Published online Dec 21, 2012. doi: 10.3748/wjg.v18.i47.6894
Revised: June 13, 2012
Accepted: June 28, 2012
Published online: December 21, 2012
In addition to squamous cell carcinoma, the incidence of Barrett’s esophagus with high-grade dysplasia and esophageal adenocarcinoma is rapidly increasing worldwide. Unfortunately, the current standard of care for esophageal pathology involves resection of the affected tissue, sometimes involving radical esophagectomy. Without exception, these procedures are associated with a high morbidity, compromised quality of life, and unacceptable mortality rates. Regenerative medicine approaches to functional tissue replacement include the use of biological and synthetic scaffolds to promote tissue remodeling and growth. In the case of esophageal repair, extracellular matrix (ECM) scaffolds have proven to be effective for the reconstruction of small patch defects, anastomosis reinforcement, and the prevention of stricture formation after endomucosal resection (EMR). More so, esophageal cancer patients treated with ECM scaffolds have shown complete restoration of a normal, functional, and disease-free epithelium after EMR. These studies provide evidence that a regenerative medicine approach may enable aggressive resection of neoplastic tissue without the need for radical esophagectomy and its associated complications.
- Citation: Londono R, Jobe BA, Hoppo T, Badylak SF. Esophagus and regenerative medicine. World J Gastroenterol 2012; 18(47): 6894-6899
- URL: https://www.wjgnet.com/1007-9327/full/v18/i47/6894.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i47.6894
The default tissue response to injury in adult mammals is characterized by hemostasis, inflammation, and subsequent deposition of dense collagenous connective tissue (i.e., scar tissue)[1-5]. The deposited scar tissue serves as a partial volume replacement for the missing native tissue and maintains the structural integrity of the tissue, albeit at a loss of normal function in many instances. This mechanism is adequate in most, but not all, tissues.
Some tissues in adults retain the ability to regenerate either as part of normal physiologic events or in response to injury. For example, the epidermis is completely replaced approximately every 40 d[6-9]. The bone marrow sustains a regenerating population of cells to continuously replenish the hematopoietic cell population[10-13], and the intestinal epithelium regenerates from a well described crypt stem cell population[14-16]. The liver can respond to injury by a nonblastemal epimorphic regenerative mechanism[17] and can replace most if not all of its lost hepatocellular mass if the native stroma remains intact[18-20]. Skeletal muscle has limited regenerative potential and can respond to mild or repetitive injury with full return to structure and function[21,22]. However, volumetric muscle loss (i.e., loss of greater the 20% of the muscle mass) results in deposition of scar tissue[23]. Therefore regenerative potential is encoded into the genome of adult mammals but only functionally expressed in selected tissues or to a limited extent. It should also be noted that all of these examples of tissue/organ regeneration involve the participation of a reserve stem/progenitor cell population.
Those tissues with the inability to regenerate functional mass following injury are the cause of significant morbidity, aesthetic deformity, mortality, and are causally associated with a large fraction of the health care burden worldwide. For example, the inability to regenerate functional myocardium following ischemic coronary artery disease[24-27], the dysfunctional central nervous system tissue following ischemic stroke or spinal cord injury[28-35], and the lack of functional pancreatic beta cells following immune mediated destruction[36-39] are the cause for a group of diseases that affects a large percentage of the aging population. Esophageal pathology, especially neoplasia, affects a rapidly increasing number of individuals in North America[40,41] and worldwide[42]. The lack of regenerative ability in the esophagus relegates this tubular structure to an inflammation/scarring response following injury, which in turn results in stricture and loss of function. Therefore, the standard of care for many esophageal diseases, especially overt cancer and its’ precursor Barrett’s disease with high grade dysplasia (HGD) involves esophagectomy; a procedure associated with a complication incidence approaching 50%[43-46]. A regenerative medicine approach which can recreate functional esophageal tissue, preserve the integrity of the esophagus, and avoid the necessity for esophagectomy would offer a significant advancement in the arsenal of treatment methods available to affected patients.
There are 5000 to 10 000 patients identified annually with non-neoplastic esophageal disease[47] including congenital anomalies such as esophageal atresia, tracheoesophageal fistulas[48], and corrosive injuries[49,50]. The incidence of Barrett’s esophagus (BE) and esophageal adenocarcinoma has increased dramatically and esophageal cancer now represents the world’s sixth leading cause of cancer death with 300 000 new cases each year[41,42,51]. The management of Barrett’s disease with HGD and intramucosal adenocarcinoma remains controversial. Esophagectomy has been the standard of care for HGD based on the high incidence of progression to subsequent neoplasia[52,53]. However, the majority of patients in which esophageal neoplasia is diagnosed have disease limited to the mucosa and the involvement of regional lymph nodes is unlikely. Because esophagectomy is associated with high morbidity rates and a marked compromise in quality of life, there has been a great deal of interest and success in minimally invasive endoscopic approaches which involve esophageal preserving techniques in patients with superficial malignancy[54].
There are several endoscopic ablation techniques for BE with HGD and for superficial adenocarcinoma. Radiofrequency ablation (RFA) has become accepted as a viable treatment for BE, especially flat BE, in light of a recent sham controlled randomized trial[55]. RFA has been shown to effectively ablate BE with very low rate of stricture formation. For BE with nodularity, endomucosal resection (EMR) with or without ablation therapy has been shown to be safe and effective to eradicate BE and prevent the recurrence of BE with minimal complications[56]. Excellent survival has been found in long term follow up studies in which endoscopic approaches were used to treat HGD and intramucosal adenocarcinoma[57,58].
However, the development of metachronous lesions is common (21.5%) with risk factors that include piecemeal resection, no ablation therapy of flat BE after EMR, long-segment BE, multifocal neoplasia, and the prolonged time required for complete eradication of the lesions[59]. Currently used techniques invariably include one or more of the stated risk factors. These risk factors are compounded by the inability to remove all affected tissue as an en bloc specimen by endoscopic techniques; thus less than optimal specimens are available for histopathologic examination of the removed tissue.
A stepwise radical endoscopic resection (SRER) has been proposed to treat BE refractory to RFA and/or EMR. A recent multicenter randomized trial[60] has demonstrated encouraging results of SRER but the technique involved a greater number of therapeutic sessions and complications such as esophageal stenosis requiring dilation in up to 50% of cases.
In summary, the limitations of currently used endoscopic techniques include the necessity for numerous interventions, the high incidence of metachronous lesions, the absence of a suitable tissue specimen for histologic assessment, and the unavoidable sampling error that occurs especially in patients with long segment Barrett’s. Ideally, en bloc resection of the entire abnormal epithelium in a single procedure without any compromise of tissue specimens collected for histopathologic examination would be possible. A regenerative medicine strategy that would facilitate restitution of the resected esophageal tissue without concomitant stenosis would represent a significant advancement in the treatment of esophageal disease.
Classic tissue engineering and regenerative medicine approaches involve either cell based therapies, utilization of a scaffold material, and/or use of bioactive molecules such as growth factors, cytokines and chemokines. In reality, the goal of all approaches is to alter or avoid the default inflammatory/scar tissue response to esophageal injury, and either replace the missing tissue with engineered normal tissue or stimulate the endogenous formation of new, site appropriate functional tissue.
Although an esophageal epithelial stem cell population located in the basal layer of the esophagus has been identified[61-65], their use in a cell based approach to functional esophageal reconstruction has not been described. Sheets of esophageal epithelial cells can be cultured[66-68], but practical application of such cell sheet technology to resurface the esophageal lumen following ablative procedures has not been successful. An approach which involves the placement of xenogeneic extracellular matrix (ECM) showed that full thickness defects that included approximately 40%-50% of the circumference and 5 cm of length could facilitate a constructive, non-stenotic healing response with formation of all layers of the esophageal wall in a preclinical dog model[47]. However, when reconstruction of complete circumferential full thickness defects was attempted with the same ECM scaffold approach, there was the uniform occurrence of severe stricture[69]. Of note however, if the complete circumferential defects were not full thickness in nature and lesions were limited to the mucosa, then placement of the ECM scaffold upon the subjacent muscularis externa supported the endogenous regeneration of a functional mucosa without clinical stricture[47,69-71].
These results suggested that a combination of the biologic scaffold material in contact with a native esophageal cell population (i.e., skeletal and smooth muscle plus adventitial cells) was required for a constructive remodeling response to occur. Further studies showed that as little as 30% of the normal esophageal muscle tissue was required to support the constructive type of esophageal remodeling outcome which allowed for normal dietary habits and absence of any signs of esophageal disease[69].
The promising results of these preclinical studies were the basis of successful endoscopic treatment for five patients with esophageal adenocarcinoma[72]. All patients had long segment disease limited to the mucosa. Complete circumferential en bloc mucosal resection, ranging from 8 cm to 14 cm in length, was performed on these patients with subsequent placement of a xenogeneic ECM scaffold (SurgiSisTM, Cook Biotech, Lafayatte, IN) held in place by an expandable stent. The stent was removed within 9-17 d during which time the ECM scaffold integrated with the underlying muscular wall of the esophagus and supported complete epithelialization and formation of a new submucosal layer. All patients required transient post operative dilation for mild stricture but were able to then eat a normal diet without recurrence of disease. Several of these patients have had subsequent reflux surgery and require no further treatment (unreported data). In the context of classic approaches to regenerative medicine, one could consider the successful approach in these patients as a combination of scaffold plus the bioactive factors inherent in the ECM, plus the required endogenous host cells in contact with the scaffold.
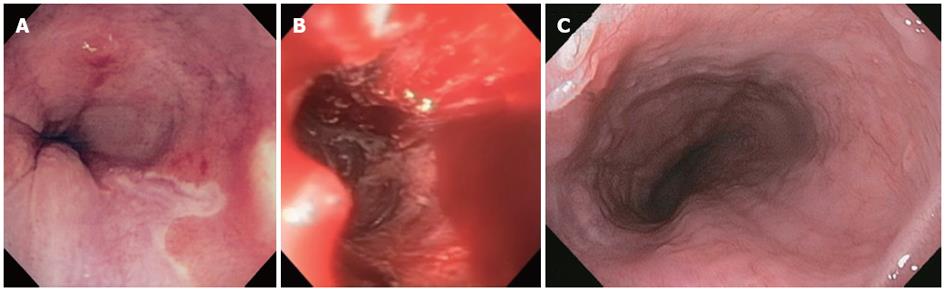
Using a similar approach, three additional patients recently were subjected to endoscopic, circumferential en bloc resection of Barrett’s with HGD, followed by fundoplication (Figure 1).The results support the findings from the previous study and provide further evidence for the use of this procedure as a feasible alternative to surgery for the treatment of HGD and intramucosal adenocarcinoma.
Alternative regenerative medicine approaches to creating esophageal tissue have been explored. Grikscheit et al[73] adapted a technique previously used in intestinal engineering whereby organoid units, mesenchymal cores surrounded by epithelial cells, were isolated from neonatal and adult rats, labeled with green fluorescent protein (GFP), and paratopically transplanted on biodegradable polyglycolic acid tubes before implantation within the omentum of syngeneic hosts. Four weeks later, the engineered esophageal tissue was either harvested or anastomosed as an onlay patch or total interposition graft[73]. Histologic examination of these organoids showed a complete esophageal wall including mucosa, submucosa, and muscularis propria. These findings were confirmed with immunohistochemical staining for actin smooth muscle. Furthermore, the tissue-engineered esophagus architecture was maintained after interposition or use as a patch, and animals gained weight on a normal diet. GFP-labeled tissue-engineered esophagus preserved its fluorescent label, proving the donor origin of the tissue-engineered esophagus. The maximal amount of esophageal tissue that could be replaced by this method remains to be explored and the application of this technique to full circumferential lesions has not been investigated.
Similar cell based and/or scaffold based approaches to construct functional esophageal tissue have been investigated by others. In 2006, Marzaro et al[74] used esophageal ECM seeded with smooth muscle cells (SMCs) to repair a 2 cm defect in the tunica muscularis in a porcine model. They reported the ingrowth of SMCs with early organization into small fascicles. Two years later, Nakase et al[75] explored replacement of a full circumference esophageal defect with polyglycolic acid scaffolds seeded with epithelial cells. Good distensibility of the construct following implantation was reported although peristaltic activity of the new tissue was absent. The thickness of both the squamous epithelial layer and the smooth muscle layer of the engineered esophagus were similar to that of the native esophagus. These results confirmed the concept of biomaterials seeded with cells, either differentiated cells or stem/progenitor cells, as a potentially viable approach for the repair of damaged esophageal tissue.
The mechanisms by which ECM bioscaffolds alter the default proinflammatory esophageal healing response and instead promote a more constructive remodeling response are only partially understood. However it is known that degradation of the ECM releases a variety of growth factors including vascular endothelial growth factor and basic fibroblast growth factor, among others[76]. The critical amounts of active growth factor and the specific factors required to support constructive tissue remodeling are unknown. ECM scaffold degradation in vivo occurs rapidly based upon results of preclinical studies in non-esophageal sites[77-80] and the endoscopic procedures to remove the temporary stents in the patients treated for esophageal cancer suggests that degradation is also very rapid in this location. Scaffold degradation is considered important because it removes a persistent foreign material against which the host can mount a chronic inflammatory reaction and, perhaps more importantly, scaffold degradation results in the generation of bioactive cryptic peptides from component structural molecules of the ECM such as collagen[81]. These cryptic peptides, typically no larger than 10-12 amino acids in length, have been shown to have potent chemotactic and mitogenic activity for selected stem and progenitor cells in vitro[81-84]. The role of this chemotactic phenomenon in constructive remodeling is not fully understood but logically it provides a method for supporting a regenerative type of response.
It is also known that the host response to the presence of a xenogeneic ECM scaffold includes local modulation of the innate immune response from proinflammatory M1 macrophage mediated events toward more dominant constructive tissue remodeling M2 macrophage mediated processes[77,85-87]. However, it is unknown which, if any, of these mechanisms occur or are important in the esophageal location.
Esophageal disease is an increasingly important problem and has very limited satisfactory treatment options. The default inflammatory and scarring response of the nonregenerating esophageal tissue not only creates severe morbidity from the disease process itself, but also limits the therapeutic options since manipulation and tissue injury are unavoidable sequelae of either invasive or minimally invasive endoscopic techniques. Regenerative medicine strategies that utilize cell based, scaffold based, and bioactive molecule based approaches potentially provide a viable alternative for both physicians and the affected patients. Preliminary early results of a bioactive ECM scaffold based approach have been promising.
Peer reviewer: Dr. Jianyuan Chai, Department of Research, VA Long Beach Healthcare System, 5901 E. 7th St 09-151, Long Beach, CA 90822, United States
S- Editor Gou SX L- Editor A E- Editor Li JY
| 1. | Metz CN. Fibrocytes: a unique cell population implicated in wound healing. Cell Mol Life Sci. 2003;60:1342-1350. [PubMed] |
| 2. | Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 566] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 3. | Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283-289. [PubMed] |
| 4. | Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89:219-229. [PubMed] |
| 5. | Adamson R. Role of macrophages in normal wound healing: an overview. J Wound Care. 2009;18:349-351. [PubMed] |
| 6. | Halprin KM. Epidermal "turnover time"--a re-examination. Br J Dermatol. 1972;86:14-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 106] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Iizuka H. Epidermal architecture that depends on turnover time. J Dermatol Sci. 1995;10:220-223. [PubMed] |
| 8. | Fuchs E. Skin stem cells: rising to the surface. J Cell Biol. 2008;180:273-284. [PubMed] |
| 9. | Adolphe C, Wainwright B. Pathways to improving skin regeneration. Expert Rev Mol Med. 2005;7:1-14. [PubMed] |
| 10. | Moffatt DJ, Rosse C, Yoffey JM. Identity of the haemopoietic stem cell. Lancet. 1967;2:547-548. [PubMed] |
| 12. | Loutit JF. Versatile haemopoietic stem cells. Br J Haematol. 1968;15:333-336. [PubMed] |
| 13. | Dunn CD. The differentiation of haemopoietic stem cells. Ser Haematol. 1971;4:1-71. [PubMed] |
| 14. | Leblond CP, Clermont Y, Nadler NJ. The pattern of stem cell renewal in three epithelia: (esophagus, intestine and testis). Proc Can Cancer Conf. 1967;7:3-30. [PubMed] |
| 15. | Yau HC, Cairnie AB. Cell-survival characteristics of intestinal stem cells and crypts of gamm-irradiated mice. Radiat Res. 1979;80:92-107. [PubMed] |
| 16. | Potten CS, Chadwick C, Ijiri K, Tsubouchi S, Hanson WR. The recruitability and cell-cycle state of intestinal stem cells. Int J Cell Cloning. 1984;2:126-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Morgan TH. Regeneration. New York and London: Macmillan Co 1901; 170. |
| 18. | Vracko R. Basal lamina scaffold-anatomy and significance for maintenance of orderly tissue structure. Am J Pathol. 1974;77:314-346. [PubMed] |
| 19. | Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1246] [Cited by in RCA: 1158] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 20. | Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol. 2010;176:2-13. [PubMed] |
| 21. | Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528-1530. [PubMed] |
| 22. | Grounds MD. Muscle regeneration: molecular aspects and therapeutic implications. Curr Opin Neurol. 1999;12:535-543. [PubMed] |
| 23. | Grogan BF, Hsu JR. Volumetric muscle loss. J Am Acad Orthop Surg. 2011;19 Suppl 1:S35-S37. [PubMed] |
| 24. | Canto JG, Kiefe CI, Rogers WJ, Peterson ED, Frederick PD, French WJ, Gibson CM, Pollack CV, Ornato JP, Zalenski RJ. Number of coronary heart disease risk factors and mortality in patients with first myocardial infarction. JAMA. 2011;306:2120-2127. [PubMed] |
| 25. | Thelle DS. Case fatality of acute myocardial infarction: an emerging gender gap. Eur J Epidemiol. 2011;26:829-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Allison MA, Hsi S, Wassel CL, Morgan C, Ix JH, Wright CM, Criqui MH. Calcified atherosclerosis in different vascular beds and the risk of mortality. Arterioscler Thromb Vasc Biol. 2012;32:140-146. [PubMed] |
| 27. | Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46-e215. [PubMed] |
| 28. | Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O'Donnell C, Kittner S. Heart disease and stroke statistics--2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85-151. [PubMed] |
| 30. | Baryan HK, Allan SM, Vail A, Smith CJ. Systematic review and meta-analysis of the efficacy of statins in experimental stroke. Int J Stroke. 2012;7:150-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Jaffer H, Morris VB, Stewart D, Labhasetwar V. Advances in Stroke Therapy. Drug Deliv Transl Res. 2011;1:409-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 32. | Burns AS, O'Connell C. The challenge of spinal cord injury care in the developing world. J Spinal Cord Med. 2012;35:3-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 33. | Devivo MJ. Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord. 2012;50:365-372. [PubMed] |
| 34. | Bracken MB. Steroids for acute spinal cord injury. Cochrane Database Syst Rev. 2012;1:CD001046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 155] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 35. | O'Dowd JK. Basic principles of management for cervical spine trauma. Eur Spine J. 2010;19 Suppl 1:S18-S22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Greenbaum CJ. Type 1 diabetes intervention trials: what have we learned? A critical review of selected intervention trials. Clin Immunol. 2002;104:97-104. [PubMed] |
| 37. | Allen S, Huber J, Devendra D. Prevalence of organ-specific autoantibodies in childhood- and adult-onset type 1 diabetes. Ann N Y Acad Sci. 2008;1150:260-262. [PubMed] |
| 38. | Aly T, Devendra D, Eisenbarth GS. Immunotherapeutic approaches to prevent, ameliorate, and cure type 1 diabetes. Am J Ther. 2005;12:481-490. [PubMed] |
| 39. | Devendra D, Liu E, Eisenbarth GS. Type 1 diabetes: recent developments. BMJ. 2004;328:750-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 186] [Article Influence: 8.9] [Reference Citation Analysis (2)] |
| 40. | Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol. 2007;17:2-9. [PubMed] |
| 41. | Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241-2252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2115] [Cited by in RCA: 2219] [Article Influence: 100.9] [Reference Citation Analysis (0)] |
| 42. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [PubMed] |
| 43. | Orringer MB, Marshall B, Iannettoni MD. Transhiatal esophagectomy for treatment of benign and malignant esophageal disease. World J Surg. 2001;25:196-203. [PubMed] |
| 44. | Alcantara PS, Spencer-Netto FA, Silva-Júnior JF, Soares LA, Pollara WM, Bevilacqua RG. Gastro-esophageal isoperistaltic bypass in the palliation of irresectable thoracic esophageal cancer. Int Surg. 1997;82:249-253. [PubMed] |
| 45. | Ellis FH. Standard resection for cancer of the esophagus and cardia. Surg Oncol Clin N Am. 1999;8:279-294. [PubMed] |
| 46. | Gawad KA, Hosch SB, Bumann D, Lübeck M, Moneke LC, Bloechle C, Knoefel WT, Busch C, Küchler T, Izbicki JR. How important is the route of reconstruction after esophagectomy: a prospective randomized study. Am J Gastroenterol. 1999;94:1490-1496. [PubMed] |
| 47. | Badylak S, Meurling S, Chen M, Spievack A, Simmons-Byrd A. Resorbable bioscaffold for esophageal repair in a dog model. J Pediatr Surg. 2000;35:1097-1103. [PubMed] |
| 48. | Haight C. Some observations on esophageal atresias and tracheoesophageal fistulas of congenital origin. J Thorac Surg. 1957;34:141-172. [PubMed] |
| 49. | Katzka DA. Caustic Injury to the Esophagus. Curr Treat Options Gastroenterol. 2001;4:59-66. [PubMed] |
| 50. | Luostarinen M, Isolauri J. Esophageal perforation and caustic injury: approach to instrumental perforations of the esophagus. Dis Esophagus. 1997;10:86-89. [PubMed] |
| 51. | Kohler BA, Ward E, McCarthy BJ, Schymura MJ, Ries LA, Eheman C, Jemal A, Anderson RN, Ajani UA, Edwards BK. Annual report to the nation on the status of cancer, 1975-2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst. 2011;103:714-736. [PubMed] |
| 52. | Collard JM. High-grade dysplasia in Barrett's esophagus. The case for esophagectomy. Chest Surg Clin N Am. 2002;12:77-92. [PubMed] |
| 53. | Falk GW, Rice TW, Goldblum JR, Richter JE. Jumbo biopsy forceps protocol still misses unsuspected cancer in Barrett's esophagus with high-grade dysplasia. Gastrointest Endosc. 1999;49:170-176. [PubMed] |
| 54. | Rice TW, Zuccaro G, Adelstein DJ, Rybicki LA, Blackstone EH, Goldblum JR. Esophageal carcinoma: depth of tumor invasion is predictive of regional lymph node status. Ann Thorac Surg. 1998;65:787-792. [PubMed] |
| 55. | Keeley SB, Pennathur A, Gooding W, Landreneau RJ, Christie NA, Luketich J. Photodynamic therapy with curative intent for Barrett's esophagus with high grade dysplasia and superficial esophageal cancer. Ann Surg Oncol. 2007;14:2406-2410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 56. | Shaheen NJ, Sharma P, Overholt BF, Wolfsen HC, Sampliner RE, Wang KK, Galanko JA, Bronner MP, Goldblum JR, Bennett AE. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med. 2009;360:2277-2288. [PubMed] |
| 57. | Dumot JA, Vargo JJ, Falk GW, Frey L, Lopez R, Rice TW. An open-label, prospective trial of cryospray ablation for Barrett's esophagus high-grade dysplasia and early esophageal cancer in high-risk patients. Gastrointest Endosc. 2009;70:635-644. [PubMed] |
| 58. | May A, Gossner L, Behrens A, Kohnen R, Vieth M, Stolte M, Ell C. A prospective randomized trial of two different endoscopic resection techniques for early stage cancer of the esophagus. Gastrointest Endosc. 2003;58:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 131] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 59. | Yamamoto H. Technology insight: endoscopic submucosal dissection of gastrointestinal neoplasms. Nat Clin Pract Gastroenterol Hepatol. 2007;4:511-520. [PubMed] |
| 60. | Sharma VK, Wang KK, Overholt BF, Lightdale CJ, Fennerty MB, Dean PJ, Pleskow DK, Chuttani R, Reymunde A, Santiago N. Balloon-based, circumferential, endoscopic radiofrequency ablation of Barrett's esophagus: 1-year follow-up of 100 patients. Gastrointest Endosc. 2007;65:185-195. [PubMed] |
| 61. | Croagh D, Phillips WA, Redvers R, Thomas RJ, Kaur P. Identification of candidate murine esophageal stem cells using a combination of cell kinetic studies and cell surface markers. Stem Cells. 2007;25:313-318. [PubMed] |
| 62. | Croagh D, Thomas RJ, Phillips WA, Kaur P. Esophageal stem cells--a review of their identification and characterization. Stem Cell Rev. 2008;4:261-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 63. | Seery JP. Stem cells of the oesophageal epithelium. J Cell Sci. 2002;115:1783-1789. [PubMed] |
| 64. | Seery JP, Watt FM. Asymmetric stem-cell divisions define the architecture of human oesophageal epithelium. Curr Biol. 2000;10:1447-1450. [PubMed] |
| 65. | Souza RF, Schwartz RE, Mashimo H. Esophageal stem cells and 3D-cell culture models. Ann N Y Acad Sci. 2011;1232:316-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 66. | Takagi R, Murakami D, Kondo M, Ohki T, Sasaki R, Mizutani M, Yamato M, Nishida K, Namiki H, Yamamoto M. Fabrication of human oral mucosal epithelial cell sheets for treatment of esophageal ulceration by endoscopic submucosal dissection. Gastrointest Endosc. 2010;72:1253-1259. [PubMed] |
| 67. | Ohki T, Yamato M, Murakami D, Takagi R, Yang J, Namiki H, Okano T, Takasaki K. Treatment of oesophageal ulcerations using endoscopic transplantation of tissue-engineered autologous oral mucosal epithelial cell sheets in a canine model. Gut. 2006;55:1704-1710. [PubMed] |
| 68. | Takagi R, Yamato M, Murakami D, Kondo M, Ohki T, Sasaki R, Nishida K, Namiki H, Yamamoto M, Okano T. Fabrication and validation of autologous human oral mucosal epithelial cell sheets to prevent stenosis after esophageal endoscopic submucosal dissection. Pathobiology. 2011;78:311-319. [PubMed] |
| 69. | Badylak SF, Vorp DA, Spievack AR, Simmons-Byrd A, Hanke J, Freytes DO, Thapa A, Gilbert TW, Nieponice A. Esophageal reconstruction with ECM and muscle tissue in a dog model. J Surg Res. 2005;128:87-97. [PubMed] |
| 70. | Nieponice A, McGrath K, Qureshi I, Beckman EJ, Luketich JD, Gilbert TW, Badylak SF. An extracellular matrix scaffold for esophageal stricture prevention after circumferential EMR. Gastrointest Endosc. 2009;69:289-296. [PubMed] |
| 71. | Nieponice A, Gilbert TW, Badylak SF. Reinforcement of esophageal anastomoses with an extracellular matrix scaffold in a canine model. Ann Thorac Surg. 2006;82:2050-2058. [PubMed] |
| 72. | Badylak SF, Hoppo T, Nieponice A, Gilbert TW, Davison JM, Jobe BA. Esophageal preservation in five male patients after endoscopic inner-layer circumferential resection in the setting of superficial cancer: a regenerative medicine approach with a biologic scaffold. Tissue Eng Part A. 2011;17:1643-1650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 163] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 73. | Grikscheit T, Ochoa ER, Srinivasan A, Gaissert H, Vacanti JP. Tissue-engineered esophagus: experimental substitution by onlay patch or interposition. J Thorac Cardiovasc Surg. 2003;126:537-544. [PubMed] |
| 74. | Marzaro M, Vigolo S, Oselladore B, Conconi MT, Ribatti D, Giuliani S, Nico B, Perrino G, Nussdorfer GG, Parnigotto PP. In vitro and in vivo proposal of an artificial esophagus. J Biomed Mater Res A. 2006;77:795-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 75. | Nakase Y, Nakamura T, Kin S, Nakashima S, Yoshikawa T, Kuriu Y, Sakakura C, Yamagishi H, Hamuro J, Ikada Y. Intrathoracic esophageal replacement by in situ tissue-engineered esophagus. J Thorac Cardiovasc Surg. 2008;136:850-859. [PubMed] |
| 76. | Voytik-Harbin SL, Brightman AO, Kraine MR, Waisner B, Badylak SF. Identification of extractable growth factors from small intestinal submucosa. J Cell Biochem. 1997;67:478-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 77. | Badylak SF, Gilbert TW. Immune response to biologic scaffold materials. Semin Immunol. 2008;20:109-116. [PubMed] |
| 78. | Gilbert TW, Stewart-Akers AM, Badylak SF. A quantitative method for evaluating the degradation of biologic scaffold materials. Biomaterials. 2007;28:147-150. [PubMed] |
| 79. | Record RD, Hillegonds D, Simmons C, Tullius R, Rickey FA, Elmore D, Badylak SF. In vivo degradation of 14C-labeled small intestinal submucosa (SIS) when used for urinary bladder repair. Biomaterials. 2001;22:2653-2659. [PubMed] |
| 80. | Badylak S, Kokini K, Tullius B, Whitson B. Strength over time of a resorbable bioscaffold for body wall repair in a dog model. J Surg Res. 2001;99:282-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 162] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 81. | Reing JE, Zhang L, Myers-Irvin J, Cordero KE, Freytes DO, Heber-Katz E, Bedelbaeva K, McIntosh D, Dewilde A, Braunhut SJ. Degradation products of extracellular matrix affect cell migration and proliferation. Tissue Eng Part A. 2009;15:605-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 278] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 82. | Beattie AJ, Gilbert TW, Guyot JP, Yates AJ, Badylak SF. Chemoattraction of progenitor cells by remodeling extracellular matrix scaffolds. Tissue Eng Part A. 2009;15:1119-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 169] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 83. | Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301-313. [PubMed] |
| 84. | Agrawal V, Tottey S, Johnson SA, Freund JM, Siu BF, Badylak SF. Recruitment of progenitor cells by an extracellular matrix cryptic peptide in a mouse model of digit amputation. Tissue Eng Part A. 2011;17:2435-2443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 139] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 85. | Badylak SF, Valentin JE, Ravindra AK, McCabe GP, Stewart-Akers AM. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A. 2008;14:1835-1842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 555] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 86. | Brown BN, Londono R, Tottey S, Zhang L, Kukla KA, Wolf MT, Daly KA, Reing JE, Badylak SF. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater. 2012;8:978-987. [PubMed] |
| 87. | Brown BN, Valentin JE, Stewart-Akers AM, McCabe GP, Badylak SF. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30:1482-1491. [PubMed] |