analyzed with Statistical Analysis System. One-way repeated measures analysis of variance with the Student-Newman-Keuls test was performed for multiple comparisons to test the effect of time, groups, and the interaction between the time and groups. A P value of < 0.05 was considered significant.
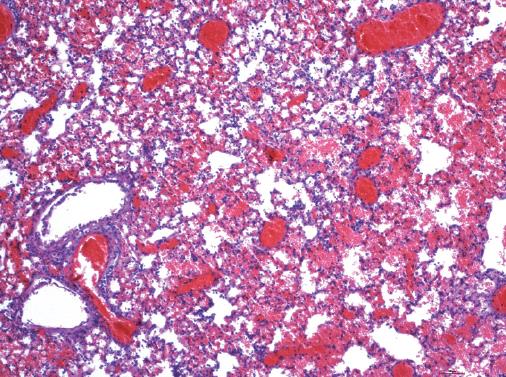
The cold perfusion time in each group was eight minutes with the use of an infusion pump. No significant difference was shown in warm ischemia time, anhepatic phase and operative duration for biliary external drainage among the four groups (P > 0.05). Five of the 40 rats in this study evaluating the one-week survival rate died, including one death of bleeding caused by unsuccessful abdominal aorta repair (group III), one of sudden respiratory arrest after HA reperfusion (group IV) and three of severe pulmonary infection (group IV). The result of autopsy after the death of three rats in group IV showed serious pulmonary vascular exudation and edema accompanied with pulmonary consolidation. Pathological examination revealed obvious leucocyte infiltration in alveolar cavity and pulmonary interstitium, numerous inflammatory exudates around bronchi, and alveolar wall damage with microvascular endothelial cell injury and bleeding (Figure 2). A significant decrease of one-week survival rate in group IV was noted compared with the other three groups (P < 0.05, Table 1).
| Group | Warm ischemia time (min) | Anhepatic phase (min) | Operative duration for biliary external drainage (min) | 1-wk survival rate (%) |
| I | 3.1 ± 0.5 | 18.1 ± 1.2 | 6.2 ± 1.2 | 100 |
| II | 3.0 ± 0.4 | 17.6 ± 1.5 | 7.1 ± 1.4 | 100 |
| III | 3.3 ± 0.3 | 18.5 ± 1.1 | 6.7 ± 1.6 | 90 |
| IV | 3.2 ± 0.5 | 17.9 ± 1.7 | 6.5 ± 2.0 | 60 |
A significant increase of ALT, AST, AKP, TB and DB was observed at 6 h, 24 h, day 3 and day 7 (P < 0.05) after operation in the three groups compared with the results in group I. The increased indexes of liver function test in group IV were higher than in groups II and III, and there were significant differences among these three groups at 6 h, 24 h, day 3 and day 7 after operation (P < 0.05).
ALT and AST reached the peak at 6 h after autologous liver transplantation, and very significant decrease was observed on post-operative days 3 and 7 (P < 0.01). Other liver function indexes, including AKP, TB and DB, reached the peak at post-operative 24 h, and there was significant decrease on post-operative days 3 and 7 (P < 0.05, Table 2).
| Liver function | Normal value | Group | 6 h | 24 h | 3 d | 7 d |
| ALT | 5-40 | I | 165.71 ± 31.42 | 140.22 ± 21.08 | 24.30 ± 12.04 | 20.15 ± 8.12 |
| (U/L) | II | 356.15 ± 52.52a | 246.31 ± 22.93a | 46.43 ± 4.69a | 38.24 ± 3.52a | |
| III | 632.23 ± 32.28bc | 444.32 ± 27.80bc | 79.58 ± 17.93ac | 56.12 ± 10.23ac | ||
| IV | 931.27 ± 20.21bde | 801.81 ± 25.17bde | 108.95 ± 11.81bde | 71.26 ± 9.14bde | ||
| AST | 8-40 | I | 855.11 ± 28.20 | 553.62 ± 17.66 | 82.02 ± 21.14 | 52.13 ± 17.26 |
| (U/L) | II | 1027.05 ± 42.02a | 837.31 ± 47.72a | 117.33 ± 30.86a | 84.24 ± 18.92a | |
| III | 1560.46 ± 68.39bc | 1129.26 ± 137.09bc | 173.48 ± 33.61ac | 113.13 ± 25.72ac | ||
| IV | 2620.13 ± 123.68bde | 2194.53 ± 297.70bde | 250.10 ± 48.54bde | 165.31 ± 41.26bde | ||
| AKP | 47-185 | I | 124.52 ± 26.26 | 154.13 ± 32.32 | 121.22 ± 14.21 | 91.52 ± 11.56 |
| (U/L) | II | 206.54 ± 27.69a | 270.85 ± 28.48a | 189.74 ± 24.28a | 126.51 ± 16.15a | |
| III | 297.40 ± 34.14bc | 363.36 ± 30.73bc | 295.90 ± 22.04bc | 163.26 ± 13.29bc | ||
| IV | 417.06 ± 10.82bde | 445.81 ± 33.49bde | 327.92 ± 12.36bce | 227.84 ± 32.16bce | ||
| TB | 0.3-1.2 | I | 0.82 ± 0.26 | 1.42 ± 0.27 | 0.82 ± 0.24 | 0.70 ± 0.21 |
| (mg/dL) | II | 1.13 ± 0.17a | 1.52 ± 0.10 | 0.93 ± 0.11 | 0.81 ± 0.16 | |
| III | 2.12 ± 0.20ac | 2.54 ± 0.19ac | 1.37 ± 0.20ac | 1.04 ± 0.18ac | ||
| IV | 2.64 ± 0.28bce | 3.43 ± 0.19bde | 2.34 ± 0.27bce | 1.96 ± 0.28bce | ||
| DB | 0.1-0.4 | I | 0.83 ± 0.17 | 1.06 ± 0.11 | 0.64 ± 0.11 | 0.52 ± 0.21 |
| (mg/dL) | II | 1.02 ± 0.11 | 1.33 ± 0.12a | 0.83 ± 0.12 | 0.71 ± 0.16 | |
| III | 2.05 ± 0.22ac | 2.36 ± 0.20ac | 1.45 ± 0.22ac | 1.32 ± 0.19ac | ||
| IV | 2.36 ± 0.12bce | 2.75 ± 0.12ace | 2.23 ± 0.20bde | 2.02 ± 0.26bde |
The apoptosis index of bile duct epithelia in groups I at 6 and 24 h after hepatic artery reperfusion was 5.87 ± 0.50 and 7.13 ± 0.60. Compared with group I, there was a significant increase of apoptosis index at 6 and 24 h in groups II and III (P < 0.05), and a very significant increase in group IV (P < 0.01). The increased apoptosis index in group IV was higher than in groups II and III, and there were significant differences among these three groups at post-operative 6 and 24 h (P < 0.05). In all groups, the apoptosis index at 24 h after hepatic artery reperfusion was higher than at 6 h, but no significant differences were noted (P > 0.05) (Table 3; Figure 3A and B).
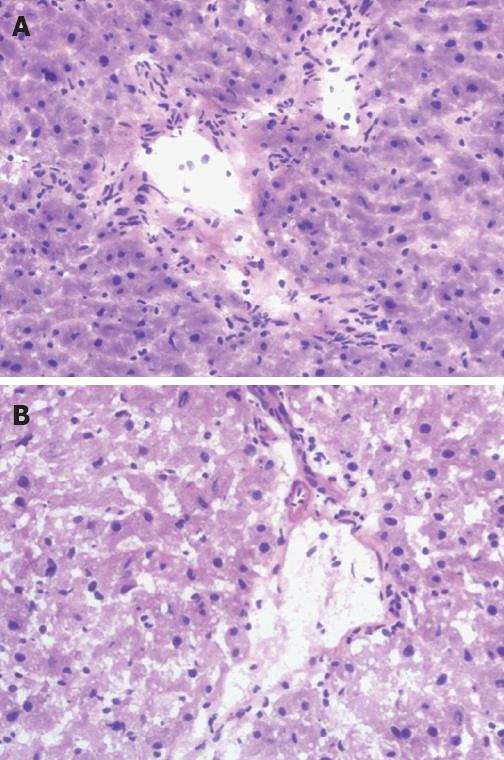
The predominant injuries of bile duct in group I included cholangiocytes lined in disorder, diversified morphous and edema, inflammatory cell infiltration, migrated chromatin, and the necrotic and caducous cell debris in the lumen under light microscope. The bile duct showed more histological changes in groups II and III, and the most significant injury occurred in group IV (Figure 4A and B). Microthrombi were found in the micrangium around the biliary tract in some sections from groups III and IV.
Compared with group I, there was a significant increase of BDISS at 6 and 24 h in groups II and III (P < 0.05), and a very significant increase of apoptosis in group IV (P < 0.01). The BDISS in group IV was higher than in groups II and III, and there were significant differences among the three groups at post-operative 6 and 24 h (P < 0.05). In all groups, BDISS at 24 h after hepatic artery reperfusion was higher than at 6 h, but no significant differences were noted (P > 0.05, Table 3).
Grafts from DCD are used to increase the number of organs available for liver transplantation, and warm ischemic time in the donor in addition to subsequent cold ischemia-reperfusion injury is believed to result in increased damage to biliary epithelial cells[7,8]. It is associated with a higher risk of biliary strictures, and the incidence of NAS after DCD ranged from 10% to 30% compared with an incidence of 1%-10% after brain death[9-11]. The present experiment used a rat model of autologous orthotopic liver transplantation to simulate I/R injury of the biliary tract, which simulated the whole process of clinical liver transplantation. This model decreased the possibility of blood vessel or vascular anastomosis damage compared with allogeneic orthotopic liver transplantation, and it avoided the effects of immunologic rejection. This model is simple and has a high successful rate, better reflects the pathophysiologic process of bile ducts, and provides an approach for investigating the intrahepatic bile duct injury in liver transplantation caused directly by I/R injury[12].
More and more studies have focused on the role of bile salt toxicity in the development of bile duct injury after transplantation, and bile composition analysis may be important in evaluating the liver function[13-15]. Establishing a stable model of orthotopic liver transplantation with external biliary drainage in rats is needed to provide the possibility for dynamic detection of bile characteristics after transplantation. But the lack of bile in the gut lumen is one of the factors associated with an increase in translocation of bacteria through the intestinal mucosa. It has been proposed that the intestinal bile flow is important to the immunity because bile salt has a dispersion effect on lipopolysaccharide and endotoxin, which had been proved to correlate with suppression of cellular immunity[16]. In experimental models, internal biliary drainage resulted in better systemic immune status, and improved intestinal barrier and mucosal immune functions[17]. The operative duration for biliary external drainage in this study was 6.7 ± 1.7 min, and no rat died of biliary complication. The biliary extra-drainage model in rat autologous liver transplantation in this study provides a simple and reliable method for dynamic collection of bile, and it could be applied in various experimental studies.
Hepatocytes are supplied by both the hepatic artery and the portal vein, but bile ducts entirely depend on arterial blood supply for oxygenation. The terminal arteriole of hepatic artery branches off into the peribiliary plexus (PBP), which supplies the intrahepatic bile ducts. Therefore, the changes of PBP usually altered the intrahepatic bile duct structure. Post-transplantational hepatic artery ischemia induces ischemia and occlusion of PBP, which is the vital reason leading to aggravation of ischemia of intrahepatic bile ducts[18-20]. The pathomorphologic changes of biliary tract showed that the relationship between secondary ischemia time and pathological structural injury was time-dependent, and the biliary tract injury in group IV was most serious among the four groups in this study. Microthrombi were found in the micrangium around the biliary tract in some sections from groups III and IV. Accordingly, the serum levels of liver function increased with the prolonged second warm ischemia time. The levels of AKP, TB and DB, in particular, revealed the injury of the bile duct, which became worse with the longer second warm ischemia time.
Bile duct epithelia are highly susceptible to reoxygenation after anoxia[21]. The increased susceptibility to reoxygenation injury by cholangiocytes is associated with increased production of toxic reactive oxygen species by cholangiocytes during reoxygenation with concomitant low basal levels of the antioxidant glutathione in these epithelial cells[22]. Apoptosis is one of the two mechanisms by which cell death occurs (the other is the pathological process of necrosis). Accumulating evidence suggests that apoptosis plays an important role in ischemia-reperfusion injury in organ transplantation[23,24] and it is widely taken as a reference index to evaluate bile duct epithelial injury. With the prolongation of the biliary warm ischemia time, the biliary epithelial cell apoptosis index was significantly elevated, and the apoptosis index at 24 h after hepatic artery reperfusion was higher than at 6h, but with no significant differences.
In conclusion, the relationship between secondary warm ischemia time and the bile duct injury degree is time-dependent. Because of a lower one-week survival rate in the 40 min group, 20 min of secondary biliary warm ischemia time is feasible for the study of bile duct injury in a rat autologous liver transplantation model with external bile drainage.
We want to thank Dr. Chen Jun for his work in pathological analysis.
With the improvement of surgical techniques, the incidence of anastomotic biliary strictures after liver transplantation decreased remarkably, whereas the nonanastomotic biliary strictures became the major type of biliary complications of liver allograft. Diffuse non-anastomotic biliary strictures remain the most challenging type of biliary complication as they are frequently therapy-resistant and often associated with long-term consequences.
Warm ischemic time in donation after cardiac death in addition to subsequent cold ischemia-reperfusion injury is believed to result in increased damage to biliary epithelial cells. Compared with liver cells, the bile duct epithelial cells experience an extra ischemia process, which includes the time from portal vein recanalization to hepatic artery recanalization, and this is “secondary warm ischemia time in the biliary tract” or “relative warm ischemia time in the biliary tract”. This is the special phase of biliary tract warm ischemia in the grafts, and more and more studies have focused on the effect of secondary warm ischemia time on bile duct injury.
Because warm ischemic time in the harvesting of donor liver after cardiac death is inevitable, more and more studies have investigated the effect of secondary warm ischemia time on bile duct injury. In this study, the authors investigated the impact of different time points of secondary warm ischemia in a rat autologous liver transplantation model with external bile drainage. This model provides a simple and reliable method for dynamic collection of bile, and can be applied in various experimental studies.
This study demonstrated that the relationship between secondary warm ischemia time and the bile duct injury degree is time-dependent. Because of the lower one-week survival rate in the 40 min group, the authors proposed that 20 min of secondary biliary warm ischemia time should be feasible for the study of bile duct injury in a rat autologous liver transplantation model with external bile drainage.
This is a well designed and conducted experimental study with very interesting results. The literature review was adequate. The materials and methods were adequate and coincident to the objective of the study. Newer studies with your rat model with prolonged secondary biliary will help us to elucidate unanswered questions in hepatobiliary surgery.
Peer reviewers: Sheng-Lei Yan, MD, Division of Gastroenterology, Department of Internal Medicine, Chang Bing Show Chwan Memorial Hospital, No. 6, Lugong Rd., Lugang Township, Changhua County 505, Taiwan, China; José LS Souza, MD, Clinics Hospital, University of Sao Paulo, 255 Eneas de Carvalho Ave. 9 th Floor, Room 9159, Sao Paulo 1009, Brazil
S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Buck DG, Zajko AB. Biliary complications after orthotopic liver transplantation. Tech Vasc Interv Radiol. 2008;11:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Wojcicki M, Milkiewicz P, Silva M. Biliary tract complications after liver transplantation: a review. Dig Surg. 2008;25:245-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 3. | Sharma S, Gurakar A, Jabbour N. Biliary strictures following liver transplantation: past, present and preventive strategies. Liver Transpl. 2008;14:759-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 275] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 4. | Howell JA, Gow PJ, Angus PW, Jones RM, Wang BZ, Bailey M, Fink MA. Early-onset versus late-onset nonanastomotic biliary strictures post liver transplantation: risk factors reflect different pathogenesis. Transpl Int. 2012;25:765-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Abou Abbass A, Abouljoud M, Yoshida A, Kim DY, Slater R, Hundley J, Kazimi M, Moonka D. Biliary complications after orthotopic liver transplantation from donors after cardiac death: broad spectrum of disease. Transplant Proc. 2010;42:3392-3398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Geuken E, Visser D, Kuipers F, Blokzijl H, Leuvenink HG, de Jong KP, Peeters PM, Jansen PL, Slooff MJ, Gouw AS. Rapid increase of bile salt secretion is associated with bile duct injury after human liver transplantation. J Hepatol. 2004;41:1017-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Saidi RF, Bradley J, Greer D, Luskin R, O'Connor K, Delmonico F, Kennealey P, Pathan F, Schuetz C, Elias N. Changing pattern of organ donation at a single center: are potential brain dead donors being lost to donation after cardiac death? Am J Transplant. 2010;10:2536-2540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Abt P, Crawford M, Desai N, Markmann J, Olthoff K, Shaked A. Liver transplantation from controlled non-heart-beating donors: an increased incidence of biliary complications. Transplantation. 2003;75:1659-1663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 239] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 9. | Chan EY, Olson LC, Kisthard JA, Perkins JD, Bakthavatsalam R, Halldorson JB, Reyes JD, Larson AM, Levy AE. Ischemic cholangiopathy following liver transplantation from donation after cardiac death donors. Liver Transpl. 2008;14:604-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 176] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 10. | Grewal HP, Willingham DL, Nguyen J, Hewitt WR, Taner BC, Cornell D, Rosser BG, Keaveny AP, Aranda-Michel J, Satyanarayana R. Liver transplantation using controlled donation after cardiac death donors: an analysis of a large single-center experience. Liver Transpl. 2009;15:1028-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 143] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 11. | Foley DP, Fernandez LA, Leverson G, Anderson M, Mezrich J, Sollinger HW, D'Alessandro A. Biliary complications after liver transplantation from donation after cardiac death donors: an analysis of risk factors and long-term outcomes from a single center. Ann Surg. 2011;253:817-825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 308] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 12. | Zhou B, Zhang PJ, Tian T, Jin C, Li Y, Feng M, Liu XY, Jie L, Tao LD. Role of vascular endothelial growth factor in protection of intrahepatic cholangiocytes mediated by hypoxic preconditioning after liver transplantation in rats. Transplant Proc. 2010;42:2457-2462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Hoekstra H, Porte RJ, Tian Y, Jochum W, Stieger B, Moritz W, Slooff MJ, Graf R, Clavien PA. Bile salt toxicity aggravates cold ischemic injury of bile ducts after liver transplantation in Mdr2+/- mice. Hepatology. 2006;43:1022-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Yska MJ, Buis CI, Monbaliu D, Schuurs TA, Gouw AS, Kahmann ON, Visser DS, Pirenne J, Porte RJ. The role of bile salt toxicity in the pathogenesis of bile duct injury after non-heart-beating porcine liver transplantation. Transplantation. 2008;85:1625-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Buis CI, Geuken E, Visser DS, Kuipers F, Haagsma EB, Verkade HJ, Porte RJ. Altered bile composition after liver transplantation is associated with the development of nonanastomotic biliary strictures. J Hepatol. 2009;50:69-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Parks RW, Clements WD, Smye MG, Pope C, Rowlands BJ, Diamond T. Intestinal barrier dysfunction in clinical and experimental obstructive jaundice and its reversal by internal biliary drainage. Br J Surg. 1996;83:1345-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 97] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Sano T, Ajiki T, Takeyama Y, Kuroda Y. Internal biliary drainage improves decreased number of gut mucosal T lymphocytes and MAdCAM-1 expression in jaundiced rats. Surgery. 2004;136:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Buis CI, Hoekstra H, Verdonk RC, Porte RJ. Causes and consequences of ischemic-type biliary lesions after liver transplantation. J Hepatobiliary Pancreat Surg. 2006;13:517-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 166] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 19. | Nishida S, Nakamura N, Kadono J, Komokata T, Sakata R, Madariaga JR, Tzakis AG. Intrahepatic biliary strictures after liver transplantation. J Hepatobiliary Pancreat Surg. 2006;13:511-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Dacha S, Barad A, Martin J, Levitsky J. Association of hepatic artery stenosis and biliary strictures in liver transplant recipients. Liver Transpl. 2011;17:849-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Feng L, Pang L, Guo Y, Ke N, Li S, Wei L, Li Q, Li Y. Hypoxia/reoxygenation up-regulates death receptor expression and enhances apoptosis in human biliary epithelial cells. Life Sci. 2009;85:401-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Fouassier L, Beaussier M, Schiffer E, Rey C, Barbu V, Mergey M, Wendum D, Callard P, Scoazec JY, Lasnier E. Hypoxia-induced changes in the expression of rat hepatobiliary transporter genes. Am J Physiol Gastrointest Liver Physiol. 2007;293:G25-G35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Wang Z, Zhou J, Lin J, Wang Y, Lin Y, Li X. RhGH attenuates ischemia injury of intrahepatic bile ducts relating to liver transplantation. J Surg Res. 2011;171:300-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Rüdiger HA, Graf R, Clavien PA. Liver ischemia: apoptosis as a central mechanism of injury. J Invest Surg. 2003;16:149-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 76] [Article Influence: 3.6] [Reference Citation Analysis (0)] |