Published online Dec 21, 2012. doi: 10.3748/wjg.v18.i47.7026
Revised: September 29, 2012
Accepted: October 30, 2012
Published online: December 21, 2012
AIM: To quantitatively assess the ability of double contrast-enhanced ultrasound (DCUS) to detect tumor early response to pre-operative chemotherapy.
METHODS: Forty-three patients with gastric cancer treated with neoadjuvant chemotherapy followed by curative resection between September 2011 and February 2012 were analyzed. Pre-operative chemotherapy regimens of fluorouracil + oxaliplatin or S-1 + oxaliplatin were administered in 2-4 cycles over 6-12 wk periods. All patients underwent contrast-enhanced computed tomography (CT) scan and DCUS before and after two courses of pre-operative chemotherapy. The therapeutic response was assessed by CT using the response evaluation criteria in solid tumors (RECIST 1.1) criteria. Tumor area was assessed by DCUS as enhanced appearance of gastric carcinoma due to tumor vascularity during the contrast phase as compared to the normal gastric wall. Histopathologic analysis was carried out according to the Mandard tumor regression grade criteria and used as the reference standard. Receiver operating characteristic (ROC) analysis was used to evaluate the efficacy of DCUS parameters in differentiating histopathological responders from non-responders.
RESULTS: The study population consisted of 32 men and 11 women, with mean age of 59.7 ± 11.4 years. Neither age, sex, histologic type, tumor site, T stage, nor N stage was associated with pathological response. The responders had significantly smaller mean tumor size than the non-responders (15.7 ± 7.4 cm vs 33.3 ± 14.1 cm, P < 0.01). According to Mandard’s criteria, 27 patients were classified as responders, with 11 (40.7%) showing decreased tumor size by DCUS. In contrast, only three (18.8%) of the 16 non-responders showed decreased tumor size by DCUS (P < 0.01). The area under the ROC curve was 0.64, with a 95%CI of 0.46-0.81. The effects of several cut-off points on diagnostic parameters were calculated in the ROC curve analysis. By maximizing Youden’s index (sensitivity + specificity - 1), the best cut-off point for distinguishing responders from non-responders was determined, which had optimal sensitivity of 62.9% and specificity of 56.3%. Using this cut-off point, the positive and negative predictive values of DCUS for distinguishing responders from non-responders were 70.8% and 47.4%, respectively. The overall accuracy of DCUS for therapeutic response assessment was 60.5%, slightly higher than the 53.5% for CT response assessment with RECIST criteria (P = 0.663). Although the advantage was not statistically significant, likely due to the small number of cases assessed. DCUS was able to identify decreased perfusion in responders who showed no morphological change by CT imaging, which can be occluded by such treatment effects as fibrosis and edema.
CONCLUSION: DCUS may represent an innovative tool for more accurately predicting histopathological response to neoadjuvant chemotherapy before surgical resection in patients with locally-advanced gastric cancer.
- Citation: Ang J, Hu L, Huang PT, Wu JX, Huang LN, Cao CH, Zheng YX, Chen L. Contrast-enhanced ultrasonography assessment of gastric cancer response to neoadjuvant chemotherapy. World J Gastroenterol 2012; 18(47): 7026-7032
- URL: https://www.wjgnet.com/1007-9327/full/v18/i47/7026.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i47.7026
Neoadjuvant chemotherapy (NAC) is a particularly promising component of the current multidisciplinary model for treating locally-advanced gastric cancer. Recent studies have shown that pre-operative NAC can increase the likelihood of curative resection, thereby improving long-term survival[1]. However, in cases where patients prove unresponsive to the pre-operative NAC regimen, the result is higher risk of tumor progression and death since the surgical treatment is delayed. Thus, in contrast to a post-operative NAC regimen, pre-operative NAC requires an accurate, non-invasive technique to assess short-term therapeutic response. Such a technique should not only identify unresponsive cases in a timely manner to initiate individualized treatment options but also provide alternative endpoints for identifying non-responders and prognostic parameters for assessing individual prognosis[2].
Currently, computed tomography (CT) is a key method for evaluating chemotherapy response in patients with advanced gastric cancer. The parameters of CT imaging to assess tumor response to treatment are well defined and established in the standard workup for gastric cancer staging[3]. The response evaluation criteria in solid tumors (RECIST 1.1) were developed in 2009 as a robust and standardized guideline for clinical assessment of tumor response to treatment[4]. Unfortunately, the morphologic imaging techniques, including CT, magnetic resonance image (MRI) and endoscopic ultrasonography (EUS), have limited accuracy for detecting residual tumorous tissue within chemotherapy-treated areas due to occlusion by chemotherapy-induced fibrosis[3,4].
Double contrast-enhanced ultrasound (DCUS) was recently developed as a complementary tool for the existing imaging modalities to help improve assessment of gastric cancer[5,6]. In clinical application of this technology, use of an oral ultrasound contrast agent reveals the three-layered structure of the gastric wall, while use of intravenous contrast reveals the dynamic features of tumor vascularity. Thus, patient evaluation with double contrast provides qualitative and quantitative measures by which changes in gastric cancer pathophysiology may be evaluated and used to determine a patient’s prognosis or treatment responsiveness[5]. In addition, DCUS is superior to the traditional staging methods for gastric cancer since it can assess the depth of tumor penetration and the presence of lymph node metastases. However, no study to date has systematically evaluated the DCUS parameters for differentiating histopathological responders from non-responders after NAC and prior to surgical treatment.
The purpose of this study was to determine whether DCUS parameters are able to differentiate histopathological responders from non-responders early in the NAC course of treatment. In addition, the predictive value of DCUS was compared with the routine CT imaging technique.
Forty-five consecutive patients who underwent pre-operative NAC followed by curative resection at the Second Affiliated Hospital at Zhejiang University College of Medicine between September 2011 and February 2012 were enrolled in the study. NAC was recommended for patients according to the following criteria: (1) a diagnosis of histologically proven gastric adenocarcinoma; (2) clinical stage of T4a or greater, and/or any T stage with lymph node metastasis; (3) Eastern Cooperative Oncology Group performance status of 2 or less; (4) adequate organ function; and (5) no active concomitant malignancy. All patients underwent pre-operative staging by both CT and DCUS. During the NAC treatment period, one of the study participants developed an irresectable tumor and a second developed metastatic disease; both of these patients were removed from the study and excluded from analysis.
The response to chemotherapy was evaluated for each patient after two courses of chemotherapy had been completed, according to the RECIST criteria. For cases showing partial response (PR), one or two more courses were administered or the patient underwent surgical resection. The study was carried out with pre-approval by the institute’s Medical Ethics Committee. All subjects provided written informed consent prior to study participation.
Two NAC regimens were randomly used: either fluorouracil + oxaliplatin (FOLFOX) or S-1 + oxaliplatin (SOX) were administered for 2-4 cycles (as noted above) over a period of 6-12 wk. The fluorouracil was given on day 1 of each cycle at 400 mg/m2 IVP, followed by 2400 mg/m2 IVCI over 48 h. The S-1 was given at 80 mg/m2 per day for 14 d, and then repeated three weeks later. For both regimens, the oxaliplatin was given on day 1 of each cycle at 130 mg/m2 IV over 2 h.
CT scanning and DCUS were performed before and after two courses of the pre-operative NAC (either FOLFOX or SOX). For contrast-enhanced abdominal CT (Somatom Definition AS scanner; Siemens Medical Solutions, Forchheim, Germany), patients were fasted for six hours and given a 450 mL bolus of pure water immediately before the CT scanning to induce gastric distention. Contrast medium [OmnipaqueTM (iohexol); GE Healthcare, Cork, Ireland] was delivered intravenously at a rate of 3 mL/s by using an automatic injector to achieve a total injection volume of 2 mL/kg. For DCUS, the Sequoia 512 Acuson sonographic system (Siemens Medical Solutions) equipped with CadenceTM contrast pulse sequencing visualization technology and a 4V1 vector transducer to deliver low acoustic pressure frequencies (1.0-4.0 MHz) was used. Oral contrast agent and intravenous contrast medium were administered sequentially, as previously described[5,6]. Briefly, the patients drank the Xinzhang oral contrast agent (Huqingyutang, Zhejiang, China; http://dazzy007.cn.makepolo.com/product/8891276.html) diluted in 500 mL of warm water to distend the stomach immediately before the procedure. Each dose of the microvesicle intravenous contrast medium (SonoVue, Bracco, Italy) was dissolved in 5 mL of saline and a 2.4 mL bolus was injected into the patient’s superficial elbow vein.
CT and DCUS images were interpreted and reviewed by two independent diagnostic specialists, each with more than 10 years of experience in gastric imaging. All specialists were blinded to the histological findings and the same-sample findings from the other technique for evaluating the therapeutic response. The therapeutic response indicated by CT assessment was classified using the RECIST 1.1 criteria[4] as: complete response, PR, stable disease, or progressive disease. Tumor area was assessed by DCUS as enhanced appearance of gastric carcinoma due to tumor vascularity during the contrast phase as compared to the normal gastric wall. Thus, the DCUS detected NAC response was evaluated according to: (1) the static change of ultrasonic echo; and (2) the dynamic (real-time) assessment of tumor vascularity and lymph nodes.
Surgery was performed between weeks 3 and 5 after the completion of pre-operative NAC. All surgical procedures were carried out using an open laparotomy approach. The resectability of the tumors, the extent of lymph node dissection, and the type of gastrectomy procedure were determined according to the perioperative observations. The gastrectomy procedure (total or subtotal) was selected based on the location and extent of the primary lesion. For successful resection, the resection lines had to be at least 5 cm from the edge of the macroscopic tumor. D2 (extended) or D3/D4 (super-extended) lymphadenectomies were performed according to the guidelines of the Japanese Research Society for Gastric Cancer (14th ed).
Pathological findings served as the reference standard for all patients. The pathological response to NAC was evaluated according to the criteria of Mandard’s tumor regression grade, which was based on the percentage of viable residual tumor cells in relation to fibrosis/necrosis[7]. Patients with TRG1-2 were defined as responders, while patients with TRG3-5 were defined as non-responders.
All statistical analysis were carried out using SPSS software (version 16.0 for Windows; Chicago, IL, United States). The χ2 or Fisher’s exact tests were used to determine the significance of associations between pathologic findings and categorical variables. Receiver operating characteristic (ROC) curves were constructed to evaluate the ability of DCUS to identify a histopathological therapeutic response, with the area under the curves and the corresponding 95%CIs being calculated. The effects of several cut-off points on diagnostic parameters were determined by the ROC curve analysis. To compare the CT and DCUS procedures, the summary accuracy measure of Youden’s index (sensitivity + specificity - 1) was used. By maximizing Youden’s index, the best cut-off for distinguishing pathological responders from non-responders was identified. All tests were two-sided with P values of < 0.05 considered as indicating statistical significance.
The study population consisted of 32 men and 11 women, with a mean age of 59.7 ± 11.4 years (range: 34-79 years). The baseline patient and tumor characteristics are summarized in Table 1, with patients stratified according to the status as histopathological responders or non-responders.
| Characteristic | Assessable patients with Mandard’s TRG | P value | |
| Histopathologic responders | Histopathologic non-responders | ||
| No. | 27 | 16 | |
| Age (yr), mean (range) | 68.7 (34-75) | 62.2 (39-79) | 0.370 |
| Gender | 0.429 | ||
| Male | 19 | 13 | |
| Female | 8 | 3 | |
| Histological type | 0.934 | ||
| Well differentiated | 4 | 2 | |
| Moderately differentiated | 6 | 5 | |
| Poorly differentiated | 12 | 5 | |
| Signet ring cell type | 5 | 4 | |
| Tumour size, cm (mean ± SD) | 15.7 ± 7.4 | 33.3 ± 14.1 | 0.003 |
| Tumour site | 0.376 | ||
| Fundus and cardia | 3 | 4 | |
| Body | 7 | 5 | |
| Antrum and pylorus | 17 | 7 | |
| Pathological T classification | 0.475 | ||
| T4a | 22 | 13 | |
| T4b | 5 | 3 | |
| Pathological N classification | |||
| N- | 8 | 4 | 0.744 |
| N+ | 19 | 12 | |
| Chemotherapy | |||
| FOLFOX | 15 | 9 | 0.965 |
| SOX | 12 | 7 | |
| Chemotherapy cycle | 0.432 | ||
| 2 | 12 | 4 | |
| 3 | 12 | 10 | |
| 4 | 3 | 2 | |
Neither age, sex, histologic type, tumor site, T stage, nor N stage was significantly associated with the pathological response (χ2 test, P > 0.05). However, the mean tumor size was significantly smaller in the responders than in the non-responders (15.7 ± 7.4 cm vs 33.3 ± 14.1 cm, P < 0.01).
The individual patient data of change in tumor size showed that 40.7% (11/27) of the responders experienced a decrease in tumor size that was detected by DCUS. However, significantly less, only 18.8% (3/16), of the non-responders showed a decrease in tumor size, as detected by DCUS (P < 0.01).
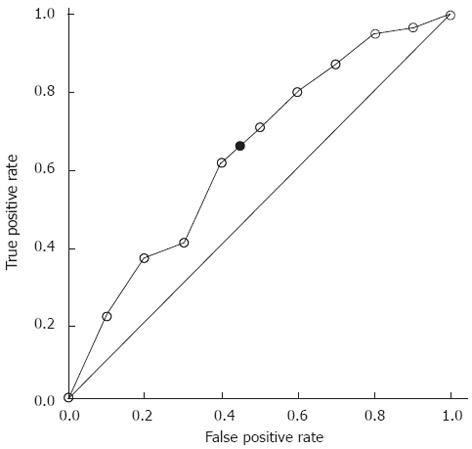
The ROC curve analysis for identifying histopathologic responders based on DCUS-detected changes in tumor size is shown in Figure 1. The area under the receiver-operating characteristic curve (AUC) was 0.64 (95%CI: 0.46-0.81). Using ROC curve analysis with Youden’s index maximization, the best cut-off for distinguishing the responders from the non-responders was identified, which showed optimal sensitivity of 62.9% and specificity of 56.3%. For this cut-off point, the positive and negative predictive values of DCUS for distinguishing the responders from the non-responders were 70.8% and 47.4%, respectively. The overall accuracy of DCUS for therapeutic response assessment was 60.5%, compared with the slightly lower overall accuracy (53.5%) of CT assessment with RECIST criteria (P = 0.663, Table 2).
| Sensitivity | Specificity | PPV | NPV | Accuracy | χ2 | P value | |
| CT | 13 (48.1) | 10 (62.5) | 13 (66.7) | 10 (41.7) | 23 (53.5) | 0.427 | 0.663 |
| DCUS | 17 (62.9) | 9 (56.3) | 17 (70.8) | 9 (47.4) | 26 (60.5) |
Interestingly, we found that DCUS was able to identify decreased perfusion in the tumors of responders who showed no morphological changes by the CT imaging technique. We believe the false negative findings of CT were likely due to occlusion by chemotherapy-induced effects, such as fibrosis and edema.
Given the generally poor long-term survival (< 20%-30%) achieved in advanced gastric cancer patients who undergo surgery alone, clinicians and researchers have been actively pursuing methods to improve the survival rates of these patients. The approach of providing chemotherapy prior to the resection surgery (pre-operative/neoadjuvant) has proven beneficial for locally-advanced tumors[8-10]. Patients receiving pre-operative NAC have shown increased likelihood of curative resection and improved overall survival. Although several studies have demonstrated the significant benefits of NAC, compared to surgery alone, for patients with resectable locally-advanced gastric cancer, the major clinical response rate has only reached 38%-69.7%[11,12].
The current standard method for discriminating chemotherapeutic responders from non-responders is histopathologic analysis, which measures the extent of the residual tumor. This method, however, is applicable only in a post-operative setting and cannot be used for either the pre-operative design of personalized treatment or the planning of intra-operative strategies[13]. Previous studies have revealed that the conventional imaging modalities (EUS, CT and MRI) to detect tumor volumetry lack reliability for predicting response to chemotherapy[14,15]. Moreover, the response evaluation methods based on the World Health Organization or RECIST criteria were reported to be highly inaccurate for gastric cancer[16]. The recently developed technology of functional imaging, which detects or measures changes in metabolism, blood flow, regional chemical composition, and absorption, appears to be a promising alterative for monitoring chemotherapeutic effects in gastric tumors[17]. In addition, the semi-quantitative approach of measuring glucose metabolism by means of positron emission tomography (PET) was shown to have clinical relevance in determining the response to chemotherapy for several tumor types, including gastric carcinomas[18]. While both PET and PET/CT are well-established methods of molecular imaging, they are each limited by poor spatial resolution[19]. In this regard, the CT and MRI technologies are preferable; however, these imaging technologies are restricted from widespread use due to their technical complexity and high cost which is often not amenable to smaller, underfunded clinics, especially those in rural areas.
Zhou et al[20] was the first to suggest that quantifying tumor perfusion with contrast-enhanced ultrasound may help detect changes in tumor perfusion after chemotherapy, based on their findings in an animal model. Our present study assessed the clinical value of a new functional imaging modality, DCUS, for non-invasive assessment of response to NAC in locally-advanced gastric cancer patients. The routine ultrasound techniques, US and EUS, rely purely on acoustic shadowing for visualization. As such, they are unable to differentiate conditions of inflammation and fibrosis in tumorous tissues, which leads to misinterpretation of the tumor depth[3]. Chemotherapy- induced death of cancer cells results in reduced blood perfusion and decreased metabolic activity of the tumor. Since DCUS is capable of assessing the physiological blood flow within a tumor, it is also able to assess the depth of tumor penetration and lymph node metastasis[21].
Quantitative assessment of tumor perfusion with contrast-enhanced ultrasound has already been successfully applied in both animal model[22-24] and clinical[25,26] studies. However, to the best of our knowledge, our study presented herein is the first to evaluate the efficiency of DCUS-detected tumor perfusion to monitor gastric cancer tumor response to chemotherapy. Although the indicated value of this technique in early assessment of tumor response to chemotherapy must be further tested in larger and more heterogeneous study populations, our preliminary results indicate that DCUS provides better diagnostic performance (AUC: 0.64) and accuracy (65.1%) for the assessment of a histopathological response than the standard CT imaging technique. Moreover, the results from the current study have clinical implications for customizing gastric cancer treatments to individual risk profiles, which should be explored in future studies.
In conclusion, in this study of patients with locally-advanced gastric cancer undergoing NAC, we found that DCUS may be as an innovative tool for predicting pathological response at an early stage of the NAC regimen and prior to definitive resection. Because this technique is non-invasive and does not cause patient discomfort, it is particularly promising for repeated monitoring during the chemotherapy treatment period. Some limitations exist in the current study, however, that may impact the generalization of our findings. Despite the fact that the present study is the first to investigate the potential of DCUS response assessment during neoadjuvant chemotherapy in patients with gastric cancer, the total number of cases assessed was small (n = 43). We found that tumor size was affected by chemotherapy, and considered this a parameter of the assessment method, but we did not investigate any fluctuations in tumor size over time or in response to features of the chemotherapy regimen (doses, drug type, or cycle duration). Finally, our institute does not routinely use ultrasonography techniques as an evaluation modality for gastric cancer, so we were unable to draw conclusions about which analysis method is most useful (i.e., EUS vs DCUS). In order to determine whether DCUS can actually be used for accurate response prediction, a larger, multi-institute study is required.
Gastric cancer is currently the fourth most commonly diagnosed cancer worldwide, and it ranks second among cancer-related deaths. Currently, almost two-thirds of the gastric cancer cases occur in developing countries, with China alone accounting for 42%. Neoadjuvant chemoradiotherapy (NACRT) before surgery can improve survival in patients with locally-advanced gastric cancer, but not all patients respond to this treatment. An accurate method to assess short-term response to NACRT is critical for identifying responsive and non-responsive patients to design appropriate individualized treatment strategies.
The criteria of computed tomography (CT) imaging for assessing tumor response to chemotherapy is well defined, and a well-established component of the standard workup for gastric cancer staging. However, CT imaging is usually not accurate for identifying the presence of residual tumorous tissues within areas with chemotherapy-induced effects, such as fibrosis. Their previous studies showed that double contrast-enhanced ultrasound (DCUS) is superior to the traditional CT-based gastric cancer staging methods to assess tumor penetration depth and lymph node metastases. In the current study, authors aimed to determine whether DCUS parameters are able to differentiate histopathological responders from non-responders in the early stage after neoadjuvant chemotherapy (NAC), and if so to compare the predictive efficacy of DCUS with that of CT imaging.
The results of the current study demonstrate that DCUS has optimal sensitivity and specificity for distinguishing histopathological responders from non-responders among NAC-treated gastric cancer patients. The overall accuracy of 60.5% was slightly higher than that of 53.5% for CT assessment with the response evaluation criteria in solid tumors criteria. Moreover, DCUS was able to identify decreased perfusion in cases of responders who showed no morphological changes by CT imaging, which had been likely occluded by treatment effects, such as fibrosis and edema.
DCUS is a feasible addition to the preoperative workup for NAC response assessment in gastric cancer patients. The results of DCUS may be beneficial and additive for formulating appropriate treatment plans for individual patients. Finally, ultrasound is a low-cost technique that is amenable to widespread application in small clinical setting with restricted funding and may prove a valuable technique for assessing the patients served by such clinics.
Contrast-enhanced ultrasound is based on the traditional medical sonography imaging technique but combined with contrast agents to enhance and differentiate various sections of an object under investigation. The substance composition of each of the various ultrasound contrast agents reflects sound waves in a distinctive manner. Neoadjuvant therapy is the administration of therapeutic agents prior to the main treatment regimen being initiated. The objective of neoadjuvant chemotherapy for gastric carcinoma is to reduce the size or extent of the cancer lesion prior to the curative resection surgery; smaller tumors are easier to resect and less extensive tumors have a higher chance of complete removal. Use of neoadjuvant chemotherapy is expected to reduce the potential risk and side effects of more radical surgical interventions required for larger and more extensive tumors.
This is an interesting study in which authors evaluate the efficacy of double contrast-enhanced ultrasound for assessing tumor response to pre-operative chemotherapy in patients with gastric cancer. The results suggest that DCUS may represent an innovative tool for accurate predicting of histopathological response to neoadjuvant chemotherapy in patients with locally-advanced gastric cancer.
Peer reviewers: Misha Luyer, MD, PhD, Department of Surgery, Orbis Medical Centre, Postbus 5500, 6130 MB Sittard, The Netherlands; Stephen M Kavic, MD, FACS, Assistant Professor of Surgery, Department of Surgery, University of Maryland School of Medicine, 22 South Greene Street, Room S4B09, Baltimore, MD 21201, United States
S- Editor Gou SX L- Editor A E- Editor Li JY
| 1. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4899] [Cited by in RCA: 4600] [Article Influence: 242.1] [Reference Citation Analysis (0)] |
| 2. | Langer R, Ott K, Feith M, Lordick F, Siewert JR, Becker K. Prognostic significance of histopathological tumor regression after neoadjuvant chemotherapy in esophageal adenocarcinomas. Mod Pathol. 2009;22:1555-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Afaq A, Akin O. Imaging assessment of tumor response: past, present and future. Future Oncol. 2011;7:669-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 21581] [Article Influence: 1348.8] [Reference Citation Analysis (1)] |
| 5. | Huang P, Li S, Aronow WS, Wang Z, Nair CK, Xue N, Shen X, Chen C, Cosgrove D. Double contrast-enhanced ultrasonography evaluation of preoperative Lauren classification of advanced gastric carcinoma. Arch Med Sci. 2011;7:287-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Zheng Z, Yu Y, Lu M, Sun W, Wang F, Li P, Zhang Y, Lin L, Huang P, Chen J. Double contrast-enhanced ultrasonography for the preoperative evaluation of gastric cancer: a comparison to endoscopic ultrasonography with respect to histopathology. Am J Surg. 2011;202:605-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, Roussel A, Jacob JH, Segol P, Samama G. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680-2686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 8. | Lowy AM, Mansfield PF, Leach SD, Pazdur R, Dumas P, Ajani JA. Response to neoadjuvant chemotherapy best predicts survival after curative resection of gastric cancer. Ann Surg. 1999;229:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 187] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 9. | Schuhmacher C, Gretschel S, Lordick F, Reichardt P, Hohenberger W, Eisenberger CF, Haag C, Mauer ME, Hasan B, Welch J. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol. 2010;28:5210-5218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 532] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 10. | Fushida S, Nashimoto A, Fukushima N, Kawachi Y, Fujimura T, Kuwabara S, Musha N. Phase II trial of preoperative chemotherapy with docetaxel, cisplatin and S-1 for T4 locally advanced gastric cancer. Jpn J Clin Oncol. 2012;42:131-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | De Vita F, Orditura M, Matano E, Bianco R, Carlomagno C, Infusino S, Damiano V, Simeone E, Diadema MR, Lieto E. A phase II study of biweekly oxaliplatin plus infusional 5-fluorouracil and folinic acid (FOLFOX-4) as first-line treatment of advanced gastric cancer patients. Br J Cancer. 2005;92:1644-1649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 142] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Li ZY, Koh CE, Bu ZD, Wu AW, Zhang LH, Wu XJ, Wu Q, Zong XL, Ren H, Tang L. Neoadjuvant chemotherapy with FOLFOX: improved outcomes in Chinese patients with locally advanced gastric cancer. J Surg Oncol. 2012;105:793-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Guerra L, Niespolo R, Di Pisa G, Ippolito D, De Ponti E, Terrevazzi S, Bovo G, Sironi S, Gardani G, Messa C. Change in glucose metabolism measured by 18F-FDG PET/CT as a predictor of histopathologic response to neoadjuvant treatment in rectal cancer. Abdom Imaging. 2011;36:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Mallery S, DeCamp M, Bueno R, Mentzer SJ, Sugarbaker DJ, Swanson SJ, Van Dam J. Pretreatment staging by endoscopic ultrasonography does not predict complete response to neoadjuvant chemoradiation in patients with esophageal carcinoma. Cancer. 1999;86:764-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Brown WA, Thomas J, Gotley D, Burmeister BH, Lim KH, Martin I, Walpole ET, Thomson DB, Harvey JA, Smithers BM. Use of oesophagogastroscopy to assess the response of oesophageal carcinoma to neoadjuvant therapy. Br J Surg. 2004;91:199-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Wang LB, Teng RY, Jiang ZN, Hu WX, Dong MJ, Yuan XM, Chen WJ, Jin M, Shen JG. Clinicopathologic variables predicting tumor response to neoadjuvant chemotherapy in patients with locally advanced gastric cancer. J Surg Oncol. 2012;105:293-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Marcus CD, Ladam-Marcus V, Cucu C, Bouché O, Lucas L, Hoeffel C. Imaging techniques to evaluate the response to treatment in oncology: current standards and perspectives. Crit Rev Oncol Hematol. 2009;72:217-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 18. | Lorenzen S, von Gall C, Stange A, Haag GM, Weitz J, Haberkorn U, Lordick F, Weichert W, Abel U, Debus J. Sequential FDG-PET and induction chemotherapy in locally advanced adenocarcinoma of the Oesophago-gastric junction (AEG): the Heidelberg Imaging program in Cancer of the oesophago-gastric junction during Neoadjuvant treatment: HICON trial. BMC Cancer. 2011;11:266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Miller JC, Pien HH, Sahani D, Sorensen AG, Thrall JH. Imaging angiogenesis: applications and potential for drug development. J Natl Cancer Inst. 2005;97:172-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 227] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 20. | Zhou JH, Cao LH, Zheng W, Liu M, Han F, Li AH. Contrast-enhanced gray-scale ultrasound for quantitative evaluation of tumor response to chemotherapy: preliminary results with a mouse hepatoma model. AJR Am J Roentgenol. 2011;196:W13-W17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Kanamori A, Hirooka Y, Itoh A, Hashimoto S, Kawashima H, Hara K, Uchida H, Goto J, Ohmiya N, Niwa Y. Usefulness of contrast-enhanced endoscopic ultrasonography in the differentiation between malignant and benign lymphadenopathy. Am J Gastroenterol. 2006;101:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | McCarville MB, Streck CJ, Dickson PV, Li CS, Nathwani AC, Davidoff AM. Angiogenesis inhibitors in a murine neuroblastoma model: quantitative assessment of intratumoral blood flow with contrast-enhanced gray-scale US. Radiology. 2006;240:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Lavisse S, Lejeune P, Rouffiac V, Elie N, Bribes E, Demers B, Vrignaud P, Bissery MC, Brulé A, Koscielny S. Early quantitative evaluation of a tumor vasculature disruptive agent AVE8062 using dynamic contrast-enhanced ultrasonography. Invest Radiol. 2008;43:100-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Broillet A, Hantson J, Ruegg C, Messager T, Schneider M. Assessment of microvascular perfusion changes in a rat breast tumor model using SonoVue to monitor the effects of different anti-angiogenic therapies. Acad Radiol. 2005;12 Suppl 1:S28-S33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Bertolotto M, Pozzato G, Crocè LS, Nascimben F, Gasparini C, Cova MA, Tiribelli C. Blood flow changes in hepatocellular carcinoma after the administration of thalidomide assessed by reperfusion kinetics during microbubble infusion: preliminary results. Invest Radiol. 2006;41:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Schirin-Sokhan R, Winograd R, Roderburg C, Bubenzer J, do Ó NC, Guggenberger D, Hecker H, Trautwein C, Tischendorf JJ. Response evaluation of chemotherapy in metastatic colorectal cancer by contrast enhanced ultrasound. World J Gastroenterol. 2012;18:541-545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |