Published online Dec 21, 2012. doi: 10.3748/wjg.v18.i47.6974
Revised: July 19, 2012
Accepted: July 28, 2012
Published online: December 21, 2012
AIM: To investigate the utility of immunohistochemical (IHC) staining with an antibody to Mycobacterium tuberculosis (M. tuberculosis) for the diagnosis of intestinal tuberculosis (TB).
METHODS: We retrospectively identified 10 patients (4 males and 6 females; mean age = 65.1 ± 13.6 years) with intestinal TB. Clinical characteristics, including age, gender, underlying disease, and symptoms were obtained. Chest radiograph and laboratory tests, including sputum Ziehl-Neelsen (ZN) staining, M. tuberculosis culture, and sputum polymerase chain reaction (PCR) for tubercle bacilli DNA, as well as Tuberculin skin test (TST) and QuantiFERON-TB gold test (QFT), were examined. Colonoscopic records recorded on the basis of Sato’s classification were also reviewed, in addition to data from intestinal biopsies examined for histopathological findings, including hematoxylin and eosin staining, and ZN staining, as well as M. tuberculosis culture, and PCR for tubercle bacilli DNA. For the present study, archived formalin-fixed paraffin-embedded (FFPE) intestinal tissue samples were immunohistochemically stained using a commercially available species-specific monoclonal antibody to the 38-kDa antigen of the M. tuberculosis complex. These sections were also stained with the pan-macrophage marker CD68 antibody.
RESULTS: From the clinical data, we found that no patients were immunocompromised, and that the main symptoms were diarrhea and weight loss. Three patients displayed active pulmonary TB, six patients (60%) had a positive TST, and 4 patients (40%) had a positive QFT. Colonoscopic findings revealed that all patients had type 1 findings (linear ulcers in a circumferential arrangement or linear ulcers arranged circumferentially with mucosa showing multiple nodules), all of which were located in the right hemicolon and/or terminal ileum. Seven patients (70%) had concomitant healed lesions in the ileocecal area. No acid-fast bacilli were detected with ZN staining of the intestinal tissue samples, and both M. tuberculosis culture and PCR for tubercle bacilli DNA were negative in all samples. The histopathological data revealed that tuberculous granulomas were present in 4 cases (40%). IHC staining in archived FFPE samples with anti-M. tuberculosis monoclonal antibody revealed positive findings in 4 patients (40%); the same patients in which granulomas were detected by hematoxylin and eosin staining. M. tuberculosis antigens were found to be mostly intracellular, granular in pattern, and primarily located in the CD68+ macrophages of the granulomas.
CONCLUSION: IHC staining with a monoclonal antibody to M. tuberculosis may be an efficient and simple diagnostic tool in addition to classic examination methods for the diagnosis of intestinal TB.
-
Citation: Ihama Y, Hokama A, Hibiya K, Kishimoto K, Nakamoto M, Hirata T, Kinjo N, Cash HL, Higa F, Tateyama M, Kinjo F, Fujita J. Diagnosis of intestinal tuberculosis using a monoclonal antibody to
Mycobacterium tuberculosis . World J Gastroenterol 2012; 18(47): 6974-6980 - URL: https://www.wjgnet.com/1007-9327/full/v18/i47/6974.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i47.6974
Tuberculosis (TB), a chronic granulomatous infectious disease caused by Mycobacterium tuberculosis (M. tuberculosis), is still a significant cause of morbidity and mortality worldwide. Due to the increasing prevalence of human immunodeficiency virus (HIV) infection and immunosuppressive therapy for various diseases, the global incidence of TB has increased[1].
The gastrointestinal tract is a common site of extrapulmonary TB. The ileocecal region is frequently involved in most patients diagnosed with intestinal TB, although the diagnosis of intestinal TB is often difficult because of its diverse clinical manifestations and very low positivity using current diagnostic tests including Ziehl-Neelsen (ZN) staining and M. tuberculosis culture from intestinal tissue samples[2]. More recently, detection of tubercle bacilli DNA by polymerase chain reaction (PCR) has been developed as a diagnostic tool with excellent sensitivity and specificity in respiratory specimens. However, diagnosis by PCR in clinical settings still requires validation[3]. Therefore, diagnosis is generally made on the basis of the classical histopathological demonstration of a caseating epithelioid cell granuloma, which is suggestive of TB. However, it may be difficult to differentiate intestinal TB from Crohn’s disease based on this technique due to the fact that intestinal TB and CD have similar clinical, colonoscopic, and pathological findings. Although it is well known that caseating granulomas are a feature of TB, and non-caseating granulomas are that of CD, the prevalence of caseation is low in clinical settings for intestinal tuberculous granulomas[4,5].
The present study was conducted to investigate the utility of immunohistochemical (IHC) staining with a species-specific monoclonal antibody to the 38-kDa antigen of the M. tuberculosis complex to diagnose intestinal TB in archived formalin-fixed paraffin-embedded (FFPE) intestinal tissue sections of suspected intestinal TB patients.
We retrospectively identified 10 patients (4 males and 6 females; mean age, 65.1 ± 13.6 years) with intestinal TB between 1996 and 2011. All cases were obtained from the archives of the Department of Infectious, Respiratory, and Digestive Medicine at the University of the Ryukyus Hospital, Okinawa, Japan. The diagnosis of intestinal TB was made by at least one of the following criteria: (1) a positive culture of M. tuberculosis from the intestinal tissue; (2) histopathological demonstration of acid-fast bacilli (AFB) in the intestinal tissue; (3) histopathological demonstration of a caseating epithelioid cell granuloma in the intestinal tissue; (4) detection of tubercle bacilli DNA by PCR from the intestinal tissue; and (5) typical endoscopic features together with a favorable response to a trial of antituberculous therapy. These patients were all treated with a full course of anti-tuberculosis therapy (rifampicin, isoniazid, ethambutol, pyrazinamide) following diagnosis. The clinical and colonoscopic records of these patients were obtained, as well as archived FFPE intestinal tissue sections. This study was approved by the Ethics Committee of our institute.
Colonoscopy was performed with standard colonoscopes (Olympus, Tokyo, Japan). All patients diagnosed with intestinal TB were examined from the rectum to terminal ileum after lavage bowel preparation with a polyethylene glycol electrolyte solution. Colonoscopic findings were recorded on the basis of Sato’s classification[6]. Open ulcers or erosions were classified into 4 types: type 1 (linear ulcers in a circumferential arrangement or linear ulcers arranged circumferentially with mucosa showing multiple nodules), type 2 (round or irregular-shaped isolated small ulcers arranged circumferentially without nodules), type 3 (multiple erosions restricted to the colon), and type 4 (small aphthous ulcers or erosions restricted to the ileum). Healed lesions in the ileocecal area were also recorded, including the patulous ileocecal valve (PV), pseudodiverticular deformity (PD), and atrophic mucosal area (AMA) with multiple ulcer scars[6]. During colonoscopy, biopsy specimens were obtained in a routine fashion using standard forceps. The specimens were prepared for ZN staining, tuberculous culture, PCR for tubercle bacilli DNA, and hematoxylin and eosin (HE) staining.
IHC staining was performed using the IgG1 type mouse monoclonal antibody against the 38-kDa antigen of the M. tuberculosis complex (Vector Laboratories, Burlingame, CA, United States). 5 μm thick sections were prepared from formalin-fixed, paraffin-embedded tissue. IHC was carried out using the VECTASTAIN ABC kit (Vector Laboratories, Burlingame, CA, United States) as described elsewhere[7-11]. Briefly, after deparaffinization and rehydration, the sections were exposed to antigen retrieval (Target retrieval solution pH 6.0, DakoCytomation, CA, United States) in high temperature water (98 °C) for 30 min, and then cooled for 20 min at room temperature. Endogenous peroxidase activity was inhibited by incubating the sections with hydrogen peroxide for 20 min. To prevent non-specific binding, these sections were incubated in normal mouse serum for 20 min. Primary antibody (anti-M. tuberculosis mouse monoclonal antibody in 1:80 dilutions) was applied to the sections overnight. This step was followed by washing and 40-min incubation with a biotinylated secondary antibody. These sections were then subjected to an avidin biotin-peroxidase complex for 40 min. Visualization was performed using ImmPACT DAB (Vector Laboratories, Burlingame, CA, United States), which was applied for 10 s. Sections were counter-stained with hematoxylin. A negative control in which the primary antibody was substituted with antibody diluent was used. IHC expression of the M. tuberculosis antigen was evaluated under light microscopy for the distribution of stain in the cytoplasm of epithelioid histiocytes and multinucleated giant cells. Additional IHC staining was performed to evaluate the relationship between M. tuberculosis antigen and macrophage distribution in the colonic specimens. These paraffin sections were stained with anti-M. tuberculosis rabbit antibody (Abcam, Cambridge, MA) at a 1:200 dilution, and the pan-macrophage marker CD68 antibody (Leica Microsystem, Buckinghamshire, United Kingdom) at a 1:80 dilution using the streptavidin-biotin peroxidase method as described previously.
Clinical features of the 10 patients are summarized in Table 1. No patients were immunocompromised, including immunosuppressive medication use and HIV infection. Primary symptoms were diarrhea and weight loss. Although no patients had respiratory symptoms, chest radiograph and sputum ZN staining in 3 patients revealed active pulmonary TB. Six patients (60%) had a positive Tuberculin skin test (TST) and four patients (40%) had a positive QuantiFERON-TB gold test (QFT), of which three patients had both a positive TST and QFT test.
| Case | Age/gender | Underlying disease | Symptoms | Chest radiograph | Sputum ZN stain | Sputum culture | Sputum PCR | TST | QFT |
| 1 | 38/F | Epilepsy | Fever | Active TB | + | - | + | + | ND |
| 2 | 66/F | Lumber neuralgia | Diarrhea | Normal | - | - | - | + | ND |
| 3 | 81/F | Cecal cancer | Diarrhea, weight loss | Active TB | + | - | - | ND | ND |
| 4 | 72/M | Post-herpes neuralgia | Abdominal pain | Normal | - | - | - | + | + |
| 5 | 43/M | Diabetes mellitus | Diarrhea | Normal | - | - | - | - | ND |
| 6 | 76/M | Gout | Diarrhea | Normal | - | - | - | + | + |
| 7 | 58/M | Ulcerative colitis | Diarrhea | Normal | - | - | - | - | - |
| 8 | 74/F | Gastric ulcer | Weight-loss | Active TB | + | - | - | + | + |
| 9 | 74/F | Colonic polyp | None | Normal | - | - | - | ND | + |
| 10 | 70/F | Rectal cancer | Diarrhea | Inactive TB | - | - | - | + | ND |
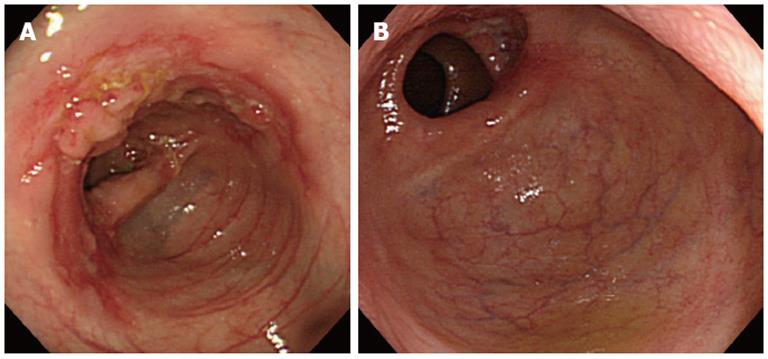
Colonoscopic, bacteriological, and histopathological findings of the 10 patients are summarized in Table 2. All patients had type 1 findings (linear ulcers in a circumferential arrangement or linear ulcers arranged circumferentially with mucosa showing multiple nodules), all of which were located in the right hemicolon and/or terminal ileum (Figure 1A). Seven patients (70%) had concomitant healed lesions, including PV, PD, or AMA, in the ileocecal area (Figure 1B). No AFB was detected with ZN staining of the intestinal tissue samples. Tuberculous culture and PCR for tubercle bacilli DNA were negative in all intestinal tissue samples.
| Case | Type | Colonoscopic or macroscopic findings | Histopathological findings | ||||
| Location | PV, PD, AMA | AFB in ZN staining | Culture/PCR | Granuloma | TB by IHC staining | ||
| 1 | 1 | TI, C, A | PV, AMA | - | - | - | - |
| 2 | 1 | TI, C, A | PV, AMA | - | - | + | + |
| 3 | 1 | TI | - | - | - | + | + |
| 4 | 1 | C, A | PV, PD, AMA | - | - | - | - |
| 5 | 1 | TI, C, A, T, D, S | PV, PD, AMA | - | - | - | - |
| 6 | 1 | TI | - | - | - | - | - |
| 7 | 1 | TI, C, A, T, D, S | PV, PD, AMA | - | - | - | - |
| 8 | 1 | TI, C | PV, AMA | - | - | + | + |
| 9 | 1 | TI, C, A, S | PD, AMA | - | - | - | - |
| 10 | 1 | TI | - | - | - | + | + |
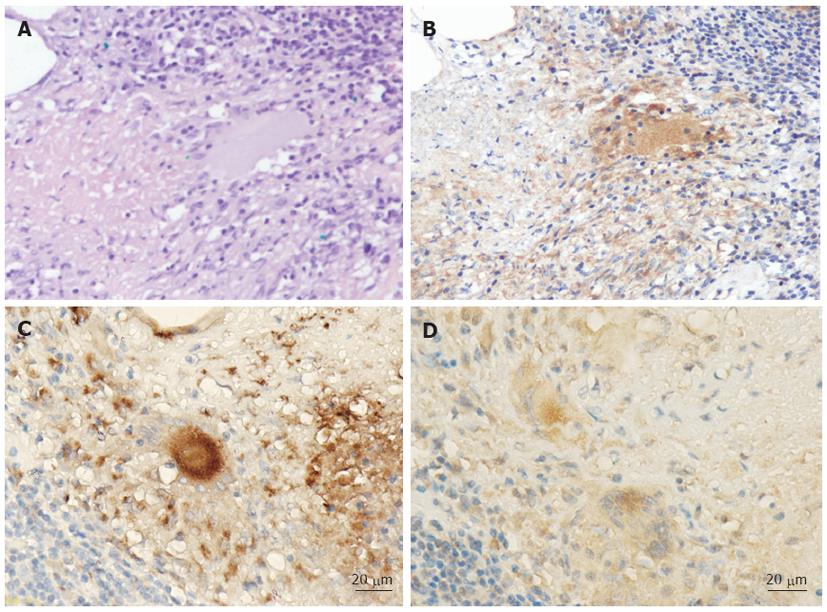
In the histopathological studies, tuberculous granulomas were identified in 4 cases (40%) (Figure 2A). IHC staining with anti-M. tuberculosis monoclonal antibody was positive in these same 4 patients (40%). The mycobacterial antigens were mainly detected as granular cytoplasmic staining in the epithelioid cells and giant cells within granulomas (Figure 2B). All samples in which granulomas were detected by HE staining were positive for TB by IHC staining. As for the relationship between M. tuberculosis antigens and macrophage distribution in the colonic consecutive specimens, the mycobacterial antigens were seen predominantly as coarse granular immunopositive material in CD68+ macrophage cytoplasm (Figure 2C, D).
All patients were suspected to have intestinal TB on the basis of clinicopathologic findings. The regimen for TB was combined chemotherapy containing isoniazid, rifampicin, and ethambutol for six months and pyrazinamide for two months. All patients responded to antituberculous therapy and follow-up colonoscopies showed improvement of the colonic lesions.
This study has highlighted several important considerations. Most patients in this study had abdominal symptoms, and although no patients had respiratory symptoms, 4 (40%) had concomitant active or inactive pulmonary TB, consistent with prior reports in which pulmonary TB was apparent in less than 25% of patients with intestinal TB[2,12]. Based on these findings, we should be aware of the possibility of tuberculous involvement in multiple organs despite apparent symptoms.
Although the TST has long been used as a reliable diagnostic examination, the recently developed QFT has been increasingly applied. There is controversy as to whether or not the QFT is effective for the diagnosis of extrapulmonary TB. Kim et al[13] reported that in a prospective study of 128 patients, QFT was a limited but useful aid in combination with the TST in the diagnosis of intestinal TB. In agreement with their findings, the QFT and TST had a good agreement in our study.
In this study, the vast majority of cases were colonoscopically diagnosed with TB. Similar to previous studies[6,14], all patients in our study had a type 1 appearance among colonoscopic findings. Although this type has been established as a reliable colonoscopic feature, recent studies have emphasized that healed lesions in the ileocecal area, including PV, PD, and AMA with multiple ulcer scars, can coexist with active tuberculous inflammation. Sato et al[6] reported that 91% of patients in their study have AMA lesions, and so concluded that the AMA was the most frequently recognized endoscopic manifestation of intestinal TB. Our data, in which 60% of patients had these lesions, correlates strongly with their findings.
Numerous studies have compared the diagnostic methods of intestinal TB[15]. A study by Sekar et al[16] evaluated the role of PCR in the laboratory diagnosis of different forms of extrapulmonary TB in comparison with conventional bacteriologic techniques. They found an 18%, 22%, and 63% sensitivity for smear, culture, and PCR, respectively. A recent study clearly demonstrated that using real-time PCR technology with fluorescence resonance energy transfer probes has much higher sensitivity for the detection of tubercle bacilli DNA in tissue biopsy samples and FFPE surgically resected tissues of the gastrointestinal tract than traditional PCR[17]. Our negative AFB and PCR tests may be due to a small biopsy sample size.
There have been several studies on IHC staining for the diagnosis of TB in which the most used polyclonal antibodies[18,19] resulted in false-positive reactions. There are only two studies that have evaluated IHC staining with a species-specific monoclonal antibody to 38-kDa antigen of M. tuberculosis complex in archival FFPE tissue sections of intestinal TB. Goel et al[7] demonstrated that 2/2 samples tested had positive IHC staining (100%), whereas Ince et al[8] demonstrated IHC positivity in 6/8 (75%) intestinal tuberculous granulomas. Our results revealed 4 positive samples using IHC staining out of 4 tuberculous granulomas, whereas ZN staining for AFB was negative in all of these sections. Our results demonstrating that granulomas detected with HE staining are all positive with IHC have confirmed these previous findings. It is important to note that this was a retrospective study on previously diagnosed TB; hence we cannot determine the false positivity rate of IHC staining for intestinal TB.
Granular cytoplasmic staining of M. tuberculosis in IHC staining is considered to be due to fragments or debris of the bacilli[7]. Low positivity of AFB could be due to the fact that only the intact bacilli take up the stain. The fact that fragments or debris of M. tuberculosis can be detected easily using IHC staining may be a great advantage. To the best of our knowledge, this is the first report that demonstrates that M. tuberculosis antigens are located as coarse granular immunopositive material in the cytoplasm of CD68+ macrophages within human intestinal TB. This finding may help to uncover the unknown relationship between M. tuberculosis and macrophages[20].
Distinguishing intestinal TB from CD is still challenging[21-25], and the treatments of TB and CD are quite different. Corticosteroids, immunosuppressive and anti-tumor necrosis factor (TNF) agents are widely applied in the treatment of CD, whereas they may be harmful in TB. For example, anti-TNF agents can induce reactivation of TB; therefore differentiating TB from CD is extremely important. Histopathological features encountered frequently in intestinal TB include granulomas that are confluent, large (> 200 μm), and multiple in number (> 5 per section)[26]. The classical histological picture of tuberculous granulomatous inflammation is not a diagnostic problem in tissue samples; however, when sections show non-caseous epithelioid granulomas mimicking TB as is the case in CD, it creates a diagnostic dilemma. In the present study, we did not conduct any experiments using intestinal samples of CD; however Ince et al[8] concluded that positive IHC staining with species-specific antibodies to TB can rule out the diagnosis of CD with high sensitivity and specificity. We plan to conduct a large-scale study to confirm these findings in the future.
In conclusion, IHC staining using a monoclonal antibody to M. tuberculosis antigen, which is a novel translational implication, can potentially be an efficient and simple diagnostic tool to compliment classic clinicopathological examinations for the diagnosis of intestinal TB.
We thank the clinical staff of the Department of Infectious, Respiratory, and Digestive Medicine, Faculty of Medicine, University of the Ryukyus for their aid in collecting clinical data.
The diagnosis of intestinal tuberculosis (TB) is often difficult because of its diverse clinical manifestations and very low positivity using current diagnostics, including acid-fast bacilli in Ziehl-Neelsen staining and culture of Mycobacterium tuberculosis (M. tuberculosis) from intestinal tissue samples.
Detection of M. tuberculosis DNA by polymerase chain reaction has been established as a diagnostic tool with excellent sensitivity and specificity in pulmonary TB. However, the diagnosis of intestinal TB still requires validation.
In this study, the authors have shown that immunohistochemical (IHC) staining using a monoclonal antibody to M. tuberculosis antigen in archived formalin-fixed paraffin-embedded intestinal tissue samples, which is a novel translational implication, can be an efficient and simple diagnostic tool to compliment classic clinicopathological examinations for the diagnosis of intestinal TB.
Although further studies are needed, IHC staining may be beneficial to differentiate TB from Crohn’s disease (CD) with similar microscopic features, including the presence of granulomas.
Monoclonal antibodies are monospecific antibodies which have monovalent affinity. IHC staining is widely applied to investigate the distribution of antigens in biological tissues by using specific antibodies.
In this manuscript, the authors have clearly illustrated the potential advantage for the diagnosis of intestinal tuberculous granuloma. The importance of the research and its significance show that this topic is an important issue. The differentiation between TB and CD is very important in gastroenterology.
Peer reviewers: Pradyumna Kumar Mishra, MS, PhD, Professor, Division of Translational Research, Tata Memorial Centre, Advanced Centre for Treatment, Research and Education in Cancer, Navi Mumbai 410210, India; Fariborz Mansour-Ghanaei, Professor, Gastrointestinal and Liver Disease Research Center, Razi Hospital Sardar Jangle Ave, Rasht 41448-95655, Iran
S- Editor Gou SX L- Editor Rutherford A E- Editor Zhang DN
| 1. | Lawn SD, Zumla AI. Tuberculosis. Lancet. 2011;378:57-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 546] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 2. | Sheer TA, Coyle WJ. Gastrointestinal tuberculosis. Curr Gastroenterol Rep. 2003;5:273-278. [PubMed] |
| 3. | Almadi MA, Aljebreen AM, Sanai FM, Marcus V, Almeghaiseeb ES, Ghosh S. New insights into gastrointestinal and hepatic granulomatous disorders. Nat Rev Gastroenterol Hepatol. 2011;8:455-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Kim KM, Lee A, Choi KY, Lee KY, Kwak JJ. Intestinal tuberculosis: clinicopathologic analysis and diagnosis by endoscopic biopsy. Am J Gastroenterol. 1998;93:606-609. [PubMed] |
| 5. | Khan R, Abid S, Jafri W, Abbas Z, Hameed K, Ahmad Z. Diagnostic dilemma of abdominal tuberculosis in non-HIV patients: an ongoing challenge for physicians. World J Gastroenterol. 2006;12:6371-6375. [PubMed] |
| 6. | Sato S, Yao K, Yao T, Schlemper RJ, Matsui T, Sakurai T, Iwashita A. Colonoscopy in the diagnosis of intestinal tuberculosis in asymptomatic patients. Gastrointest Endosc. 2004;59:362-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Goel MM, Budhwar P. Immunohistochemical localization of mycobacterium tuberculosis complex antigen with antibody to 38 kDa antigen versus Ziehl Neelsen staining in tissue granulomas of extrapulmonary tuberculosis. Indian J Tuberc. 2007;54:24-29. [PubMed] |
| 8. | Ince AT, Güneş P, Senateş E, Sezikli M, Tiftikçi A, Ovünç O. Can an immunohistochemistry method differentiate intestinal tuberculosis from Crohn's disease in biopsy specimens? Dig Dis Sci. 2011;56:1165-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Hibiya K, Tateyama M, Tasato D, Nakamura H, Atsumi E, Higa F, Tamai K, Fujita J. Mechanisms involved in the extension of pulmonary Mycobacterium avium infection from the pulmonary focus to the regional lymph nodes. Kekkaku. 2011;86:1-8. [PubMed] |
| 10. | Hibiya K, Tateyama M, Teruya H, Nakamura H, Tasato D, Kazumi Y, Hirayasu T, Tamaki Y, Haranaga S, Higa F. Immunopathological characteristics of immune reconstitution inflammatory syndrome caused by Mycobacterium parascrofulaceum infection in a patient with AIDS. Pathol Res Pract. 2011;207:262-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Hibiya K, Shigeto E, Iida K, Kaibai M, Higa F, Tateyama M, Fujita J. Distribution of mycobacterial antigen based on differences of histological characteristics in pulmonary Mycobacterium avium infectious diseases--consideration of the extent of surgical resection from the pathological standpoint. Pathol Res Pract. 2012;208:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Almadi MA, Ghosh S, Aljebreen AM. Differentiating intestinal tuberculosis from Crohn's disease: a diagnostic challenge. Am J Gastroenterol. 2009;104:1003-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 181] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 13. | Kim BJ, Choi YS, Jang BI, Park YS, Kim WH, Kim YS, Jung SA, Han DS, Kim JS, Choi JH. Prospective evaluation of the clinical utility of interferon-γ assay in the differential diagnosis of intestinal tuberculosis and Crohn's disease. Inflamm Bowel Dis. 2011;17:1308-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Li X, Liu X, Zou Y, Ouyang C, Wu X, Zhou M, Chen L, Ye L, Lu F. Predictors of clinical and endoscopic findings in differentiating Crohn's disease from intestinal tuberculosis. Dig Dis Sci. 2011;56:188-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Yönal O, Hamzaoğlu HO. What is the most accurate method for the diagnosis of intestinal tuberculosis? Turk J Gastroenterol. 2010;21:91-96. [PubMed] |
| 16. | Sekar B, Selvaraj L, Alexis A, Ravi S, Arunagiri K, Rathinavel L. The utility of IS6110 sequence based polymerase chain reaction in comparison to conventional methods in the diagnosis of extra-pulmonary tuberculosis. Indian J Med Microbiol. 2008;26:352-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Mishra PK, Gorantla VR, Bhargava A, Varshney S, Vashistha P, Maudar KK. Molecular detection of Mycobacterium tuberculosis in formalin-fixed, paraffin-embedded tissues and biopsies of gastrointestinal specimens using real-time polymerase chain reaction system. Turk J Gastroenterol. 2010;21:129-134. [PubMed] |
| 18. | Mustafa T, Wiker HG, Mfinanga SG, Mørkve O, Sviland L. Immunohistochemistry using a Mycobacterium tuberculosis complex specific antibody for improved diagnosis of tuberculous lymphadenitis. Mod Pathol. 2006;19:1606-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Purohit MR, Mustafa T, Wiker HG, Mørkve O, Sviland L. Immunohistochemical diagnosis of abdominal and lymph node tuberculosis by detecting Mycobacterium tuberculosis complex specific antigen MPT64. Diagn Pathol. 2007;2:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Pieters J. Mycobacterium tuberculosis and the macrophage: maintaining a balance. Cell Host Microbe. 2008;3:399-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 353] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 21. | Makharia GK, Srivastava S, Das P, Goswami P, Singh U, Tripathi M, Deo V, Aggarwal A, Tiwari RP, Sreenivas V. Clinical, endoscopic, and histological differentiations between Crohn's disease and intestinal tuberculosis. Am J Gastroenterol. 2010;105:642-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 145] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 22. | Kirsch R, Pentecost M, Hall Pde M, Epstein DP, Watermeyer G, Friederich PW. Role of colonoscopic biopsy in distinguishing between Crohn's disease and intestinal tuberculosis. J Clin Pathol. 2006;59:840-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Pulimood AB, Peter S, Ramakrishna B, Chacko A, Jeyamani R, Jeyaseelan L, Kurian G. Segmental colonoscopic biopsies in the differentiation of ileocolic tuberculosis from Crohn's disease. J Gastroenterol Hepatol. 2005;20:688-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Gan HT, Chen YQ, Ouyang Q, Bu H, Yang XY. Differentiation between intestinal tuberculosis and Crohn's disease in endoscopic biopsy specimens by polymerase chain reaction. Am J Gastroenterol. 2002;97:1446-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 88] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Jin XJ, Kim JM, Kim HK, Kim L, Choi SJ, Park IS, Han JY, Chu YC, Song JY, Kwon KS. Histopathology and TB-PCR kit analysis in differentiating the diagnosis of intestinal tuberculosis and Crohn's disease. World J Gastroenterol. 2010;16:2496-2503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Pulimood AB, Ramakrishna BS, Kurian G, Peter S, Patra S, Mathan VI, Mathan MM. Endoscopic mucosal biopsies are useful in distinguishing granulomatous colitis due to Crohn's disease from tuberculosis. Gut. 1999;45:537-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 98] [Article Influence: 3.8] [Reference Citation Analysis (0)] |