Published online Dec 21, 2012. doi: 10.3748/wjg.v18.i47.6951
Revised: September 17, 2012
Accepted: September 22, 2012
Published online: December 21, 2012
AIM: To investigate the effects of hexahydrocurcumin (HHC), and its combination with 5-fluorouracil (5-FU) on dimethylhydrazine (DMH)-induced colon cancer in rats.
METHODS: Male Wistar rats weighing 100-120 g were used as subject models. Aberrant crypt foci (ACF), early preneoplastic lesions of colon cancer, were induced by subcutaneous injection of DHM (40 mg/kg) twice a week for two weeks. After the first DMH injection, rats were treated daily with vehicle (n = 12), curcumin (CUR) (50 mg/kg) (n = 12), HHC (50 mg/kg) orally (n = 12), and treated weekly with an intraperitoneal injection of 5-FU (50 mg/kg) (n = 12), or a combination of 5-FU plus CUR (n = 12) and HHC (n = 12) at the same dosage(s) for 16 wk. The total number of ACF and large ACF were assessed. Cyclooxygenase (COX)-1 and COX-2 expression were detected by immunohistochemistry in colon tissues. The quantitative data of both COX-1 and COX-2 expression were presented as the percentage of number of positive-stained cells to the total number of cells counted. Apoptotic cells in colon tissues were also visualized using the dUTP-biotin nick end labeling method. Apoptotic index (AI) was determined as the percentage of labeled nuclei with respect to the total number of nuclei counted.
RESULTS: The total number of ACF was highest in the DMH-vehicle group (1558.20 ± 17.37), however, the number of ACF was significantly reduced by all treatments, 5-FU (1231.20 ± 25.62 vs 1558.20 ± 17.37, P < 0.001), CUR (1284.20 ± 25.47 vs 1558.20 ± 17.37, P < 0.001), HHC (1086.80 ± 53.47 vs 1558.20 ± 17.37, P < 0.001), DMH-5-FU + CUR (880.20 ± 13.67 vs 1558.20 ± 17.37, P < 0.001) and DMH-5-FU + HHC (665.80 ± 16.64 vs 1558.20 ± 17.37, P < 0.001). Interestingly, the total number of ACF in the combined treatment groups, the DMH-5-FU + CUR group (880.20 ± 13.67 vs 1231.20 ± 25.62, P < 0.001; 880.20 ± 13.67 vs 1284.20 ± 25.47, P < 0.001) and the DMH-5-FU + HHC group (665.80 ± 16.64 vs 1231.20 ± 25.62, P < 0.001; 665.80 ± 16.64 vs 1086.80 ± 53.47, P < 0.001) were significantly reduced as compared to 5-FU or each treatment alone. Large ACF were also significantly reduced in all treatment groups, 5-FU (111.00 ± 7.88 vs 262.20 ± 10.18, P < 0.001), CUR (178.00 ± 7.33 vs 262.20 ± 10.18, P < 0.001), HHC (186.60 ± 21.51 vs 262.20 ± 10.18, P < 0.001), DMH-5-FU + CUR (122.00 ± 5.94 vs 262.20 ± 10.18, P < 0.001) and DMH-5-FU + HHC (119.00 ± 17.92 vs 262.20 ± 10.18, P < 0.001) when compared to the vehicle group. Furthermore, in the DMH-5-FU + CUR and DMH-5-FU + HHC groups the formation of large ACF was significantly reduced when compared to CUR (122.00 ± 5.94 vs 178.00 ± 7.33, P < 0.005) or HHC treatment alone (119.00 ± 17.92 vs 186.60 ± 21.51, P < 0.001), however, this reduction was not statistically different to 5-FU monotherapy (122.00 ± 5.94 vs 111.00 ± 7.88, P = 0.217; 119.00 ± 17.92 vs 111.00 ± 7.88, P = 0.619, respectively). The levels of COX-1 protein after all treatments were not different from normal rats. A marked increase in the expression of COX-2 protein was observed in the DMH-vehicle group. Over-expression of COX-2 was not significantly decreased by 5-FU treatment alone (95.79 ± 1.60 vs 100 ± 0.00, P = 0.198). However, over-expression of COX-2 was significantly suppressed by CUR (77.52 ± 1.68 vs 100 ± 0.00, P < 0.001), HHC (71.33 ± 3.01 vs 100 ± 0.00, P < 0.001), 5-FU + CUR (76.25 ± 3.32 vs 100 ± 0.00, P < 0.001) and 5-FU + HHC (68.48 ± 2.24 vs 100 ± 0.00, P < 0.001) in the treated groups compared to the vehicle group. Moreover, CUR (77.52 ± 1.68 vs 95.79 ± 1.60, P < 0.001), HHC (71.33 ± 3.01 vs 95.79 ± 1.60, P < 0.001), 5-FU + CUR treatments (76.25 ± 3.32 vs 95.79 ± 1.60, P < 0.001) and 5-FU + HHC (68.48 ± 2.24 vs 95.79 ± 1.60, P < 0.001) markedly decreased COX-2 protein expression more than 5-FU alone. Furthermore, the AI in all treated groups, 5-FU (38.86 ± 4.73 vs 23.56 ± 2.12, P = 0.038), CUR (41.78 ± 6.92 vs 23.56 ± 2.12, P < 0.001), HHC (41.06 ± 4.81 vs 23.56 ± 2.12, P < 0.001), 5-FU + CUR (49.05 ± 6.75 vs 23.56 ± 2.12, P < 0.001) and 5-FU + HHC (53.69 ± 8.59 vs 23.56 ± 2.12, P < 0.001) significantly increased when compared to the DMH-vehicle group. However, the AI in the combination treatments, 5-FU + CUR (49.05 ± 6.75 vs 41.78 ± 6.92, P = 0.192; 49.05 ± 6.75 vs 38.86 ± 4.73, P = 0.771) and 5-FU + HHC (53.69 ± 8.59 vs 41.06 ± 4.81, P = 0.379; 53.69 ± 8.59 vs 38.86 ± 4.73, P = 0.245) did not reach significant levels as compared with each treatment alone and 5-FU monotherapy, respectively.
CONCLUSION: The combined effects of HHC with 5-FU exhibit a synergistic inhibition by decreasing ACF formation mediated by down-regulation of COX-2 expression.
- Citation: Srimuangwong K, Tocharus C, Tocharus J, Suksamrarn A, Yoysungnoen Chintana P. Effects of hexahydrocurcumin in combination with 5-fluorouracil on dimethylhydrazine-induced colon cancer in rats. World J Gastroenterol 2012; 18(47): 6951-6959
- URL: https://www.wjgnet.com/1007-9327/full/v18/i47/6951.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i47.6951
5-fluorouracil (5-FU) therapy is a conventional treatment for colorectal cancer and has been used for five decades. However, the toxicity of this agent to normal tissues is a major obstacle to successful cancer chemotherapy. To reduce its toxicity, combination treatment of 5-FU with minimally toxic substances derived from plants, such as genistein and geraniol, have been employed. While these are beneficial, they are also considered counterproductive in enhancing overall 5-FU efficacy[1-3]. Curcumin (CUR), the major active substance in turmeric, which is derived from the rhizome of Curcuma longa L. may be an alternative[4]. CUR is considered a suitable replacement for genistein and geraniol as it promotes higher 5-FU efficacy in the treatment of various cancer types, including colon cancer[5,6]. The properties of CUR also highlight its usefulness, as it specifically inhibits the expression of cyclooxygenase (COX)-2, an enzyme highly expressed in a variety of human cancers, but does not suppress the expression of COX-1[7,8]. CUR also has a low level of toxicity and is safe for the prevention/treatment of human colorectal cancer compared to other chemopreventive agents such as non-steroidal inflammatory drugs (NSAIDs)[9]. Recently, Du et al[3] showed that the synergistic effect of CUR combined with 5-FU inhibited the growth and reduced the level of COX-2 protein expression in HT-29 human colon cancer cells. However, while CUR is a very important agent in preventing and treating colorectal cancer, its characteristics-such as poor solubility, poor absorption in the gastrointestinal tract and rapid decomposition in human blood-make it unsuitable for pharmaceutical development[10,11]. Therefore, CUR metabolites were synthesized in order to solve these problems[12]. Hexahydrocurcumin (HHC) is one of the major metabolites of CUR. Previous studies found that HHC exhibited stronger antioxidant activity than CUR[13]. Moreover, this compound inhibited the biosynthesis of prostaglandin (PGE2) in lipopolysaccharide (LPS)-stimulated macrophages[14]. PGE2 is also a major product of COX-2 enzymes, which are involved in colorectal carcinogenesis. Interestingly, HHC decreased PGE2 levels in phorbol ester-induced PGE2 production in human colonic epithelial cells[15]. Recently, our previous in vitro study showed that HHC markedly decreased the viability of HT-29 human colon cancer cells and down-regulated COX-2 mRNA production. Moreover, the combined effect of HHC with a low dose of 5-FU exerted a synergistic effect on the growth of HT-29 cells by markedly reducing cell viability and COX-2 mRNA to a greater degree than monotherapy[16]. Therefore, the present study aimed to investigate the anti-colon carcinogenic effects of HHC in combination with 5-FU, with a focus on the expression of COX-2 in dimethylhydrazine (DMH)-induced rat colon cancer.
Male Wistar rats (National Animal Center, Salaya, Nakorn Pathom, Thailand) weighing 100-120 g were used. Animals were housed at 25 ± 2 °C under a 12-h light/dark cycle. All procedures were carried out in accordance with the Guidelines of Animal Care described by the Animal Center, Faculty of Medical Science, Naresuan University, Phitsanulok, Thailand.
CUR and HHC were prepared from C. longa L. Briefly, the curcuminoid mixture obtained from the rhizomes of C. longa L. was subjected to silica-gel column chromatography, using hexane-dichloromethane, dichloromethane and dichloromethane-methanol as eluents to afford CUR as the major constituent. Recrystallization was accomplished by dissolving the evaporated eluate with a small quantity of dichloromethane and ethanol was then added. CUR crystallized out as yellow needles with a melting point (m.p.) of 181-183 °C. HHC was synthesized from CUR by a catalytic hydrogenation reaction in ethanol for 5 h with palladium on charcoal as a catalyst. The product was isolated from tetrahydrocurcumin and octahydrocurcumin by silica-gel column chromatography followed by recrystallization with dichloromethane-n-hexane to provide a 45% yield of HHC as a white amorphous solid (m.p. 81-82 °C). The spectroscopic (IR, 1H-NMR and mass spectra) data of the synthesized HHC were consistent with those in a previous report[17]. CUR and HHC were then separately dissolved in propylene glycol and were ready for use.
The animals were randomly divided into seven groups. The animals in group 1, designated the negative control (normal group, n = 10), were fed daily with propylene glycol (PG). Group 2 to 7 served as the carcinogenic groups. In these groups, the animals were subcutaneously injected with DMH (Sigma-Aldrich, St. Louis, MO, United States) (40 mg/kg) twice a week for two wk in order to induce aberrant crypt foci (ACF). In group 2, (vehicle group, n = 12) rats were fed daily with PG. Group 3 (5-FU group, n = 12) received an intraperitoneal injection (ip) of 5-FU at a dose of 50 mg/kg body weight weekly. Group 4 (CUR group, n = 12) and 5 (HHC group, n = 12), were treated daily with intragastric administration of CUR and HHC at a dose of 50 mg/kg, respectively, whereas group 6 (5-FU + CUR, n = 12) and 7 (5-FU + HHC, n = 12) were the combined treatment groups, and were treated with 5-FU plus CUR and 5-FU plus HHC, respectively. All treatments were started after the first DMH injection and were given daily until week 16.
Colons were dissected and washed with cold normal saline. They were then fixed in 10% buffer neutral formalin for 24 h. The colon tissues were stained with 2% methylene blue for 2 min and placed on a microscope slide with the mucosal surface up. ACF were examined under a light microscope at 40 × magnification and distinguished from surrounding normal crypts by the following characteristics: (1) increased size; (2) large luminal openings; (3) thickened epithelia; and (4) larger than adjacent normal crypts[18,19]. In this study, the number of ACF and large ACF defined as containing 4 crypts or more (> 3 crypts/ACF)[20], which represent advancing preneoplastic states, were counted.
COX-2: Paraffin sections from the distal portion of the colon were dewaxed in xylene and rehydrated through a graded series of alcohol. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide in methanol for 15 min at ambient temperature. After washing in water, nonspecific binding sites were blocked with 5% bovine serum in phosphate-buffered saline (PBS) for 30 min at ambient temperature. The primary polyclonal antibody COX-2 (Cayman Chemical, Ann Arbor, MI, United States) was diluted in 1% BSA/Tris-Cl (at a 1:500 dilution) and incubated at 4˚C overnight. The slides were then gently rinsed with PBS and incubated with the secondary antibody (goat anti-rabbit immunoglobulins) conjugated with labeled polymer-horseradish (HRP) developed by the Envision system/HRP (DAKO cytomation, Glostrup, Denmark) as follows: the slides were incubated in peroxidase-labeled polymer for 30 min and substrate-chromogen for 10 min at ambient temperature. The nuclei were counterstained with Mayer’s hematoxylin. COX-2 expression is presented as the percentage of the number of positive-stained cells to the total number of cells counted by image analysis (Image Pro®-plus).
COX-1: The paraffin sections were processed as in the COX-2 method up to the nonspecific binding sites step. The primary antibody COX-1 (Cayman Chemical, Ann Arbor, MI, United States) was diluted in 1% BSA/Tris-Cl (at a 1:1000 dilution) and incubated at 4˚C overnight. The slides were then rinsed with PBS and incubated with the secondary antibody conjugated with biotin (biotinylated anti-mouse immunoglobulins) for 1 h at ambient temperature. The slides were incubated with the ABC reagent (Vectastain, Burlingame, CA, United States) for 30 min at ambient temperature and developed by DAB peroxidase substrate (Vectastain, Burlingame, CA, United States). The nuclei were counterstained with Mayer’s hematoxylin. The positive stained cells were then counted by image analysis software (Image Pro®-plus). COX-1 expression is presented as the percentage of the number of positive-stained cells to the total number of cells counted.
Apoptotic cells in the distal colon were visualized using the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-biotin nick end labeling (TUNEL) method with the FragELTM DNA fragmentation Detection kit (Calbiochem, Darmstadt, Germany). The deparaffinized tissue sections were permeabilized by Triton-X-100 and inactivated by endogenous peroxidases with 3% H2O2 in methanol at ambient temperature for 5 min. The tissue sections were incubated with TUNEL reaction mixture containing the TdT at 37 °C for 60 min. Slides were rinsed twice in PBS for 10 min and dried around the sample. The labeled DNA was detected by DAB solution for 10-15 min at ambient temperature. The nuclei were counterstained immediately with methyl green solution. Finally, the slides were washed and analyzed under a light microscope. Apoptotic index (AI) was determined as the percentage of the labeled nuclei with respect to the total number of nuclei counted.
The differences in mean values among the different groups were tested and the values are expressed as mean ± SE. All data were tested by analysis of variance followed by the LSD test. All statistical calculations were carried out using SPSS (version 11.5) and a P-value < 0.05 was considered significant.
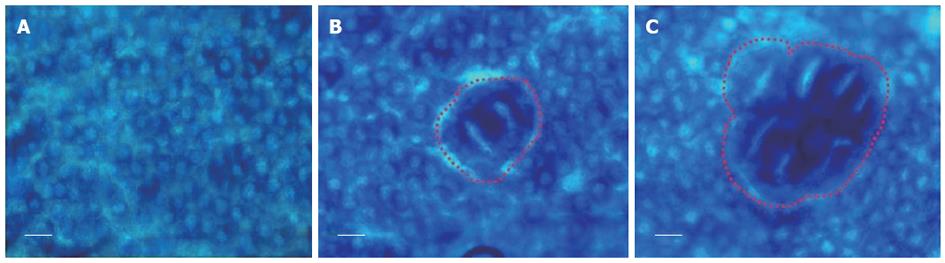
ACF were distinguished from the surrounding normal crypts by their increased size, darker epithelial lining than normal crypts and large luminal opening (Figure 1). We assessed ACF formation in the colonic epithelia of experimental models by recording two parameters of ACF: (1) number of aberrant crypts and (2) large ACF containing 4 crypts or more (> 3 crypts/ACF), which are found in advancing preneoplastic states. The results showed that all rats developed ACF in the colon after DMH induction. The total number of ACF was highest in the DMH-vehicle group (1558.20 ± 17.37) as shown in Table 1. HHC treatment alone markedly reduced the total number of ACF as compared to the vehicle group (1086.80 ± 53.47 vs 1558.20 ± 17.37, P < 0.001). In addition, the total number of ACF in the 5-FU (1231.20 ± 25.62 vs 1558.20 ± 17.37, P < 0.001) and CUR treatment alone groups (1284.20 ± 25.47 vs 1558.20 ± 17.37, P < 0.001) were significantly decreased as compared to the DMH-vehicle group. Interestingly, the total number of ACF in the combined treatment group, the DMH-5-FU + HHC group, was significantly reduced as compared to 5-FU or HHC treatment alone (665.80 ± 16.64 vs 1231.20 ± 25.62, P < 0.001; 665.80 ± 16.64 vs 1086.80 ± 53.47, P < 0.001). Similarly, the total number of ACF in the DMH-5-FU + CUR group was significantly reduced as compared to 5-FU or CUR treatment alone (880.20 ± 13.67 vs 1231.20 ± 25.62, P < 0.001; 880.20 ± 13.67 vs 1284.20 ± 25.47, P < 0.001). The large ACF were significantly reduced in all treatment groups, 5-FU (111.00 ± 7.88 vs 262.20 ± 10.18, P < 0.001), CUR (178.00 ± 7.33 vs 262.20 ± 10.18, P < 0.001), HHC (186.60 ± 21.51 vs 262.20 ± 10.18, P < 0.001), DMH-5-FU + CUR (122.00 ± 5.94 vs 262.20 ± 10.18, P < 0.001) and DMH-5-FU + HHC (119.00 ± 17.92 vs 262.20 ± 10.18, P < 0.001) when compared to the vehicle group. Furthermore, the DMH-5-FU + CUR and DMH-5-FU + HHC groups significantly reduced the formation of the large ACF when compared to CUR (122.00 ± 5.94 vs 178.00 ± 7.33, P < 0.005) or HHC treatment alone (119.00 ± 17.92 vs 186.60 ± 21.51, P < 0.001), however, this reduction was not statistically different to 5-FU monotherapy (122.00 ± 5.94 vs 111.00 ± 7.88, P = 0.217; 119.00 ± 17.92 vs 111.00 ± 7.88, P = 0.619, respectively).
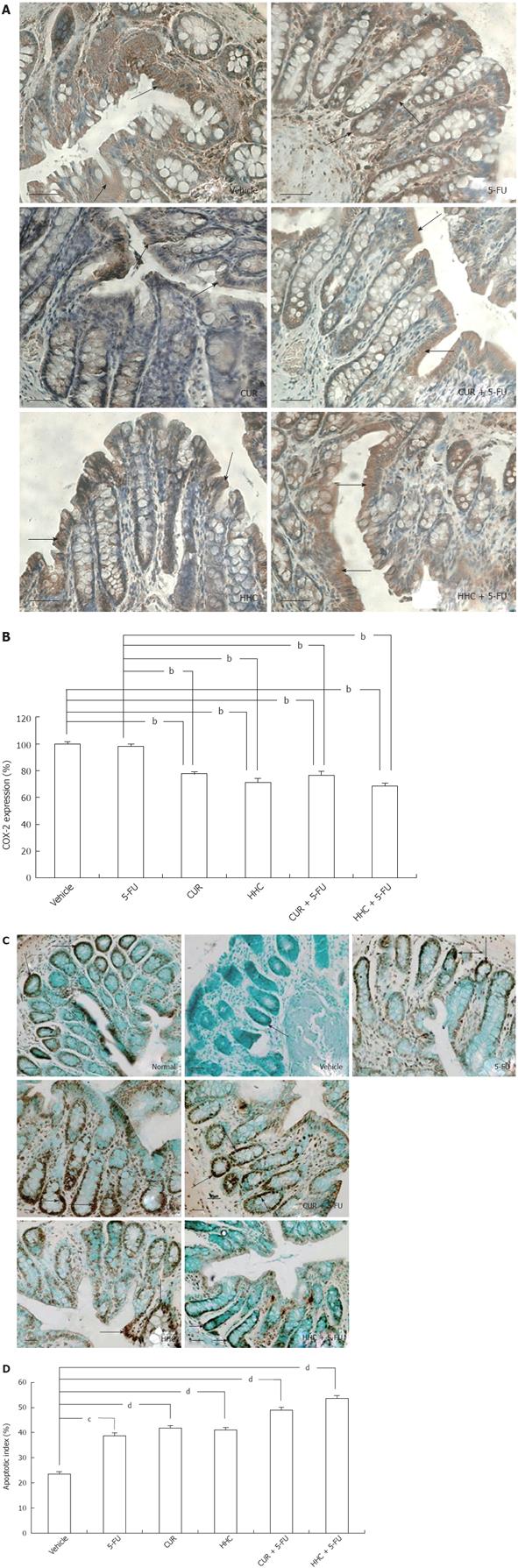
Immunohistochemical assays demonstrated that COX-2 protein was mostly localized in the cytoplasm in a diffuse-granular pattern. Positive staining was seen as a brown stain at a low-power field (20X), indicated by the arrows in Figure 2A. The COX-2 protein was not observed in normal colon mucosal tissue. The over-expression of COX-2 was most marked in DMH-vehicle rats (Figure 2A). CUR and HHC alone and their combined treatments attenuated the over-expression of COX-2. The quantitative data from image analysis showed that marked COX-2 expression was found in the vehicle group (100% ± 0.00%) (Figure 2B). Over-expression of COX-2 was not significantly decreased by 5-FU treatment alone (95.79% ± 1.60% vs 100% ± 0.00%, P = 0.198). However, over-expression of COX-2 was significantly suppressed by CUR (77.52% ± 1.68% vs 100% ± 0.00%, P < 0.001), HHC (71.33% ± 3.01% vs 100% ± 0.00%, P < 0.001), 5-FU + CUR (76.25% ± 3.32% vs 100% ± 0.00%, P < 0.001) and 5-FU + HHC (68.48% ± 2.24% vs 100% ± 0.00%, P < 0.001) compared to the vehicle group. Moreover, CUR monotherapy (77.52% ± 1.68% vs 95.79% ± 1.60%, P < 0.001), HHC monotherapy (71.33% ± 3.01% vs 95.79% ± 1.60%, P < 0.001), 5-FU + CUR (76.25% ± 3.32% vs 95.79% ± 1.60%, P < 0.001) and 5-FU + HHC (68.48% ± 2.24% vs 95.79% ± 1.60%, P < 0.001) markedly decreased COX-2 protein expression more than 5-FU treatment alone.
COX-1 expression was observed in the cytoplasm of most cells in colonic crypts in all animals. The COX-1 protein-labeled cells were analyzed by image analysis. The data revealed that the level of COX-1 protein in all treated rats was not different from the normal group (data not shown).
Apoptotic cells showed a strong dark or brown stain in normal colon mucosa (arrows in Figure 2C). In contrast, apoptotic cells were very low in DMH-vehicle group. 5-FU, CUR, and HHC alone and their combined treatments induced an increment of apoptotic cells in colon tissue. The quantitative data, as shown by the AI was highest in normal colon tissue (75.71% ± 3.59%). In Figure 2D, the results show that AI was very low in the DMH-induced colon cancer vehicle group (23.56% ± 2.12%). The AI in all treated groups were as follows: 5-FU (38.86% ± 4.73% vs 23.56% ± 2.12%, P = 0.038), CUR (41.78% ± 6.92% vs 23.56% ± 2.12%, P < 0.001), HHC (41.06% ± 4.81% vs 23.56% ± 2.12%, P < 0.001), 5-FU + CUR (49.05% ± 6.75% vs 23.56% ± 2.12%, P < 0.001) and 5-FU + HHC (53.69% ± 8.59% vs 23.56% ± 2.12%, P < 0.001) and were significantly increased when compared to the DMH-vehicle group. However, the AI in the combined treatments which were: 5-FU + CUR (49.05% ± 6.75% vs 41.78% ± 6.92%, P = 0.192; 49.05% ± 6.75% vs 38.86% ± 4.73%, P = 0.771) and 5-FU + HHC (53.69% ± 8.59% vs 41.06% ± 4.81%, P = 0.379; 53.69% ± 8.59% vs 38.86% ± 4.73%, P = 0.245) did not reach significant levels as compared with each treatment alone and 5-FU monotherapy, respectively.
This study aimed to determine the anti-colon carcinogenic effects of HHC, a major metabolite of CUR, in combination with 5-FU therapy with a focus on the expression of COX-2 in DMH-induced colorectal cancer in rats. DMH induces DNA damage in the colon, ileum, and liver, and thus is a carcinogen in colorectal tissue[21]. Several studies have reported that ACF are recognized as early preneoplastic lesions of colorectal origin. In addition, crypt multiplicity may indicate a step in colon carcinogenesis promotion[22,23]. This study thus assessed the formation of ACF in DMH-induced colorectal cancer in a rat model. The results showed that both the total number of ACF and large ACF were highest in the DMH-vehicle group. Moreover, over-expression of COX-2 protein was most marked in this group. These findings agree with a previous study where an over-expression of COX-2 was detected in ACF, adenomas and carcinomas in azoxymethane-induced rat colon cancer[24]. Several studies reported that COX-2 over-expression and up-regulation of the prostaglandins play a crucial role in carcinogenesis and angiogenesis[25-27]. COX-2 is also known to have an anti-apoptotic effect on colon cancer cells via the activation of different signal transduction pathways[28-30]. Therefore, ACF induction of COX-2 promotes colon carcinogenesis, and blockage of these processes is a strategy for colon cancer prevention and treatment.
In the present study, we demonstrated that HHC treatment alone significantly reduced the total number of ACF and large ACF compared with the vehicle. This result suggests that HHC has the ability to inhibit the initiation and promotion of steps in colorectal carcinogenesis which is similar to the property of CUR[31]. Furthermore, HHC together with 5-FU exhibited a synergistic effect, with inhibition of total ACF numbers being higher than either HHC or 5-FU treatment alone. Therefore, it is reasonable to assume that HHC augments the growth inhibitory effect of 5-FU chemotherapy by inhibiting the initiation of colorectal carcinogenesis. This result also correlates with the synergistic inhibitory effect of CUR combined with 5-FU on the growth of HT-29 human colon cancer cells[3].
The anti-colorectal cancer mechanisms of HHC have not previously been reported. However, Shao et al[14] reported that this compound inhibited the biosynthesis of PGE2 in LPS-stimulated macrophages. Our study found that HHC alone significantly decreased the COX-2 protein level compared to vehicle treatment which was similar to CUR treatment alone. The results support our recent report which showed that HHC is significantly responsible for the down-regulation of COX-2 mRNA synthesis, but does not alter the expression of COX-1 in HT-29 human colon cancer cells[16]. Furthermore, HHC in combination with 5-FU has a higher level of potency against COX-2 mRNA expression when compared with cells treated with 5-FU or HHC alone. We therefore propose that HHC plays a significant role in inhibiting the expression of COX-2 protein in colorectal carcinogenesis, and when combined with 5-FU can lead to an overall decrease in the level of COX-2 protein and a reduction in the formation of ACF in the early stage of colorectal carcinogenesis through attenuation of COX-2 expression. When compared to the vehicle treatment group, this is an important distinction. Furthermore, this study found that the level of COX-1 protein after treatment with HHC alone and in combination with 5-FU was not different from normal rats. Therefore, HHC can be classified as a selective COX-2 inhibitor in contrast to some NSAIDs such as meloxicam, diclofenac, indomethacin which inhibit both COX-2 and COX-1 expression, causing injury to the gastric mucosa[32,33]. We suggest that HHC treatment alone or in combination with 5-FU would not produce unwanted side effects in the colorectal cancer rat model, and thus HHC administration may be suitable for the long-term treatment and/or prevention of human colorectal cancer. However, we must consider that the relationship between COX-2 and carcinogenesis may involve several pathways, including conversion of procarcinogens into active carcinogens, inhibition of apoptosis, increase in tumor growth and invasiveness, and promotion of angiogenesis[25-30,34]. Therefore, it is reasonable that COX-2 inhibitors may offer an important and powerful target for cancer prevention and treatment. In the present study, we also investigated whether HHC could induce colon cell apoptosis in DMH-induced colon cancer in rats. We showed that colon cell apoptosis in the DMH-injected group was significantly lower than that in normal rats and was very low in the vehicle-treated group, however, treatment with HHC alone resulted in greater levels of apoptosis. These results suggested that HHC may also suppress colorectal carcinogenesis through its ability to induce apoptosis, possibly by inhibiting COX-2 production. The combined treatment of HHC and 5-FU significantly increased apoptosis when compared to the vehicle group, but not when compared to the groups treated with HHC and 5-FU alone. These findings suggested that HHC combined with 5-FU did not have a synergistic effect on apoptosis induction in colorectal carcinogenesis in the DMH-induced rat model. Therefore, the combined effects of these agents on inhibition of ACF formation may be due to other modes of actions which need to be explored.
In summary, the evidence presented in this in vivo study gives new insight into the anti-carcinogenic activity of HHC in this colorectal cancer model. Our findings indicate that the addition of DMH caused COX-2 over-expression resulting in resistance to apoptosis in colorectal tissues. HHC treatment alone and combined with 5-FU significantly suppressed the growth of colorectal cancer at the initiation step by down-regulating the expression of COX-2 and inducing apoptosis. Although the combined effect of HHC and 5-FU was similar to its progenitor, CUR, the greater bioavailability of HCC makes it more suitable for further pharmaceutical development in the prevention and therapy of colon cancer. Further studies should be encouraged to determine the mechanisms underlining these anti-cancer activities.
The toxicity of 5-fluorouracil (5-FU) chemotherapy in normal tissues is a major limitation of its treatment of colon cancer. To avoid the toxicity of chemotherapeutic agents, alternative treatments using chemopreventive natural medicine may be an option. The previous study showed that hexahydrocurcumin (HHC), a natural metabolite of curcumin (CUR), enhanced 5-FU in inhibiting the growth of HT-29 human colon cancer cells and down-regulated the expression of cyclooxygenase-2 (COX-2) mRNA in vitro. However, the effects of HHC combined with 5-FU in vivo have not yet been studied. The present study therefore aimed to investigate the anti-colon carcinogenic effects of HHC in combination with 5-FU on dimethylhydrazine (DMH)-induced colon cancer in rats, with a focus on the expression of the COX-2.
This study involved testing the anti-colon carcinogenesis effect of HHC combined with the standard chemotherapeutic agent, 5-FU. Areas of this research included: studying the natural substance-HHC as an alternative or an adjunctive cancer therapy; testing the anti-carcinogenic effect against DMH-induced colon cancer in rats; measuring aberrant crypt foci (ACF) which are early preneoplastic lesions of colorectal cancer; measuring COX-2 protein, an important enzyme which promotes/initiates the colon carcinogenesis process; and detecting the apoptosis of colonic cells after these treatments.
In a previous study of 5-FU combined with CUR, it was found that this combined treatment had a synergistic effect on inhibiting the growth of a colorectal cancer cell line. However, the physical properties of CUR are unsuitable for pharmaceutical development. The authors thus synthesized HHC, a major metabolite of CUR, which shows greater bioavailability compared to CUR. In this study, HHC in combination with 5-FU was tested for the treatment of colorectal cancer. The authors found that HHC in combination with 5-FU suppressed the formation of ACF more than HHC or 5-FU treatment alone, which indicates that this combination shows a synergistic effect for anti-carcinogenic activity.
The results suggest that HHC combined with 5-FU can suppress the growth of colorectal cancer without any side effects. HHC plus 5-FU also showed a synergistic inhibitory effect to reduce ACF formation. Although the combined effect of HHC and 5-FU was similar to its progenitor, CUR, its greater bioavailability in colon cancer makes it more suitable for further pharmaceutical development in the prevention of colon cancer and its treatment.
5-FU is a conventional cancer chemotherapeutic drug used to treat human colorectal cancer. Its toxicity to normal cells and long-term exposure to this drug shows higher resistance in cancer cells to treatment. CUR is the major yellow pigment in turmeric which is obtained from the rhizome of Curcuma longa L. HHC is one of the natural metabolites of CUR.
This study examines the effects of HHC, a relatively low toxic CUR metabolite, on colonic ACF formation in a rat model induced by DMH to produce colorectal cancer. The authors suggest that HHC has similar effects to CUR and reduced ACF numbers by increasing apoptosis, inhibiting COX-2 protein expression but no expression of COX-1. This is an interesting study that extends previous in vitro studies and suggests that HHC effectively reduced preneoplastic changes in an in vivo model of colorectal cancer.
Peer reviewer: Debbie Trinder, PhD, School of Medicine and Pharmacology, University of Western Australia, Fremantle Hospital, PO Box 480, Fremantle 6959, Western Australia, Australia
S- Editor Gou SX L- Editor Webster JR E- Editor Zhang DN
| 1. | Hwang JT, Ha J, Park OJ. Combination of 5-fluorouracil and genistein induces apoptosis synergistically in chemo-resistant cancer cells through the modulation of AMPK and COX-2 signaling pathways. Biochem Biophys Res Commun. 2005;332:433-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 138] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 2. | Carnesecchi S, Bras-Gonçalves R, Bradaia A, Zeisel M, Gossé F, Poupon MF, Raul F. Geraniol, a component of plant essential oils, modulates DNA synthesis and potentiates 5-fluorouracil efficacy on human colon tumor xenografts. Cancer Lett. 2004;215:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Du B, Jiang L, Xia Q, Zhong L. Synergistic inhibitory effects of curcumin and 5-fluorouracil on the growth of the human colon cancer cell line HT-29. Chemotherapy. 2006;52:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 103] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Wahl H, Tan L, Griffith K, Choi M, Liu JR. Curcumin enhances Apo2L/TRAIL-induced apoptosis in chemoresistant ovarian cancer cells. Gynecol Oncol. 2007;105:104-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Hanif R, Qiao L, Shiff SJ, Rigas B. Curcumin, a natural plant phenolic food additive, inhibits cell proliferation and induces cell cycle changes in colon adenocarcinoma cell lines by a prostaglandin-independent pathway. J Lab Clin Med. 1997;130:576-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 164] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 6. | Kawamori T, Lubet R, Steele VE, Kelloff GJ, Kaskey RB, Rao CV, Reddy BS. Chemopreventive effect of curcumin, a naturally occurring anti-inflammatory agent, during the promotion/progression stages of colon cancer. Cancer Res. 1999;59:597-601. [PubMed] |
| 7. | Lee KW, Kim JH, Lee HJ, Surh YJ. Curcumin inhibits phorbol ester-induced up-regulation of cyclooxygenase-2 and matrix metalloproteinase-9 by blocking ERK1/2 phosphorylation and NF-kappaB transcriptional activity in MCF10A human breast epithelial cells. Antioxid Redox Signal. 2005;7:1612-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Lev-Ari S, Vexler A, Starr A, Ashkenazy-Voghera M, Greif J, Aderka D, Ben-Yosef R. Curcumin augments gemcitabine cytotoxic effect on pancreatic adenocarcinoma cell lines. Cancer Invest. 2007;25:411-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Engelhardt G, Bögel R, Schnitzer C, Utzmann R. Meloxicam: influence on arachidonic acid metabolism. Part 1. In vitro findings. Biochem Pharmacol. 1996;51:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 98] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Ravindranath V, Chandrasekhara N. Absorption and tissue distribution of curcumin in rats. Toxicology. 1980;16:259-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 290] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Wang YJ, Pan MH, Cheng AL, Lin LI, Ho YS, Hsieh CY, Lin JK. Stability of curcumin in buffer solutions and characterization of its degradation products. J Pharm Biomed Anal. 1997;15:1867-1876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1140] [Cited by in RCA: 1165] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 12. | Anand P, Thomas SG, Kunnumakkara AB, Sundaram C, Harikumar KB, Sung B, Tharakan ST, Misra K, Priyadarsini IK, Rajasekharan KN. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem Pharmacol. 2008;76:1590-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 811] [Cited by in RCA: 783] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 13. | Somparn P, Phisalaphong C, Nakornchai S, Unchern S, Morales NP. Comparative antioxidant activities of curcumin and its demethoxy and hydrogenated derivatives. Biol Pharm Bull. 2007;30:74-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 237] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 14. | Shao J, Lee SB, Guo H, Evers BM, Sheng H. Prostaglandin E2 stimulates the growth of colon cancer cells via induction of amphiregulin. Cancer Res. 2003;63:5218-5223. [PubMed] |
| 15. | Ireson C, Orr S, Jones DJ, Verschoyle R, Lim CK, Luo JL, Howells L, Plummer S, Jukes R, Williams M. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001;61:1058-1064. [PubMed] |
| 16. | Srimuangwong K, Tocharus C, Yoysungnoen Chintana P, Suksamrarn A, Tocharus J. Hexahydrocurcumin enhances inhibitory effect of 5-fluorouracil on HT-29 human colon cancer cells. World J Gastroenterol. 2012;18:2383-2389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Changtam C, de Koning HP, Ibrahim H, Sajid MS, Gould MK, Suksamrarn A. Curcuminoid analogs with potent activity against Trypanosoma and Leishmania species. Eur J Med Chem. 2010;45:941-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 18. | Bouzourene H, Chaubert P, Seelentag W, Bosman FT, Saraga E. Aberrant crypt foci in patients with neoplastic and nonneoplastic colonic disease. Hum Pathol. 1999;30:66-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | McLellan EA, Bird RP. Aberrant crypts: potential preneoplastic lesions in the murine colon. Cancer Res. 1988;48:6187-6192. [PubMed] |
| 20. | Taché S, Ladam A, Corpet DE. Chemoprevention of aberrant crypt foci in the colon of rats by dietary onion. Eur J Cancer. 2007;43:454-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Pretlow TP, Barrow BJ, Ashton WS, O'Riordan MA, Pretlow TG, Jurcisek JA, Stellato TA. Aberrant crypts: putative preneoplastic foci in human colonic mucosa. Cancer Res. 1991;51:1564-1567. [PubMed] |
| 22. | Bird RP. Role of aberrant crypt foci in understanding the pathogenesis of colon cancer. Cancer Lett. 1995;93:55-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 400] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 23. | Roncucci L, Stamp D, Medline A, Cullen JB, Bruce WR. Identification and quantification of aberrant crypt foci and microadenomas in the human colon. Hum Pathol. 1991;22:287-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 210] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 24. | Takahashi M, Mutoh M, Kawamori T, Sugimura T, Wakabayashi K. Altered expression of beta-catenin, inducible nitric oxide synthase and cyclooxygenase-2 in azoxymethane-induced rat colon carcinogenesis. Carcinogenesis. 2000;21:1319-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 111] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | Harris RE. Cyclooxygenase-2 (cox-2) and the inflammogenesis of cancer. Subcell Biochem. 2007;42:93-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 143] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 26. | Williams CS, Tsujii M, Reese J, Dey SK, DuBois RN. Host cyclooxygenase-2 modulates carcinoma growth. J Clin Invest. 2000;105:1589-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 524] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 27. | Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Arico S, Pattingre S, Bauvy C, Gane P, Barbat A, Codogno P, Ogier-Denis E. Celecoxib induces apoptosis by inhibiting 3-phosphoinositide-dependent protein kinase-1 activity in the human colon cancer HT-29 cell line. J Biol Chem. 2002;277:27613-27621. [PubMed] |
| 29. | Elder DJ, Halton DE, Playle LC, Paraskeva C. The MEK/ERK pathway mediates COX-2-selective NSAID-induced apoptosis and induced COX-2 protein expression in colorectal carcinoma cells. Int J Cancer. 2002;99:323-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 83] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Sun Y, Tang XM, Half E, Kuo MT, Sinicrope FA. Cyclooxygenase-2 overexpression reduces apoptotic susceptibility by inhibiting the cytochrome c-dependent apoptotic pathway in human colon cancer cells. Cancer Res. 2002;62:6323-6328. [PubMed] |
| 31. | Shpitz B, Giladi N, Sagiv E, Lev-Ari S, Liberman E, Kazanov D, Arber N. Celecoxib and curcumin additively inhibit the growth of colorectal cancer in a rat model. Digestion. 2006;74:140-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Engelhardt G, Bögel R, Schnitzler C, Utzmann R. Meloxicam: influence on arachidonic acid metabolism. Part II. In vivo findings. Biochem Pharmacol. 1996;51:29-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 82] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Fosslien E. Adverse effects of nonsteroidal anti-inflammatory drugs on the gastrointestinal system. Ann Clin Lab Sci. 1998;28:67-81. [PubMed] |
| 34. | Fosslien E. Review: molecular pathology of cyclooxygenase-2 in cancer-induced angiogenesis. Ann Clin Lab Sci. 2001;31:325-348. [PubMed] |