Published online Dec 21, 2012. doi: 10.3748/wjg.v18.i47.7087
Revised: December 4, 2012
Accepted: December 15, 2012
Published online: December 21, 2012
Processing time: 321 Days and 21.6 Hours
AIM: To improve the outcome of orthotopic transplantation in a mouse model, we used an absorbable gelatin sponge (AGS) in nude mice to establish an orthotopic implantation tumor model.
METHODS: MHCC-97L hepatocellular carcinoma (HCC) cells stably expressing the luciferase gene were injected into the subcutaneous region of nude mice. One week later, the ectopic tumors were harvested and transplanted into the left liver lobe of nude mice. The AGS was used to establish the nude mouse orthotopic implantation tumor model. The tumor suppressor gene, paired box gene 5 (PAX5), which is a tumor suppressor in HCC, was transfected into HCC cells to validate the model. Tumor growth was measured by bioluminescence imaging technology. Semi-quantitative reverse transcription polymerase chain reaction (RT-PCR) and histopathology were used to confirm the tumorigenicity of the implanted tumor from the MHCC-97L cell line.
RESULTS: We successfully developed an orthotopic transplantation tumor model in nude mice with the use of an AGS. The success rate of tumor transplantation was improved from 60% in the control group to 100% in the experimental group using AGS. The detection of fluorescent signals showed that tumors grew in all live nude mice. The mice were divided into 3 groups: AGS-, AGS+/PAX5- and AGS+/PAX5+. Tumor size was significantly smaller in PAX5 transfected nude mice compared to control mice (P < 0.0001). These fluorescent signal results were consistent with observations made during surgery. Pathologic examination further confirmed that the tissues from the ectopic tumor were HCC. Results from RT-PCR proved that the HCC originated from MHCC-97L cells.
CONCLUSION: Using an AGS is a convenient and efficient way of establishing an indirect orthotopic liver transplantation tumor model with a high success rate.
- Citation: Zhao GJ, Xu LX, Chu ES, Zhang N, Shen JY, Damirin A, Li XX. Establishment of an orthotopic transplantation tumor model of hepatocellular carcinoma in mice. World J Gastroenterol 2012; 18(47): 7087-7092
- URL: https://www.wjgnet.com/1007-9327/full/v18/i47/7087.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i47.7087
Hepatocellular carcinoma (HCC) is one of the most common types of malignant tumors[1-3]. Thus, establishing appropriate animal models is critical to promote our understanding of the molecular, cellular and pathophysiological mechanisms of HCC, and is essential for the development of new therapeutic strategies. Most HCC patients are diagnosed at an advanced stage when available treatments are ineffective. The ideal HCC animal model should display similarities to the human disease and accurately recapitulate the disease with a high success rate[4]. Currently, the most commonly employed models of HCC are xenograft models, including subcutaneous and orthotopic transplantation in nude mice[5-9]. Orthotopic transplantation of liver tumors allows the development of tumor metastases and provides relevant sites for host-tumor interaction and a microenvironment. Although this method is well accepted, its use is still quite limited. This is because construction of the model is complex, and a high level of technical skill is required. This model would be more popular if the surgical procedure could be simplified.
The goal of this study was to investigate the value of using an absorbable gelatin sponge (AGS) in HCC orthotopic transplantation, compared with the routine procedure. Previous research has shown that paired box gene 5 (PAX5) acts as a tumor suppressor in the development of HCC[10]. Therefore, in this experiment, we also overexpressed PAX5 in the xenograft tumor to validate the efficiency of the liver orthotopic transplantation tumor model in real time.
The human hepatocellular carcinoma cell line MHCC97L (a kind gift from the Liver Cancer Institute, Fudan University) was used in this study. Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM, Gibco BRL, Rockville, MD, United States) with 10% fetal bovine serum (Life Technologies, Carlsbad, CA, United States), 100 mg/mL penicillin G, and 50 μg/mL streptomycin (Life Technologies) at 37 °C in a humidified atmosphere containing 5% CO2. MHCC97L cells stably expressing the luciferase gene were constructed.
Complementary DNA corresponding to full-length PAX5 was obtained by reverse transcription-polymerase chain reaction (RT-PCR) amplification of normal human stomach cDNA with primers specific to PAX5. The PCR aliquots were subcloned into the mammalian expression vector pcDNA3.1-TOPO TA (Life Technologies)[11]. Either pcDNA3.1 or pcDNA3.1-PAX5 was transfected into MHCC97L cells using Lipofectamine 2000 (Life Technologies). Positive clones were selected in DMEM supplemented with G418 at a concentration of 500 μg/mL.
MHCC97L cells (1 × 107 cells in 0.1 mL PBS) transfected with PAX5 or pcDNA3.1 were subcutaneously injected into the dorsal left flank of 4-wk-old male Balb/c nude mice (1/group); the body weight of all mice was between 12 and 15 g. Six-week-old athymic male Balb/c nude mice weighing 16-18 g were used for xenograft tumor transplantation. The nude mice were obtained from the Chinese University of Hong Kong Laboratory Animal Service Center and maintained under specific pathogen free (SPF) conditions. All experimental procedures were approved by the Animal Ethics Committee of the Chinese University of Hong Kong.
An orthotopic HCC mouse model was established to determine intrahepatic tumorigenicity. Subcutaneous tumors were harvested when they reached 1 cm in diameter and were cut into pieces under aseptic conditions. After washing with PBS, the tumors were rinsed in DMEM. Only intact pieces were chosen for further study. After removing thanatosis tissues, the tumors were cut into cubes 1 mm3 in size. One piece was then implanted into the left liver lobe of each mouse. The mice were divided into 3 groups. The ten mice in group I were operated without an AGS. The wounds were sutured using VICRYL Plus Suture 5-O (Ethicon Inc, Somerville, NJ, United States) Absorbable gelatin sponges were used in groups II and III. The mice in groups II (n = 6) and III (n = 6) underwent tumor transplantation with or without PAX5 expression. Intraperitoneal anesthesia was administered using 20 g/L pentobarbital sodium at 0.04 mL per gram body weight. Once anesthetized, the mice were fixed on an experiment board in the supine position. After sterilizing with 70% alcohol, a 2 mm transverse incision was made below the xiphoid, which was perpendicular to the median line and was 1-1.5 cm long. The left liver lobes were carefully pulled out of the abdominal cavity with a sterile cotton swab. A 0.2 cm incision was made in Glisson’s capsule to serve as the transplantation site. The tumor tissue mentioned above was transplanted into recipient mouse livers using extra-fine forceps.
In group I, a routine procedure was carried out to suture the incision in the liver and stop bleeding, and homoeostasis was achieved by compression. In group II and group III, in addition to compression, the transplantation site was further covered by a 5 mm × 5 mm × 1.5 mm AGS (Nanjing Jinglin Biopharming Co. Ltd., Nanjing, Jiangsu, China). The liver was placed back into the enterocoelia using a sterile cotton swab, and shifting of the AGS was not allowed. If neither bleeding nor tumor tissue leakage occurred, then the skin was sterilized with 70% alcohol and the wound sutured with a Plus 5-0 suture line. The average operation time was 10 min. Following surgery, the mice were kept warm using a heat lamp and allowed to recover. The recovery period was reduced from 30 ± 5 min to 20 ± 5 min at room temperature. Following recovery, the mice were put back into their cages and raised under SPF conditions. All mice had free access to sterilized food and autoclaved water. We inspected the living conditions of the mice daily.
For bioluminescence imaging, the animals were injected with the luciferase substrate D-luciferin at a dose of 150 mg/kg in 0.2 mL sterile isotonic saline.
Liver tumor growth rates were monitored using the Xenogen International Veterinary Information Service (IVIS) imaging system every week for 3 wk. All images were obtained after intraperitoneal injection of luciferin (100 mg/kg body weight; Synchem, Elk Grove Village, IL, United States). Ten minutes after injection of luciferin, nude mice were placed onto the Xenogen IVIS 200 imaging stage and were continuously sedated during image acquisition. Image analysis and bioluminescence quantification were performed using Living Image software (Caliper Life Sciences, Hopkinton, MA, United States). Once imaging was complete, each animal was removed from the Xenogen IVIS 200 imaging stage, placed on a heated platform in its original cage and allowed to recover. After the animals had fully recovered from the anesthesia, they were returned to the specific investigator.
After 3 wk, the mice were sacrificed. Following tumor excision from the euthanized mice, tumor weights were measured the same day. A portion of the tumor tissue was fixed in 10% formalin for subsequent histological examination, and the remaining tissue was snap-frozen in liquid nitrogen and stored at -70 °C for molecular studies.
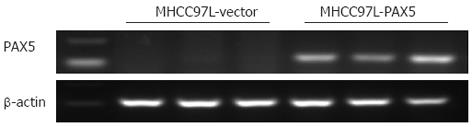
Total RNA was extracted from tissues using QIAzol reagent (Qiagen, Valencia, CA, United States). cDNA was synthesized from 2 μg total RNA using Transcriptor Reverse Transcriptase (Roche Applied Sciences, Indianapolis, IN, United States). Semi-quantitative RT-PCR analyses were performed using PAX5 forward primer 5’-GTCCATTCCATCAAGTCCTG-3’ and PAX5 reverse primer 5’-TTGCTGACACAACCATGGCT-3’. β-actin was used as an internal control for mRNA expression (5’-GTCTTCCCCTCCATCGTG-3’ and 5’-AGGGTGAGGATGCCTCTCTT-3’).
Formalin-fixed tumor was embedded in paraffin, and 4 μm sections were cut and stained with hematoxylin and eosin (HE) as previously described[12].
Data were presented as mean ± SD. A repeated measures analysis of variance was used to compare the differences between groups. All analyses were performed using SPSS software (version 13.0, Chicago, IL, United States). Statistical significance was accepted at the level of P < 0.05.
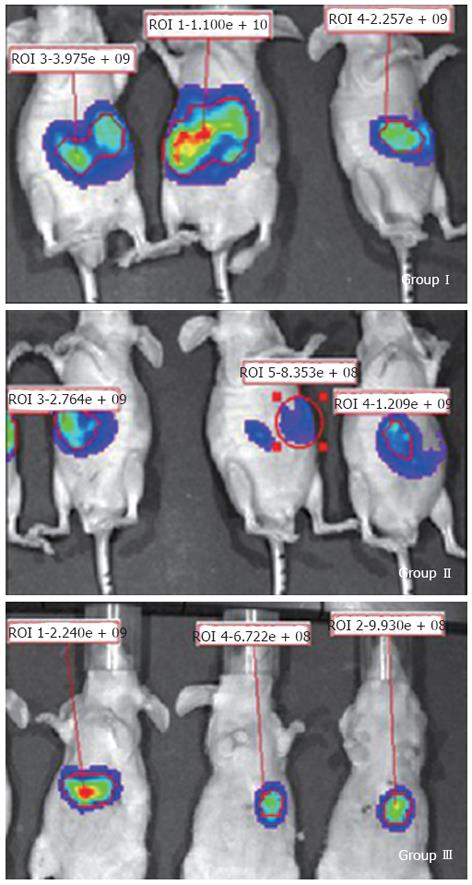
One day after surgery, only six of ten mice in group I (without AGS) were alive after transplantation. The survival rate using the routine suture method was only 60%. In contrast, all mice in groups II and III were alive and recovered well. From the 7th day after surgery, the nude mice were scanned in the Xenogen IVIS 100 imaging system for a total of 3 wk. Fluorescence signals showed that transplanted tumors grew in all nude mice (Figure 1).
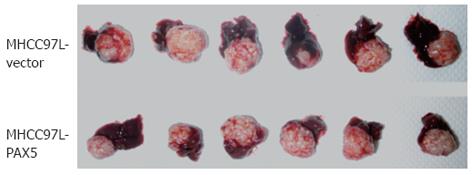
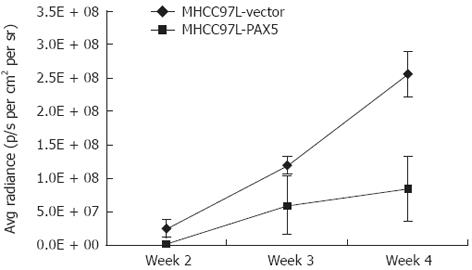
The fluorescence signal intensity was related to tumor size. The signal intensity steadily increased from the second week to the fourth week. Tumor size was significantly smaller in the PAX5-transfected nude mice from group III compared to the vector control mice from group II (P < 0.0001) (Figure 2). This indicated that PAX5 gene expression inhibited tumor growth in nude mice (Figure 3). The area around the tumor and the surrounding tissues was euangiotic, and adjacent organs were strongly adhered.
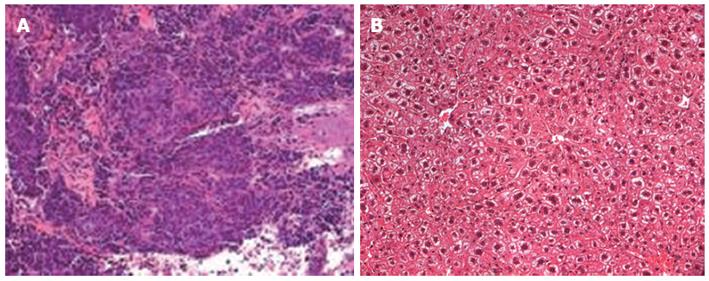
HE staining further confirmed tumorigenesis of the liver tissue (Figure 4). Tumor tissue characteristics included atypia, big core, dark color, different cell size, irregular form, and nuclear division. Many of these characteristics were similar to those of the original cancer cells.
RT-PCR results showed that no PAX5 expression was observed in the tissue from group II, and all the tissues from group III had a high PAX5 mRNA level. This demonstrated that the HCC cells originated from MHCC-97L cells transfected with or without the PAX5 expression plasmid (Figure 5), and the orthotopic liver tumor model was successfully established. We did not detect any intrahepatic or abdominal metastases.
Currently, the most common way of producing liver orthotopic tumor models is either by implantation of an established subcutaneous tumor harvested from another nude mouse, or by injecting tumor cells directly into the liver[6,7]. Both strategies require surgery following liver exposure. The tumor cell injection animal model is more relevant to clinical practice; thus, it can be a useful research model for lymph node metastasis[13]. However, the technical difficulty associated with this model is that it is hard to control tumor cell leakage during injection. Analyses are complicated by the fact that apparent metastasis may just be due to leakiness.
In the second method, the goal is to establish a subcutaneous model of human HCC in nude mice, and then to inoculate the liver in order to establish the orthotopic transplantation model. The principle is to make the HCC cells adapt to the environment and grow into tumor tissues, then implant the tumor into the host’s liver. Thus, the survival rate of the transplanted tumor is supposed to increase.
Compared with subcutaneous and intraperitoneal xenografts in nude mice, the properties of invasion and metastasis in the orthotopic liver tumor model are better[14-16]. Use of this model is limited due to the need for a highly complex surgical technique, especially in bleeding control. Routine surgery using incision suture takes a long time. This is one of the reasons why the mice in group I had a high mortality rate. If post-operative hemorrhage occurred, the mice would probably die, leading to failure of the long-term experiment. Thus the efforts to obtain the cell culture, establish a subcutaneous tumor and orthotopic tumor implantation would be in vain. An alternative method of achieving homeostasis is by suturing Glisson’s capsule at the transplantation site. However, the mouse liver has an abundant blood supply, and the tissue is fragile and easily crushed. The method is further complicated by the possibility of bleeding at the suture site. Therefore, not many laboratory staff are capable of performing this procedure. To circumvent these difficulties, we used the AGS. The AGS is commonly used in surgery to stop minor bleeding at the surgical site. It is an aseptic solid material that has good deformability. In our experiment, covering the transplantation site with an AGS was an easy and effective way of maintaining homeostasis. The sponge was slowly absorbed and the cost was minimal. In addition, the sponge prevented the implanted tumor from moving out of the host’s liver, increasing the success rate of the experiment. This procedure was safe, and none of the nude mice experienced post-operative complications or mortality, compared with the high post-operative mortality of 40% in group I. Importantly, adverse reactions to the sponge were not observed in normal tissues, as evaluated by HE staining. The gelatin was gradually absorbed and was absent when the mice were sacrificed.
The horizontal incision made during the operation should neither be too low nor too small. The optimal incision should be as small as possible to fully expose the left liver lobe. If it is too small, the liver could be crushed when pulled out, or there may not be enough space to place the AGS into the abdominal cavity. The size of the sponge should be appropriate-5 mm × 5 mm × 1.5 mm, enough to efficiently stop bleeding and be easily absorbed.
It should be noted that although we observed a high post-operative mortality in group I, all mice achieved homeostasis before closure of the abdominal cavity. One of the reasons for this high mortality may have been due to the fast post-operative recovery and frequent activities caused by pain. Administration of post-operative analgesics may reduce pain, thus might lead to a higher survival rate[17-19]. However, analgesics were not administered in this study. We would also like to point out that in models established by the direct injection of cultured human HCC cells, an AGS may not prevent leakage. In such models, HCC cells are suspended in medium rather than forming a solid tumor. The sponge is hygroscopic, thus, if leakage occurs, the sponge would only aggravate the leak.
Post-operative evaluation of orthotopic tumors is also a challenge. Monitoring subcutaneous liver transplantation tumors may be performed either by touch or by visual inspection. Orthotopic tumors can be monitored by laparotomy, computed tomography (CT)/magnetic resonance imaging (MRI)/positron emission tomography (PET)-CT scanning, or using a high frequency ultrasound mini-probe. However, there are drawbacks associated with each method. Frequent laparotomy may cause severe injury and death in mice. CT/MRI/PET-CT scanning is non-invasive, but the cost is high and is difficult to perform[20-23]. Ultrasonic examination is less expensive, but analysis of the data requires a skilled operator[24-26]. Finally, only some of the above-mentioned methods are quantitative.
Using the IVIS imaging system, bioluminescence was non-invasively measured in transplanted subcutaneous tumors using the IVIS imaging system (Xenogen Corp, Alameda, CA, United States) following injection of the luciferase substrate luciferin. Tumor growth was continuously monitored. According to the bioluminescence data, tumor growth conditions were analyzed in real time. The results showed successful tumor transplantation. Histopathology and PCR analyses showed that the transplanted tumor had the same phenotype as the liver tumor cell line MHCC-97L. Thus, the orthotopic transplantation tumor model was successfully established from the subcutaneous model of human HCC.
Based on our procedure, this operation has been simplified and can be completed in a very short period of time. The success rate is high, and reproducibility is good. Importantly, the model maintained the characteristics of the original cell line. Inspections were quantified in real time, and the model can be adapted for general use.
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors and is the third leading cause of cancer-related death. The establishment of appropriate animal models of this disease could help people to understand the mechanisms driving HCC, leading to potential therapeutics and improvements in patient survival. An absorbable gelatin sponge was used as an efficient way of establishing an indirect orthotopic liver transplantation tumor model with a high success rate.
It is reported that the conventional surgical method involves the liver capsule being sutured using microsurgery. Compared with the microsurgical method, the authors used an absorbable gelatin sponge which prevented bleeding and tissues being crushed during the experiment.
This is the first report on the use of an absorbable gelatin sponge to cover the transplantation site in the liver capsule. This experiment showed that the absorbable gelatin sponge can be used to constrict the liver and stop bleeding, and to efficiently cover tumor tissue, thus, avoiding the tumor tissue moving out of the placement site.
Using the absorbable gelatin sponge to establish the HCC model is a convenient method for researchers to study HCC. The establishment of an animal model could provide a new way to perform more successful, convenient and efficient experiments.
An absorbable gelatin sponge is a sterile, absorbable, water-insoluble, gelatin-base material which absorbs blood and provides an area for clot formation, and is used for hemostasis during surgery.
Authors found a new technique to do HCC animal model experiment. It is easy to perform and has a high success rate. The study has been well conducted and provided further validation.
Peer reviewers: Ming Kuang, Department of Hepatobiliary Surgery, Sun Yat-sen University/Institute of Diagnostic and Interventional Ultrasound, Affiliated Hospital 1, Guangzhou 510080, Guangdong Province, China; Alberta Cappelli, Azienda Osped University Bologna, Unita Operat Radiol Malpighi, Policlin St Orsola Malpighi, Via Albertoni 15, I-40138 Bologna, Italy
S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Zhao J, Zhang X, Shi M, Xu H, Jin J, Ni H, Yang S, Dai J, Wu M, Guo Y. TIP30 inhibits growth of HCC cell lines and inhibits HCC xenografts in mice in combination with 5-FU. Hepatology. 2006;44:205-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11834] [Article Influence: 845.3] [Reference Citation Analysis (4)] |
| 3. | Coleman WB. Mechanisms of human hepatocarcinogenesis. Curr Mol Med. 2003;3:573-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 172] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 4. | Li Y, Tian B, Yang J, Zhao L, Wu X, Ye SL, Liu YK, Tang ZY. Stepwise metastatic human hepatocellular carcinoma cell model system with multiple metastatic potentials established through consecutive in vivo selection and studies on metastatic characteristics. J Cancer Res Clin Oncol. 2004;130:460-468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 173] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 5. | Elinav E, Abd-Elnabi A, Pappo O, Bernstein I, Klein A, Engelhardt D, Rabbani E, Ilan Y. Suppression of hepatocellular carcinoma growth in mice via leptin, is associated with inhibition of tumor cell growth and natural killer cell activation. J Hepatol. 2006;44:529-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Wu L, Tang ZY, Li Y. Experimental models of hepatocellular carcinoma: developments and evolution. J Cancer Res Clin Oncol. 2009;135:969-981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Li ZY, Ni S, Yang X, Kiviat N, Lieber A. Xenograft models for liver metastasis: Relationship between tumor morphology and adenovirus vector transduction. Mol Ther. 2004;9:650-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Li Y, Tang Y, Ye L, Liu B, Liu K, Chen J, Xue Q. Establishment of a hepatocellular carcinoma cell line with unique metastatic characteristics through in vivo selection and screening for metastasis-related genes through cDNA microarray. J Cancer Res Clin Oncol. 2003;129:43-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 188] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 9. | Yang J, Qin LX, Li Y, Ye SL, Liu YK, Gao DM, Chen J, Tang ZY. Molecular cytogenetic characteristics of the human hepatocellular carcinoma cell line HCCLM3 with high metastatic potential: comparative genomic hybridization and multiplex fluorescence in situ hybridization. Cancer Genet Cytogenet. 2005;158:180-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Liu W, Li X, Chu ES, Go MY, Xu L, Zhao G, Li L, Dai N, Si J, Tao Q. Paired box gene 5 is a novel tumor suppressor in hepatocellular carcinoma through interaction with p53 signaling pathway. Hepatology. 2011;53:843-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Li X, Cheung KF, Ma X, Tian L, Zhao J, Go MY, Shen B, Cheng AS, Ying J, Tao Q. Epigenetic inactivation of paired box gene 5, a novel tumor suppressor gene, through direct upregulation of p53 is associated with prognosis in gastric cancer patients. Oncogene. 2012;31:3419-3430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Yu J, Hui AY, Chu ES, Cheng AS, Go MY, Chan HL, Leung WK, Cheung KF, Ching AK, Chui YL. Expression of a cyclo-oxygenase-2 transgene in murine liver causes hepatitis. Gut. 2007;56:991-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Ogawa K, Nakanishi H, Takeshita F, Futakuchi M, Asamoto M, Imaida K, Tatematsu M, Shirai T. Establishment of rat hepatocellular carcinoma cell lines with differing metastatic potential in nude mice. Int J Cancer. 2001;91:797-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Massazza G, Tomasoni A, Lucchini V, Allavena P, Erba E, Colombo N, Mantovani A, D'Incalci M, Mangioni C, Giavazzi R. Intraperitoneal and subcutaneous xenografts of human ovarian carcinoma in nude mice and their potential in experimental therapy. Int J Cancer. 1989;44:494-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Niederberger M, DeLozier-Blanchet CD, Hedinger CE, Walt H. Differences between subcutaneous and intraperitoneal forms of three human testicular teratocarcinomas in nude mice. Cancer. 1988;61:1571-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Rodolfo M, Balsari A, Clemente C, Parmiani G, Fossati G. Tumorigenicity and dissemination of primary and metastatic human melanomas implanted into different sites in athymic nude mice. Invasion Metastasis. 1988;8:317-331. [PubMed] |
| 17. | Miller AL, Wright-Williams SL, Flecknell PA, Roughan JV. A comparison of abdominal and scrotal approach methods of vasectomy and the influence of analgesic treatment in laboratory mice. Lab Anim. 2012;46:304-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Wright-Williams SL, Courade JP, Richardson CA, Roughan JV, Flecknell PA. Effects of vasectomy surgery and meloxicam treatment on faecal corticosterone levels and behaviour in two strains of laboratory mouse. Pain. 2007;130:108-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Leach MC, Klaus K, Miller AL, Scotto di Perrotolo M, Sotocinal SG, Flecknell PA. The assessment of post-vasectomy pain in mice using behaviour and the Mouse Grimace Scale. PLoS One. 2012;7:e35656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 20. | Ueno T, Imaida K, Yoshimoto M, Hayakawa T, Takahashi M, Imai T, Yanaka A, Tsuta K, Komiya M, Wakabayashi K. Non-invasive X-ray Micro-computed Tomographic Evaluation of Indomethacin on Urethane-induced Lung Carcinogenesis in Mice. Anticancer Res. 2012;32:4773-4780. [PubMed] |
| 21. | Johnston SM, Johnson GA, Badea CT. Temporal and spectral imaging with micro-CT. Med Phys. 2012;39:4943-4958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Burrell JS, Bradley RS, Walker-Samuel S, Jamin Y, Baker LC, Boult JK, Withers PJ, Halliday J, Waterton JC, Robinson SP. MRI measurements of vessel calibre in tumour xenografts: Comparison with vascular corrosion casting. Microvasc Res. 2012;84:323-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Greco A, Fiumara G, Gargiulo S, Gramanzini M, Brunetti A, Cuocolo A. High-resolution PET/CT imaging of the mouse heart. Exp Physiol. 2012;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Nguyen TM, Nakamura H, Wakabayashi A, Kanagawa T, Koyama S, Tsutsui T, Hamasaki T, Kimura T. Estimation of mouse fetal weight by ultrasonography: application from clinic to laboratory. Lab Anim. 2012;46:225-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Niu L, Qian M, Song R, Meng L, Liu X, Zheng H. A 2D non-invasive ultrasonic method for simultaneous measurement of arterial strain and flow pattern. Clin Physiol Funct Imaging. 2012;32:323-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Carpenter AR, Becknell B, Ingraham SE, McHugh KM. Ultrasound imaging of the murine kidney. Methods Mol Biol. 2012;886:403-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |