Published online Dec 28, 2012. doi: 10.3748/wjg.v18.i48.7158
Revised: October 10, 2012
Accepted: October 16, 2012
Published online: December 28, 2012
AIM: To investigate the anti-hepatofibrotic effects of Gardenia jasminoides in liver fibrosis.
METHODS: Male Sprague-Dawley rats underwent common bile duct ligation (BDL) for 14 d and were treated with Gardenia jasminoides by gavage. The effects of Gardenia jasminoides on liver fibrosis and the detailed molecular mechanisms were also assessed in human hepatic stellate cells (LX-2) in vitro.
RESULTS: Treatment with Gardenia jasminoides decreased serum alanine aminotransferase (BDL vs BDL + 100 mg/kg Gardenia jasminoides, 146.6 ± 15 U/L vs 77 ± 6.5 U/L, P = 0.0007) and aspartate aminotransferase (BDL vs BDL + 100 mg/kg Gardenia jasminoides, 188 ± 35.2 U/L vs 128 ± 19 U/L, P = 0.005) as well as hydroxyproline (BDL vs BDL + 100 mg/kg Gardenia jasminoides, 438 ± 40.2 μg/g vs 228 ± 10.3 μg/g liver tissue, P = 0.004) after BDL. Furthermore, Gardenia jasminoides significantly reduced liver mRNA and/or protein expression of transforming growth factor β1 (TGF-β1), collagen type I (Col I) and α-smooth muscle actin (α-SMA). Gardenia jasminoides significantly suppressed the upregulation of TGF-β1, Col I and α-SMA in LX-2 exposed to recombinant TGF-β1. Moreover, Gardenia jasminoides inhibited TGF-β1-induced Smad2 phosphorylation in LX-2 cells.
CONCLUSION: Gardenia jasminoides exerts antifibrotic effects in the liver fibrosis and may represent a novel antifibrotic agent.
-
Citation: Chen YH, Lan T, Li J, Qiu CH, Wu T, Gou HJ, Lu MQ.
Gardenia jasminoides attenuates hepatocellular injury and fibrosis in bile duct-ligated rats and human hepatic stellate cells. World J Gastroenterol 2012; 18(48): 7158-7165 - URL: https://www.wjgnet.com/1007-9327/full/v18/i48/7158.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i48.7158
Chronic liver injury causes the accumulation of extracellular matrix (ECM) such as α-smooth muscle actin (α-SMA) in the liver and subsequently contributes to liver fibrosis and later cirrhosis[1-6]. This eventually leads to hepatic dysfunction, portal hypertension, and hepatocellular carcinoma (HCC)[7-9]. Hepatic stellate cells (HSC) are the principal liver cells that promote liver fibrosis[3,9-12]. Upon activation by various stimuli such as transforming growth factor (TGF)-β1, HSC transdifferentiate into myofibroblasts and then produce excessive ECM proteins, resulting in liver fibrosis[13-15]. Strategies aimed at disrupting TGF-β1 synthesis and/or signaling pathways markedly ameliorates liver fibrosis in an experimental model[16].
Herbal medicines have been frequently investigated for their hepatoprotective and antifibrotic effects in both humans[17] and animal models[18]. A number of studies have shown that administration of Chinese herbs lead to a decrease in hepatic TGF-β1 expression and severity of fibrosis in rats[19,20]. Yin-Chen-Hao-Tang (YCHT) decoctions have long been used as antiinflammatory, antipyretic, choleretic and diuretic agents for liver disorders and jaundice. Several studies provide clinical evidence of its effectiveness in the treatment of various liver disease. YCHT is an aqueous extract derived from three herbs: Artemisia capillaries Thunb (Herba Artemisiae Capillaris, Yin-Cen-Hao), Gardenia jasminoides Ellis (Fructus Gardeniae, Zhi-zi) and Rheum officinale Baill (Emodin, Da-huang) with a ratio of 4:3:1 in weight. YCHT was reported to suppress liver fibrosis in rats induced by a choline-deficient diet[21]. Long-term administration of YCHT in rats ameliorated hydrophilic bile acids-induced hepatic injury presumably by reducing oxidative stress and the degree of hepatic fibrosis[22]. These studies have indicated that YCHT as a promising therapeutic agent in chronic liver disease. Recent studies showed that Artemisia capillaries and Emodin (the main compound of Rheum officinale) are well-known herbal hepatotherapeutic drugs for the treatment of liver fibrosis[23-25]. However, whether Gardenia jasminoides has an anti-fibrotic effect on liver fibrosis and the involved detailed mechanism has not been fully understood yet.
The aim of the current study is to investigate the beneficial effects of Gardenia jasminoides on liver fibrosis using the bile duct ligation (BDL) rat model in vivo and TGF-β1-stimulated HSCs in vitro.
Recombinant human TGF-β1 was obtained from R and D (Minneapolis, MN, United States). α-SMA and TGF-β1 antibodies were purchased from Abcam (Cambridge, MA, United States). Phospho-Smad2 and Smad2 antibodies were obtained from Cell Signaling Technology (Boston, MA, United States). α-tubulin antibody was purchased from Sigma-Aldrich (St. Louis, MO, United States). DMEM and fetal bovine serum (FBS), penicillin/streptomycin and trypsin were obtained from Invitrogen (Carsbad, CA, United States). Gardenia jasminoides standard (purity > 99%) was purchased from the Institute of Chinese Pharmaceutical and Biological Products.
Gardenia jasminoides was prepared as described previously[22] by boiling the dried Gardenia jasminoides fruits with distilled water for 5 h. The extract was filtered, freeze-dried, and kept at 4 °C. The yield of water extract of Gardenia jasminoides was 8.33% (w/w). The dried extract was dissolved in distilled water before use.
All animal experimental protocols were approved by the local animal care and use committee according to criteria outlined in Guide for the Care and Use of Laboratory Animals from the National Academy of Sciences (National Institutes of Health publication 86-23, 1985 revision). For experiments with BDL, rats were randomly divided into five groups (n = 8). Each day, four animals underwent BDL, and a sham-operated animal was used as a healthy control. Twenty-four hours after surgery, the four BDL animals were randomly assigned to receive 14 d of daily gavage consisting of ddH2O (the treatment control and vehicle), while treatment groups received 25, 50 and 100 mg/kg Gardenia jasminoides (suspended in ddH2O) by gavage. The sham controls also received ddH2O by gavage. At the end of experiment, rats were anesthetized, serum was collected and livers were removed. Some portions of liver tissue were fixed in 10% formalin or embedded in paraffin specimen medium. Others were snap frozen in liquid nitrogen and stored at -80 °C until use.
Serum alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were analyzed with kits from Thermo Fisher Scientific (United States). Formalin-fixed tissue was embedded in paraffin, and sections of liver (4 μm) were stained with hematoxylin and eosin (HE) to evaluate the morphological changes and stage of liver fibrosis according to the Ishak Stage Score. The liver hydroxyproline content was measured as described[26].
Total RNA was extracted using TRIzolTM Reagent (Invitrogen, United States) according to the manufacturer’s instructions. Total RNA (1 μg) was reverse transcribed, followed by quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) using the Bio-Rad iCycler Iq system. The qRT-PCR reactions were carried out in a total volume of 25 μL containing 12.5 μL of SYBR Premix Ex TaqTM (2 ×) (TaKaRa Biotechnology Co., Ltd, Dalian, China), 2 μL of cDNA, 5 pmol of forward and reverse primers, and 9.5 μL of distilled H2O. The sequences of the rat and human primers used are listed in Table 1, respectively. PCR was performed at 95 °C for 30 s, and then run for 45 cycles at 95 °C for 5 s and 60 °C for 20 s. The relative amount of mRNA was calculated using the comparative Ct (ΔΔCt)[27]. The glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene was used as a reference for normalizing data. The derived normalized values were the mean of three runs.
| Gene | Primer sequences | Size (bp) | Accession number |
| Rat TGF-β1 | Forward: 5'-TACAACAGCACCCGCGACCG-3' Reverse: 5'-TGCGTTGTTGCGGTCCACCA-3' | 117 | NM_021578.2 |
| Col I | Forward: 5'-ACCGGGCCGATGATGCCAAC-3' Reverse: 5'- ATGTGCGGGCGGGGTTCTTG-3' | 129 | NM_053304.1 |
| GAPDH | Forward: 5'-GGGCTCTCTGCTCCTCCCTGTTC-3' | 107 | NM_017008.3 |
| Reverse: 5'-ACGGCCAAATCCGTTCACACC-3' | |||
| Human TGF-β1 | Forward: 5'-TGTTCGCGCTCTCGGCAGTG-3' Reverse: 5'-GCCTCGATGCGCTTCCGCTT-3' | 184 | NM_000660.4 |
| Col I | Forward: 5'-GAGCGGACGCTAACCCCCTC-3' Reverse: 5'-AGGGCGGTGGCCGCTAAGAG-3' | 110 | NM_000088.3 |
| GAPDH | Forward: 5'-CAGCCTCCCGCTTCGCTCTC-3' | 143 | NM_002046.4 |
| Reverse: 5'-ACCAGGCGCCCAATACGACC-3' |
Total proteins were extracted and subjected to SDS-PAGE and analyzed by immunoblotting as described previously[28]. Primary antibodies against α-SMA, p-Smad2, Smad2 and α-tubulin were used. Horseradish peroxidase-coupled secondary antibodies were bought from Promega (Promega). The protein bands were detected employing ECL chemiluminescence (Thermo Scientific).
LX-2, a well-characterized cell line derived from human HSCs, was used in the in vitro studies. Recombinant TGF-β1 and Gardenia jasminoides (Standard) were used at doses shown in individual figure legends. Cells were cultured in DMEM supplemented with 10% fetal bovine serum, 1 mmol/L L-glutamine, and 100 IU/mL streptomycin/penicillin.
SPSS version 16.0 for Windows was used for all analysis. All values are expressed as mean ± SD. The statistical significance between experimental groups was determined by t test or analysis of variance. P values < 0.05 were considered statistically significant.
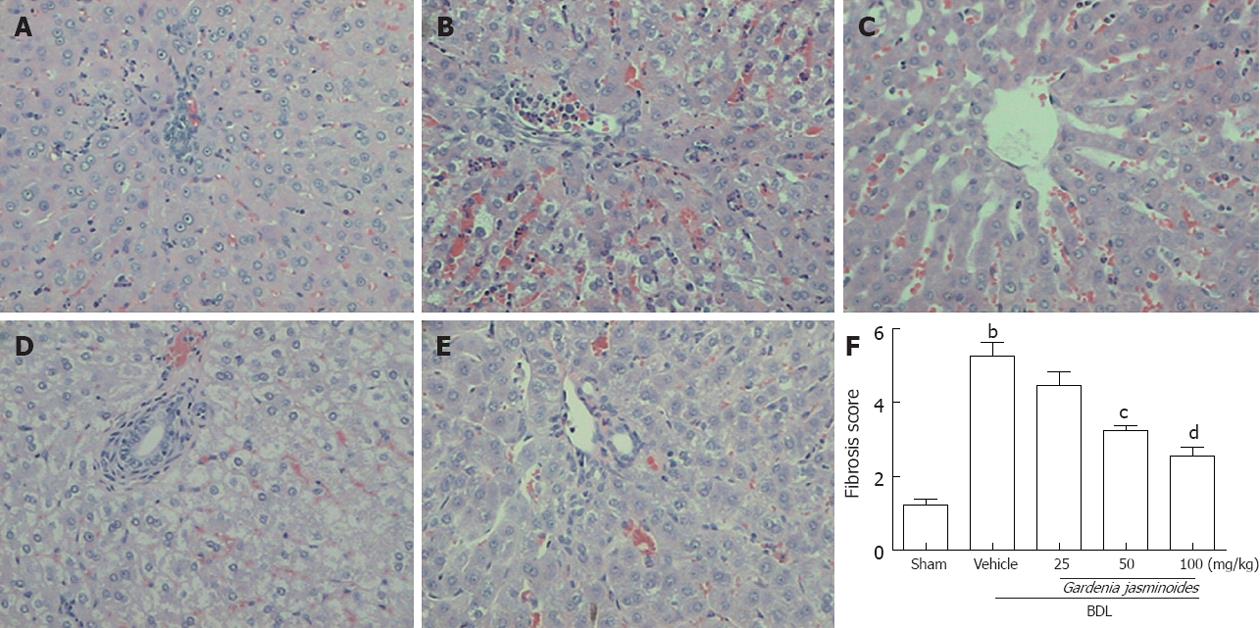
Both BDL and sham-operated rats were orally administered continuously either with the vehicle or Gardenia jasminoides (25, 50, 100 mg/kg body weight) for 2 wk. BDL in rats is associated with significant increases in liver fibrosis; this was confirmed by HE staining of liver tissues. In contrast, Gardenia jasminoides treatment groups had significantly less liver fibrosis (Figure 1), with the lowest scores in the 100 mg/kg Gardenia jasminoides treatment group.
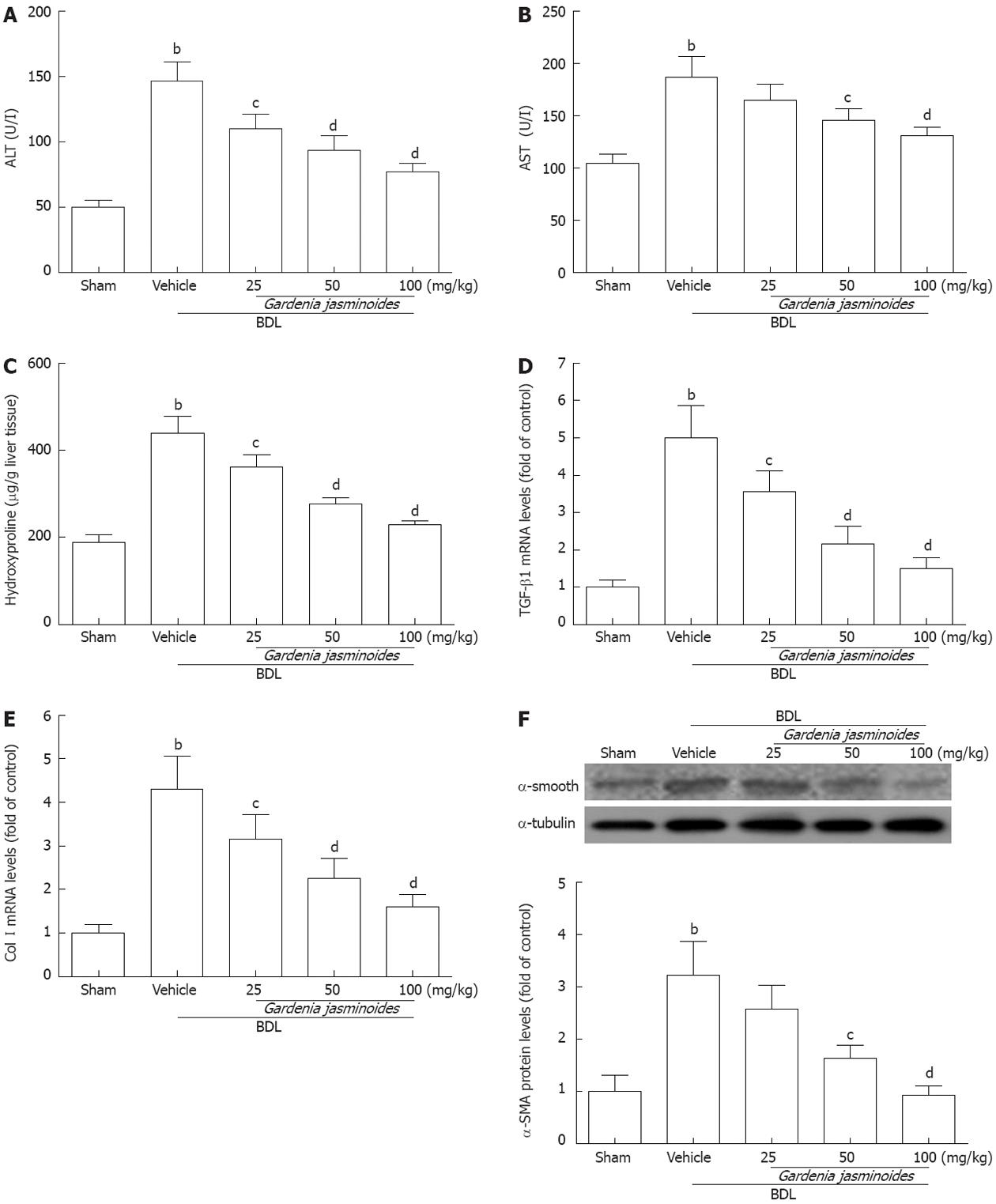
Gardenia jasminoides treatment also reduced the elevated levels of serum ALT and AST, which are indicators of hepatocellular damage induced by BDL (Figure 2A and B). Hydroxyproline analysis revealed significantly lower levels of this fibrosis marker in the livers of the Gardenia jasminoides treatment groups (Figure 2C). This was also confirmed by hepatic histology. In particular, the hydroxyproline content in the 100 mg/kg group was reduced to levels similar to those in the control group.
To uncover the mechanisms underlying this beneficial phenomenon, we investigated the effects of Gardenia jasminoides on the expression of fibrotic gene. mRNA expression of TGF-β1 and collagen I (Col I) was significantly reduced in the livers of Gardenia jasminoides treatment groups (Figure 2D and E). Western blot analysis also revealed a significant reduction in α-SMA protein expression in the livers of groups treated by 50 and 100 mg/kg Gardenia jasminoides, respectively (Figure 2F). Taken together, these data suggest that the Gardenia jasminoides therapy greatly reduces fibrosis formation in BDL rats.
Treatment with Gardenia jasminoides attenuates TGF-β1-induced HSC activation
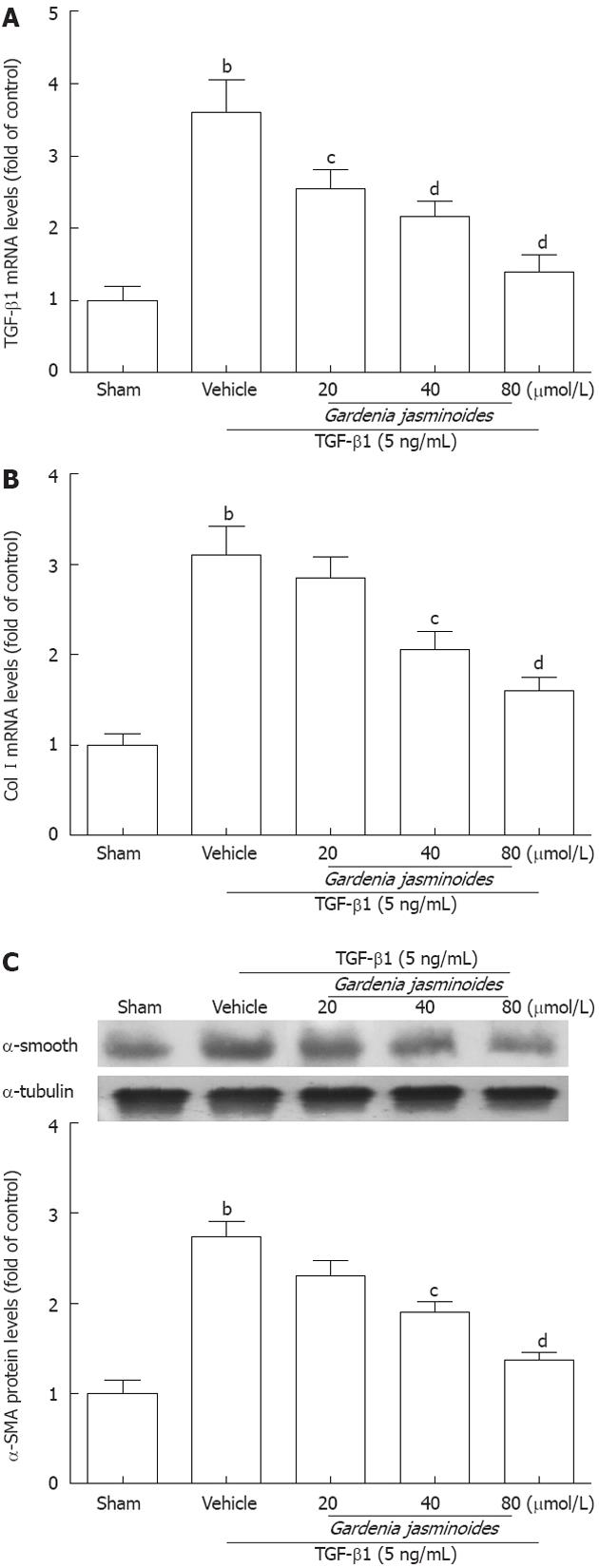
Because Gardenia jasminoides reduced markers of hepatic fibrosis in BDL rats, we next examined whether this therapy has similar antifibrotic effects in a human HSC line (LX-2 cells). The mRNA levels of TGF-β1 and Col I were significantly increased in response to recombinant TGF-β1 for 24 h. However, Gardenia jasminoides downregulated the mRNA levels of TGF-β1 and Col I in LX-2 exposed to TGF-β1 in a dose-dependent manner (Figure 3A and B). The protein expression of α-SMA as detected by Western blotting was also reduced in LX-2 cells after Gardenia jasminoides treatment (Figure 3C). Together, these results indicate that Gardenia jasminoides exerts its antifibrotic effects in the liver by repressing TGF-β1, Col I and α-SMA expression.
Gardenia jasminoides reduces Smad2 phosphorylation in TGF-β1-stimulated LX-2 cells
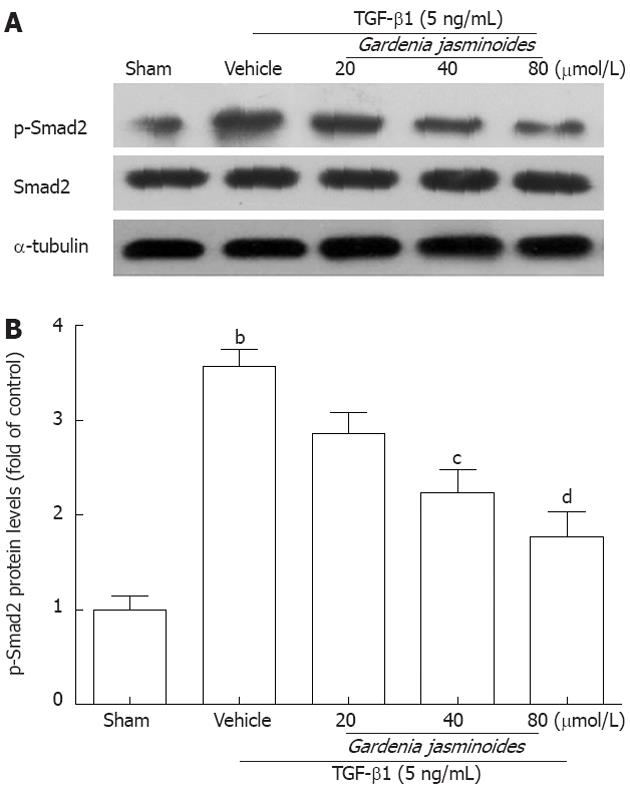
Phosphorylated Smad2 plays an important role in the activation of TGF-β1-induced Col I and α-SMA expression[29-31]. To determine whether Gardenia jasminoides attenuation of Col I and α-SMA expression is mediated through this pathway, we measured Smad2 expression and phosphorylation levels. As shown in Figure 4, Gardenia jasminoides significantly reduced Smad2 phosphorylation induced by TGF-β1 in LX-2 cells without affecting the total amount of Smad2, thereby suggesting that the inhibitory effects of Gardenia jasminoides on the expression of Col I and α-SMA is mediated by the blocking of TGF-β1-stimulated Smad2 phosphorylation.
Liver fibrosis is a key risk factor for the development of cirrhosis and chronic liver failure. Activation of HSCs is a crucial component of this process[11,12]. In the current study, we evaluated the therapeutic effects of Gardenia jasminoides in vivo in a BDL cholestatic rat model and in vitro in human hepatic cells.
Chronic cholestasis leads to liver necrosis, fibrosis, and cirrhosis, partly due to an accumulation of toxic bile acids in the liver[32]. Therapy with Gardenia jasminoides is based on the hepatoprotective properties of YCHT decoctions containing Gardenia jasminoides[22]. Because previous studies have demonstrated that the other two ingredients, Artemisia capillar Thunb and Rheum officinale Baill, have hepatoprotective properties, we hypothesized that Gardenia jasminoides might also improve hepatic function in patients with cholestatic liver diseases. As seen in this report, this hypothesis is supported by our experimental results. Biochemical and gene expression analyses demonstrated that elevated markers of liver dysfunction such as ALT and AST were reduced by Gardenia jasminoides.
Continuous accumulation of the ECM causes hepatofibrosis. Collagen is the main component of the extracellular matrix in fibrotic tissue[3]. Hydroxyproline, a major component of collagen, was used as an indicator for evaluation of the degree of liver fibrosis[33]. Gardenia jasminoides treatment (50 and 100 mg/kg) significantly attenuated collagen accumulation as evidenced by the inhibition of BDL-elevated hydroxyproline concentrations and the proportion of fibrotic tissue.
It is well-known that HSCs activation plays a pivotal role in the process of liver fibrosis, and α-SMA is a marker of activated HSCs[12,34,35]. In the current study, Western blotting indicated that Gardenia jasminoides (50 and 100 mg/kg) markedly suppressed the activation of HSCs. To determine whether human cells would show react similarly to Gardenia jasminoides, we treated human HSCs cell line, LX-2 cells, with different concentrations of Gardenia jasminoides. In contrast to vehicle, Gardenia jasminoides decreased the expression of TGF-β1, Col I and α-SMA. These data are consistent with the results of our in vivo study. Together, these results provide compelling evidence supporting the beneficial effects of Gardenia jasminoides on the rat model of cholestasis and human liver cells.
Further analysis of this antifibrotic effect suggests that Gardenia jasminoides suppressed the expression of Col I and α-SMA via the TGF-β1/Smad2 signaling pathway. HSCs are the major target of TGF-β1, which helps to stimulate the transdifferentiation of HSCs into fibrogenic myofibroblasts[36]. The production of TGF-β1 is upregulated in myofibroblasts and proliferating bile duct epithelia after BDL, which further contribute to the fibrogenic process in an autocrine/paracrine manner[37]. Downstream signaling in HSCs involves signaling transcription factors such as Smad2. In addition, TGF-β1 is thought to mediate the activation of Smad2 through its phosphorylation[38]. Here, we found that Gardenia jasminoides reduced Smad2 phosphorylation in a dose-dependent manner using LX-2 cells. These findings are consistent with a significant antifibrotic effect of Gardenia jasminoides mediated through the inhibition of the TGF-β1/Smad2 pathway. How Gardenia jasminoides represses Smad2 phosphorylation remains to be determined.
In summary, we demonstrate that Gardenia jasminoides improves the therapeutic response in a rat model of cholestasis and in vitro in human hepatic cells. These findings suggest that Gardenia jasminoides might be beneficial in patients with chronic cholestatic disorders. To further elucidate the detailed mechanisms, additional comparative studies will be needed to investigate the hepatoprotective of Gardenia jasminoides on other liver disease models as well as patients with liver fibrosis.
Liver fibrosis is a major cause of morbidity and mortality worldwide. However, there are only a few effective antifibrotic therapies for patients with liver fibrosis. Yin-Chen-Hao-Tang (YCHT) decoctions has long been used as antiinflammatory, antipyretic, choleretic and diuretic agent for liver disorders and jaundice and several studies provide clinical evidence for its effectiveness in the treatment of various liver disease. However, whether Gardenia jasminoides, one of the components of YCHT decoctions, has anti-fibrotic effect on liver fibrosis and the involved detailed mechanism has not been fully understood yet. In the present study, the anti-hepatofibrotic effects of Gardenia jasminoides were evaluated.
Strategies aimed at disrupting transforming growth factor β1 (TGF-β1) synthesis and/or signaling pathways markedly ameliorates liver fibrosis in experimental model. Inhibition of TGF-β1 signaling pathway may be related to the protective effects of Gardenia jasminoides on the bile duct ligation (BDL) rat model in vivo and TGF-β1-stimulated HSCs in vitro.
Treatment with Gardenia jasminoides decreased serum alanine aminotransferase and aspartate aminotransferase as well as hydroxyproline after BDL. Protective mechanisms of Gardenia jasminoides are associated with reduced hepatic mRNA and/or protein expression of TGF-β1, collagen type I (Col I) and α-smooth muscle actin (α-SMA). Gardenia jasminoides significantly suppressed the expression of TGF-β1, Col I and α-SMA in hepatic stellate cells exposed to recombinant TGF-β1. Moreover, Gardenia jasminoides inhibited TGF-β1-induced Smad2 phosphorylation in hepatic stellate cells.
By understanding the effects and mechanism of Gardenia jasminoides on liver fibrosis, the present study may present a promising strategy in the treatment of patients with liver fibrosis.
The present manuscript describes the effect of Gardenia jasminoides extract (YCHT) on the process of fibrosis that develops in rat liver after ligation of the common bile duct. This Chinese herbal medicine reduced the levels of serum transaminases, hydroxyproline, TGF-β1 (and its mRNA), collagen type 1 and α-smooth muscle actin. The treatment also decreases the TGF-β1-induced Smad2 phosphorylation in a human stellate cell line LX-2. This study clearly demonstrates that treatment of fibrosis, even by classical medical treatment has an effect.
Peer reviewer: Eddie Wisse, Professor, Cell Biology and Histology of the Faculty of Medicine and Pharmacy, Free University of Brussels (VUB), Laarbeeklaan 103, B 1090 Brussels-Jette, Belgium
S- Editor Lv S L- Editor Cant MR E- Editor Zhang DN
| 1. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3381] [Cited by in RCA: 4120] [Article Influence: 206.0] [Reference Citation Analysis (3)] |
| 2. | Osterreicher CH, Taura K, De Minicis S, Seki E, Penz-Osterreicher M, Kodama Y, Kluwe J, Schuster M, Oudit GY, Penninger JM. Angiotensin-converting-enzyme 2 inhibits liver fibrosis in mice. Hepatology. 2009;50:929-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655-1669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2139] [Cited by in RCA: 2164] [Article Influence: 127.3] [Reference Citation Analysis (0)] |
| 4. | Seki E, de Minicis S, Inokuchi S, Taura K, Miyai K, van Rooijen N, Schwabe RF, Brenner DA. CCR2 promotes hepatic fibrosis in mice. Hepatology. 2009;50:185-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 348] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 5. | Choi SS, Sicklick JK, Ma Q, Yang L, Huang J, Qi Y, Chen W, Li YX, Goldschmidt-Clermont PJ, Diehl AM. Sustained activation of Rac1 in hepatic stellate cells promotes liver injury and fibrosis in mice. Hepatology. 2006;44:1267-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Smart DE, Green K, Oakley F, Weitzman JB, Yaniv M, Reynolds G, Mann J, Millward-Sadler H, Mann DA. JunD is a profibrogenic transcription factor regulated by Jun N-terminal kinase-independent phosphorylation. Hepatology. 2006;44:1432-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Matsuzaki K, Murata M, Yoshida K, Sekimoto G, Uemura Y, Sakaida N, Kaibori M, Kamiyama Y, Nishizawa M, Fujisawa J. Chronic inflammation associated with hepatitis C virus infection perturbs hepatic transforming growth factor beta signaling, promoting cirrhosis and hepatocellular carcinoma. Hepatology. 2007;46:48-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 232] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 8. | Liu XE, Desmyter L, Gao CF, Laroy W, Dewaele S, Vanhooren V, Wang L, Zhuang H, Callewaert N, Libert C. N-glycomic changes in hepatocellular carcinoma patients with liver cirrhosis induced by hepatitis B virus. Hepatology. 2007;46:1426-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 142] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 9. | Trerè D, Borzio M, Morabito A, Borzio F, Roncalli M, Derenzini M. Nucleolar hypertrophy correlates with hepatocellular carcinoma development in cirrhosis due to HBV infection. Hepatology. 2003;37:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Moreno M, Chaves JF, Sancho-Bru P, Ramalho F, Ramalho LN, Mansego ML, Ivorra C, Dominguez M, Conde L, Millán C. Ghrelin attenuates hepatocellular injury and liver fibrogenesis in rodents and influences fibrosis progression in humans. Hepatology. 2010;51:974-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 11. | Son G, Hines IN, Lindquist J, Schrum LW, Rippe RA. Inhibition of phosphatidylinositol 3-kinase signaling in hepatic stellate cells blocks the progression of hepatic fibrosis. Hepatology. 2009;50:1512-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 12. | Mannaerts I, Nuytten NR, Rogiers V, Vanderkerken K, van Grunsven LA, Geerts A. Chronic administration of valproic acid inhibits activation of mouse hepatic stellate cells in vitro and in vivo. Hepatology. 2010;51:603-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Gressner AM, Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets. J Cell Mol Med. 2006;10:76-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 538] [Cited by in RCA: 610] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 14. | Park JK, Ki MR, Lee HR, Hong IH, Ji AR, Ishigami A, Park SI, Kim JM, Chung HY, Yoo SE. Vitamin C deficiency attenuates liver fibrosis by way of up-regulated peroxisome proliferator-activated receptor-gamma expression in senescence marker protein 30 knockout mice. Hepatology. 2010;51:1766-1777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Jeong WI, Park O, Radaeva S, Gao B. STAT1 inhibits liver fibrosis in mice by inhibiting stellate cell proliferation and stimulating NK cell cytotoxicity. Hepatology. 2006;44:1441-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 217] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 16. | Breitkopf K, Haas S, Wiercinska E, Singer MV, Dooley S. Anti-TGF-beta strategies for the treatment of chronic liver disease. Alcohol Clin Exp Res. 2005;29:121S-131S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Yip AY, Loo WT, Chow LW. Fructus Schisandrae (Wuweizi) containing compound in modulating human lymphatic system - a Phase I minimization clinical trial. Biomed Pharmacother. 2007;61:588-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Lin HJ, Chen JY, Lin CF, Kao ST, Cheng JC, Chen HL, Chen CM. Hepatoprotective effects of Yi Guan Jian, an herbal medicine, in rats with dimethylnitrosamine-induced liver fibrosis. J Ethnopharmacol. 2011;134:953-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Wasser S, Ho JM, Ang HK, Tan CE. Salvia miltiorrhiza reduces experimentally-induced hepatic fibrosis in rats. J Hepatol. 1998;29:760-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 116] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Lee TY, Wang GJ, Chiu JH, Lin HC. Long-term administration of Salvia miltiorrhiza ameliorates carbon tetrachloride-induced hepatic fibrosis in rats. J Pharm Pharmacol. 2003;55:1561-1568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Sakaida I, Tsuchiya M, Kawaguchi K, Kimura T, Terai S, Okita K. Herbal medicine Inchin-ko-to (TJ-135) prevents liver fibrosis and enzyme-altered lesions in rat liver cirrhosis induced by a choline-deficient L-amino acid-defined diet. J Hepatol. 2003;38:762-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Lee TY, Chang HH, Chen JH, Hsueh ML, Kuo JJ. Herb medicine Yin-Chen-Hao-Tang ameliorates hepatic fibrosis in bile duct ligation rats. J Ethnopharmacol. 2007;109:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Wang JH, Choi MK, Shin JW, Hwang SY, Son CG. Antifibrotic effects of Artemisia capillaris and Artemisia iwayomogi in a carbon tetrachloride-induced chronic hepatic fibrosis animal model. J Ethnopharmacol. 2012;140:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Dong MX, Jia Y, Zhang YB, Li CC, Geng YT, Zhou L, Li XY, Liu JC, Niu YC. Emodin protects rat liver from CCl(4)-induced fibrogenesis via inhibition of hepatic stellate cells activation. World J Gastroenterol. 2009;15:4753-4762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Imanishi Y, Maeda N, Otogawa K, Seki S, Matsui H, Kawada N, Arakawa T. Herb medicine Inchin-ko-to (TJ-135) regulates PDGF-BB-dependent signaling pathways of hepatic stellate cells in primary culture and attenuates development of liver fibrosis induced by thioacetamide administration in rats. J Hepatol. 2004;41:242-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Soroka CJ, Mennone A, Hagey LR, Ballatori N, Boyer JL. Mouse organic solute transporter alpha deficiency enhances renal excretion of bile acids and attenuates cholestasis. Hepatology. 2010;51:181-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24144] [Cited by in RCA: 26025] [Article Influence: 1084.4] [Reference Citation Analysis (0)] |
| 28. | Lan T, Liu W, Xie X, Xu S, Huang K, Peng J, Shen X, Liu P, Wang L, Xia P. Sphingosine kinase-1 pathway mediates high glucose-induced fibronectin expression in glomerular mesangial cells. Mol Endocrinol. 2011;25:2094-2105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Trojanowska M. Noncanonical transforming growth factor beta signaling in scleroderma fibrosis. Curr Opin Rheumatol. 2009;21:623-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Poncelet AC, de Caestecker MP, Schnaper HW. The transforming growth factor-beta/SMAD signaling pathway is present and functional in human mesangial cells. Kidney Int. 1999;56:1354-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 122] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 31. | Melchior-Becker A, Dai G, Ding Z, Schäfer L, Schrader J, Young MF, Fischer JW. Deficiency of biglycan causes cardiac fibroblasts to differentiate into a myofibroblast phenotype. J Biol Chem. 2011;286:17365-17375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Svegliati-Baroni G, Ridolfi F, Hannivoort R, Saccomanno S, Homan M, De Minicis S, Jansen PL, Candelaresi C, Benedetti A, Moshage H. Bile acids induce hepatic stellate cell proliferation via activation of the epidermal growth factor receptor. Gastroenterology. 2005;128:1042-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 124] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 33. | Popov Y, Patsenker E, Fickert P, Trauner M, Schuppan D. Mdr2 (Abcb4)-/- mice spontaneously develop severe biliary fibrosis via massive dysregulation of pro- and antifibrogenic genes. J Hepatol. 2005;43:1045-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 199] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 34. | Chinnadurai R, Grakoui A. B7-H4 mediates inhibition of T cell responses by activated murine hepatic stellate cells. Hepatology. 2010;52:2177-2185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Kisseleva T, Brenner DA. Mechanisms of fibrogenesis. Exp Biol Med (Maywood). 2008;233:109-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 393] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 36. | Parola M, Marra F, Pinzani M. Myofibroblast - like cells and liver fibrogenesis: Emerging concepts in a rapidly moving scenario. Mol Aspects Med. 2008;29:58-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 139] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 37. | Dranoff JA, Wells RG. Portal fibroblasts: Underappreciated mediators of biliary fibrosis. Hepatology. 2010;51:1438-1444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 216] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 38. | Liu C, Gaça MD, Swenson ES, Vellucci VF, Reiss M, Wells RG. Smads 2 and 3 are differentially activated by transforming growth factor-beta (TGF-beta ) in quiescent and activated hepatic stellate cells. Constitutive nuclear localization of Smads in activated cells is TGF-beta-independent. J Biol Chem. 2003;278:11721-11728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 154] [Article Influence: 7.0] [Reference Citation Analysis (0)] |