Published online Dec 21, 2012. doi: 10.3748/wjg.v18.i47.7122
Revised: August 13, 2012
Accepted: August 26, 2012
Published online: December 21, 2012
Processing time: 384 Days and 23.4 Hours
Hemobilia is a rare biliary complication of liver transplantation. The predominant cause of hemobilia is iatrogenic, and it is often associated with traumatic operations, such as percutaneous liver intervention, endoscopic retrograde cholangiopancreatography, cholecystectomy, biliary tract surgery, and liver transplantation. Percutaneous transhepatic cholangiography and liver biopsy are two major causes of hemobilia in liver transplant recipients. Hemobilia may also be caused by coagulation defects. It can form intracholedochal hematomas, causing obstructive jaundice. Herein we describe a patient with an intracholedochal hematoma resulting in significant obstructive jaundice after liver transplantation for fulminant hepatic failure. Previous studies have shown that percutaneous transhepatic manipulation is a major cause of hemobilia after liver transplantation, but in our case, percutaneous transhepatic intervention was used to relieve the biliary obstruction and dissolve the biliary clot, with a good outcome.
- Citation: Qin JJ, Xia YX, Lv L, Wang ZJ, Zhang F, Wang XH, Sun BC. Successful disintegration, dissolution and drainage of intracholedochal hematoma by percutaneous transhepatic intervention. World J Gastroenterol 2012; 18(47): 7122-7126
- URL: https://www.wjgnet.com/1007-9327/full/v18/i47/7122.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i47.7122
Fulminant hepatic failure is a syndrome characterized by impaired liver function in an acute setting. It is defined as the onset of hepatic encephalopathy and liver failure within 8 wk of jaundice[1]. Liver transplantation remains the exclusive choice of treatment to improve the survival in the majority of patients with fulminant hepatic failure[2]. Biliary complications following liver transplantation are a major cause of morbidity, but hemobilia is infrequent[3]. Hemobilia may result in intracholedochal hematoma, causing obstructive jaundice. The current report describes a patient with intracholedochal hematoma leading to significant obstructive jaundice after liver transplantation for fulminant hepatic failure.
A 23-year-old female was admitted on July 15, 2010 with complaints of abdominal pain and distension, accompanied by fever, dark yellow urine, and xanthochromia for the past three days. The patient had been treated for psoriasis with traditional Chinese medicine for three months prior to admission. In addition, she had a 16-year history of hepatitis B virus infection. Despite being a carrier of the hepatitis B virus, the patient had no history of hepatitis and liver function tests had been normal during the follow-up.
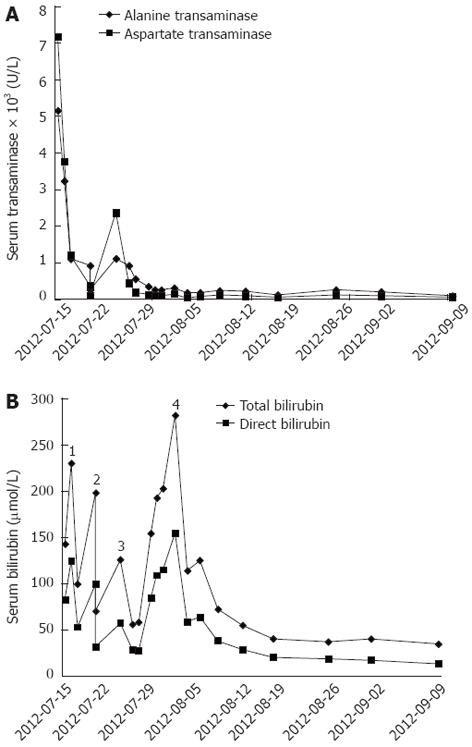
On physical examination, the patient was conscious, and her orientation and calculations were normal. Jaundice was evident. The abdomen was slightly distended and soft, with tenderness and rebound tenderness, and a shifting dullness test was positive. The serum transaminase and total bilirubin levels were markedly elevated on admission. The laboratory test results were as follows: alanine transaminase (ALT), 5190 U/L; aspartate transaminase (AST), 7230 U/L; total bilirubin (TBIL), 142.8 μmol/L; and direct bilirubin (DBIL), 83.3 μmol/L (Figure 1). The prothrombin time (PT) and activated partial thromboplastin time (APTT) were 26.5 s and 41.5 s, respectively. The international normalized ratio (INR) was 2.39. These results suggested significant impairment of coagulation function. In addition, serum hepatitis B virus DNA was present at 4.6 × 107 copies/mL.
The patient was treated with artificial liver support (ALS)-plasma exchange due to diminished liver function and impaired coagulation. Although the laboratory indicators improved (Figure 1), hepatic encephalopathy occurred and worsened progressively. Therefore, fulminant hepatic failure was diagnosed. On July 21, 2010 (post-admission day 7), the patient suffered a coma without pupillary light reflex, although the vital signs were stable. The patient underwent liver transplantation on July 23, 2010. A modified piggyback liver transplantation was carried out, and an end-to-end duct-to-duct anastomosis without a T-tube was performed to reconstruct the biliary tract. A continuous 6-0 polydioxanone suture was used for the posterior wall while interrupted sutures were applied for the anterior wall. She was maintained on a tacrolimus-based immunosuppressive regimen that included mycophenolate mofetil and glucocorticoid.
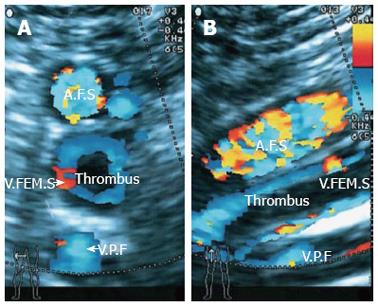
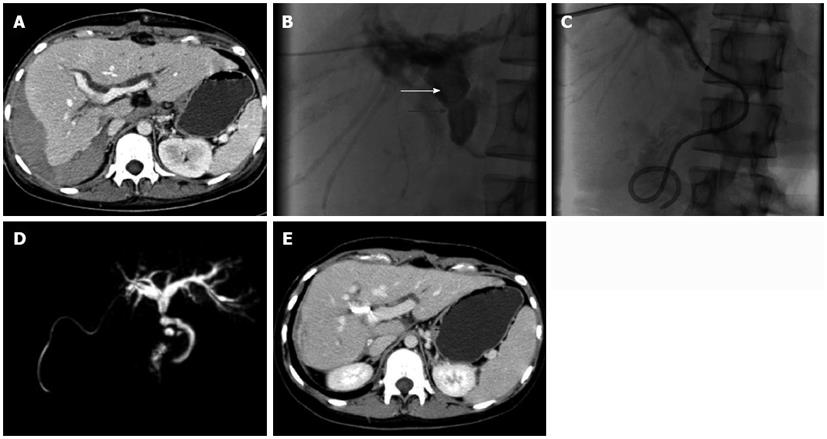
The patient regained consciousness on July 25, 2010 [post-transplantation day (PTD) 2]. The liver and coagulation functions were significantly improved. On PTD 4, the laboratory data were as follows: ALT, 553.8 U/L; AST, 187.4 U/L; TBIL, 58.7 μmol/L; DBIL, 28.5 μmol/L (Figure 1); albumin, 37.2 g/L; PT, 9.2 s; APTT, 21.9 s; INR, 0.83; serum tacrolimus, 12.2 ng/mL; hemoglobin, 100 g/L; and platelet count, 61 × 109/L. Because red blood cells, plasma, and cryoprecipitate were transfused without platelets following a massive intra-operative hemorrhage (approximately 2500 mL), dilutional thrombocytopenia was diagnosed. Platelets were then transfused to treat the thrombocytopenia; however, the right leg became edematous on PTD 3. Doppler ultrasound revealed a thrombus in the femoral vein (Figure 2), which was punctured due to the ALS-plasma exchange. Subsequently, low molecular weight heparin (LMWH) was used to treat the deep venous thrombosis which relieved the edema, but the volume of bloody ascites gradually increased. On PTD 5, the hemoglobin decreased to 77 g/L. Anticoagulation was therefore discontinued and blood was transfused. However, the jaundice became severe again. On PTD 10, the TBIL was 281.2 μmol/L, and the DBIL was 154.7 μmol/L (Figure 1). A computed tomography (CT) scan showed dilation of the intrahepatic biliary ducts and a huge hematoma below the right lobe of liver (Figure 3A). Therefore, obstructive jaundice was suspected.
To confirm the diagnosis of obstructive jaundice and relieve the biliary obstruction, endoscopic retrograde cholangiopancreatography was performed, but failed and caused pancreatitis. So as an alternative, percutaneous transhepatic intervention was administered on PTD 10. Cholangiography showed a long intrabiliary filling defect crossing the strictured biliary anastomosis (Figure 3B). As fecal occult blood testing was positive, hemobilia leading to biliary hematoma was suspected. A 0.018-inch diameter micro-guidewire, a 6F sheath, a 0.038-inch diameter micro-guidewire, and an 8F catheter were manipulated through the hematoma sequentially, making the hematoma break into pieces, enlarging the contact surface with flowing bile and making it easy to dissolve. Then the 8F biliary drainage catheter was placed across the anastomosis with one end opening into the duodenum and the other end exiting the body (Figure 3C). The amount of biliary drainage was approximately 400 mL per day, and the jaundice was markedly relieved 2 d later (TBIL, 114.1 μmol/L; DBIL, 58.9 μmol/L) (Figure 1). The patient recovered well and the jaundice gradually diminished 10 d after percutaneous transhepatic biliary drainage (PTBD); when the TBIL was 55.5 μmol/L, the external end of the biliary drainage catheter was closed. The glucocorticoid was gradually tapered and discontinued on PTD 30. On September 14, 2010 (PTD 54), she experienced no more discomfort and the TBIL was 35.3 μmol/L (Figure 1). The patient was discharged the next day with the biliary drainage catheter in situ and the external port closed.
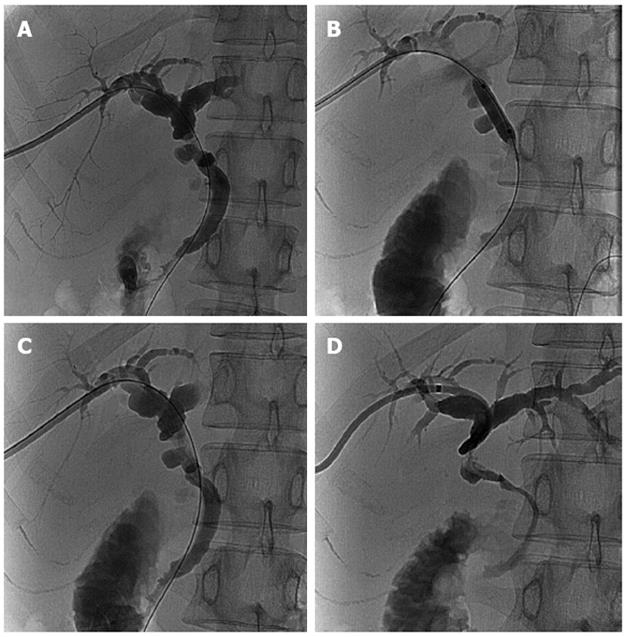
At the first follow-up evaluation on January 29, 2011, magnetic resonance cholangiopancreatography showed the intrahepatic biliary ducts were slightly dilated (Figure 3D). A CT scan revealed that the hematoma below the liver was almost absorbed and the intrahepatic biliary ducts were slightly dilated (Figure 3E). The liver function tests were within normal ranges: TBIL, 11.3 μmol/L; DBIL, 3.5 μmol/L; ALT, 15.0 U/L; AST, 22.1 U/L (Figure 1). The serum tacrolimus was 8.4 ng/mL. Cholangiography was also performed through the biliary drainage catheter, showing that the intrahepatic biliary ducts were slightly dilated and that there was a stricture at the site of the anastomosis (Figure 4A). To prevent a more severe stricture and obstructive jaundice, a balloon dilatation of the anastomotic stricture was performed (Figure 4B) to relieve the stricture (Figure 4C). To avoid the risk of infection and stenosis of the lumen, the biliary drainage catheter was replaced with a new catheter (Figure 4D). In addition, the mycophenolate mofetil was discontinued 6 mo after liver transplantation. The patient was then maintained on immunosuppressive therapy with tacrolimus (3 mg/d). Two weeks later at the second follow-up evaluation, the biliary drainage catheter was removed. During the one-year follow-up, the patient had no discomfort and her liver functions remained normal.
Biliary complications are a major cause of morbidity in liver transplant recipients, with an incidence of 10%-30% and a mortality rate of up to 10%. Biliary leaks and strictures are the most common biliary complications, followed by sphincter of Oddi dysfunction, hemobilia, and biliary obstruction from stones, sludge, casts, or cystic duct mucoceles[3]. Hemobilia is defined as bleeding into the biliary tree. Hemobilia arises when there is communication between a vascular structure and the biliary tree. The predominant cause of hemobilia is iatrogenic, such as percutaneous liver intervention, endoscopic retrograde cholangiopancreatography, cholecystectomy, biliary tract surgery, and liver transplantation[4]. Percutaneous transhepatic cholangiography and liver biopsy are two major causes of hemobilia in liver transplant recipients[3]. In addition, coagulation defects may cause hemobilia[5]. Jolobe[6] reported that hemobilia is a possible cause of jaundice in patients receiving anticoagulants, although hemobilia after liver transplantation is infrequent[3,4]. The LMWH used to treat the thrombosis within the right femoral vein may be the cause of hemobilia in the current case.
The clinical manifestations of hemobilia are determined by the amount and speed of hemorrhage in the biliary tract. Profuse hemobilia often causes severe symptoms with colicky pain and gastrointestinal hemorrhage. In contrast, occult bleeding, which is more frequent, often lacks clinical significance as the blood inconspicuously flows into the intestine. Even if the blood does coagulate within the ducts, the clots are often promptly dissolved by the fibrinolytic activity of bile. Sometimes, the clots may escape dissolution, causing obstructive jaundice[5]. Sandblom et al[7] constructed a model of the biliary tract into which blood could be injected. When hemorrhage is severe and rapid, the blood mixes with the bile and forms mushy clots; when hemobilia is minor and slow, it does not mix with the bile, but flows into the bottom of the system where it forms a solid, pure clot that is a cast of the lumen. These pure clots remain stable, whereas the mixed clots dissolve rapidly. Sandblom et al[5,7] also reported that the fate of clots is related to the bile flow, especially noting that when the clots are exposed to flowing bile, the dissolution is rapid. Our case is very interesting because hemobilia occurred with an abdominal hemorrhage after administration of LMWH, and the biliary clot and the huge subhepatic hematoma formed simultaneously after discontinuation of LMWH. The abdominal hemorrhage was massive, causing the hemoglobin to decrease sharply. The hemobilia, however, was minor because there was no evidence of significant gastrointestinal bleeding.
The principles of managing hemobilia are to resuscitate the patient, control hemorrhage, and maintain biliary patency. Resuscitation with transfusion must be administered to obtain hemodynamic stability when the hemorrhage is severe and rapid. Additionally, coagulopathies should be corrected appropriately. When hemorrhage is active and prolonged, hemostatic interventions should be considered, including transcatheter arterial embolization (TAE), percutaneous thrombin injection, and surgery. TAE is the therapy of choice to achieve hemostasis, with a success rate of 75%-100%[6]; however, TAE may not be appropriate in liver transplant patients because it can cause liver graft dysfunction and biliary ischemia. These complications are devastating, especially in the early post-transplantation period. Croutch et al[8] reported that superselective arterial embolization is a safe treatment for hemobilia caused by PTBD in liver transplant recipients, and emphasized that it is important to use a microcatheter to achieve precise subselective localization and embolization, minimize spasm, and avoid occlusion of noninvolved arterial branches. In spite of this, the cases were all due to PTBD, which often causes small branches to bleed into bile ducts. For cases of hemobilia in anastomosis or due to coagulopathy, TAE may not be appropriate.
If bleeding ceases and a biliary hematoma forms leading to biliary obstruction, it is important to maintain biliary patency. If a T-tube or PTBD is in situ, irrigation may be adequate to relieve obstruction; however, in recent years there has been a shift toward abandoning the use of T-tubes for biliary tract reconstruction because some studies have shown that biliary complications are positively related to T-tubes, such as biliary leakage after T-tube removal, anastomotic strictures, cholangitis, and biliary infections[9]. Endoscopic nasobiliary drainage can also be used to decompress the biliary tract. It has been reported that endoscopic sphincterotomy with balloon extraction of clots could be an effective method to treat biliary clots[4,10]. In addition, thrombolytic agents could be infused using a nasobiliary catheter to dissolve the biliary clots[11]. Although the role of surgery is declining in the management of hemobilia and biliary clots, surgery is still indicated when non-surgical methods are not effective. The principles of surgery for hemobilia are to control the bleeding and clear the biliary tract. Methods involving ligation of the bleeding vessels, extraction of the biliary clots, lavage and biliary tract drainage are often used[4,12].
In our case, percutaneous transhepatic intervention was used to break up the biliary hematoma and relieve bile stasis. During this intervention, a 0.018-inch diameter micro-guidewire, a 6F sheath, a 0.038-inch diameter micro-guidewire, and an 8F catheter were manipulated through the hematoma sequentially, breaking the hematoma up into pieces, enlarging the contact surface with flowing bile and making it easy to dissolve. Our case provides good proof of the principles underlying Sandblom’s clinical and experimental study. Previous studies suggested that percutaneous transhepatic manipulations were major causes of hemobilia after liver transplantation[3], but in our case, percutaneous transhepatic intervention was used to relieve the biliary obstruction and dissolve the biliary clot with a good outcome.
Peer reviewer: Lok ASF, Professor, Division of Gastroenterology, Department of Internal Medicine, University of Michigan, Ann Arbor, MI 48109, United States
S- Editor Song XX L- Editor Logan S E- Editor Zhang DN
| 1. | Trey C, Lipworth L, Chalmers TC, Davidson CS, Gottlieb LS, Popper H, Saunders SJ. Fulminant hepatic failure. Presumable contribution to halothane. N Engl J Med. 1968;279:798-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 96] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Gotthardt D, Riediger C, Weiss KH, Encke J, Schemmer P, Schmidt J, Sauer P. Fulminant hepatic failure: etiology and indications for liver transplantation. Nephrol Dial Transplant. 2007;22 Suppl 8:viii5-viii8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Wojcicki M, Milkiewicz P, Silva M. Biliary tract complications after liver transplantation: a review. Dig Surg. 2008;25:245-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 4. | Chin MW, Enns R. Hemobilia. Curr Gastroenterol Rep. 2010;12:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Sandblom P, Saegesser F, Mirkovitch V. Hepatic hemobilia: hemorrhage from the intrahepatic biliary tract, a review. World J Surg. 1984;8:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Jolobe OM. Hemobilia: a possible cause of jaundice in patients receiving anticoagulants. J Hepatol. 1995;22:597-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Sandblom P, Mirkovitch V, Saegesser F. Formation and fate of fibrin clots in the biliary tract: a clinical and experimental study. Ann Surg. 1977;185:356-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Croutch KL, Gordon RL, Ring EJ, Kerlan RK, LaBerge JM, Roberts JP. Superselective arterial embolization in the liver transplant recipient: a safe treatment for hemobilia caused by percutaneous transhepatic biliary drainage. Liver Transpl Surg. 1996;2:118-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Riediger C, Müller MW, Michalski CW, Hüser N, Schuster T, Kleeff J, Friess H. T-Tube or no T-tube in the reconstruction of the biliary tract during orthotopic liver transplantation: systematic review and meta-analysis. Liver Transpl. 2010;16:705-717. [PubMed] [DOI] [Full Text] |
| 10. | Kroser J, Rothstein RD, Kochman ML. Endoscopic management of obstructive jaundice caused by hemobilia. Gastrointest Endosc. 1996;44:618-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Moparty RK, Brown RD, Layden TJ, Chirravuri V, Wiley T, Venu RP. Dissolution of blood clots in the biliary ducts with a thrombolytic agent infused through nasobiliary catheter. Gastrointest Endosc. 2002;56:436-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Nagata S, Ikegami T, Takeishi K, Toshima T, Sugimachi K, Gion T, Soejima Y, Taketomi A, Maehara Y. Acute biliary obstruction due to a large intracholedochal hematoma after living-donor liver transplantation: Report of a case. Surg Today. 2010;40:474-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |