Published online Dec 21, 2012. doi: 10.3748/wjg.v18.i47.6865
Revised: August 27, 2012
Accepted: September 12, 2012
Published online: December 21, 2012
NR4A2 is a transcription factor belonging to the steroid orphan nuclear receptor superfamily. It was originally considered to be essential in the generation and maintenance of dopaminergic neurons, and associated with neurological disorders such as Parkinson’s disease. Recently, NR4A2 has been found to play a critical role in some inflammatory diseases and cancer. NR4A2 can be efficiently trans-activated by some proinflammatory cytokines, such as tumor necrosis factor-α, interleukin-1β, and vascular endothelial growth factor (VEGF). The nuclear factor-κB signaling pathway serves as a principal regulator of inducible NR4A expression in immune cells. NR4A2 can trans-activate Foxp3, a hallmark specifically expressed in regulatory T (Treg) cells, and plays a critical role in the differentiation, maintenance, and function of Treg cells. NR4A2 in T lymphocytes is pivotal for Treg cell induction and suppression of aberrant induction of Th1 under physiological and pathological conditions. High density of Foxp3+ Treg cells is significantly associated with gastrointestinal inflammation, tumor immune escape, and disease progression. NR4A2 is produced at high levels in CD133+ colorectal carcinoma (CRC) cells and significantly upregulated by cyclooxygenase-2-derived prostaglandin E2 in a cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA)-dependent manner in CRC cells. The cAMP/PKA signaling pathway is the common pathway of NR4A2-related inflammation and cancer. NR4A2 trans-activates osteopontin, a direct target of the Wnt/β-catenin pathway associated with CRC invasion, metastasis, and poor prognosis. Knockdown of endogenous NR4A2 expression attenuates VEGF-induced endothelial cell proliferation, migration and in vivo angiogenesis. Taken together, NR4A2 emerges as an important nuclear factor linking gastrointestinal inflammation and cancer, especially CRC, and should serve as a candidate therapeutic target for inflammation-related gastrointestinal cancer.
- Citation: Han YF, Cao GW. Role of nuclear receptor NR4A2 in gastrointestinal inflammation and cancers. World J Gastroenterol 2012; 18(47): 6865-6873
- URL: https://www.wjgnet.com/1007-9327/full/v18/i47/6865.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i47.6865
A quarter of cancer-related deaths worldwide are currently associated with chronic inflammation[1]. Most malignancies in the digestive system such as colorectal carcinoma (CRC), gastric cancer and hepatocellular carcinoma belong to the inflammation-related cancers[2-5]. The type of inflammation related to these cancers is chronic inflammation, or more precisely, nonresolving inflammation. The development and progression of these tumors in the digestive system are accompanied by angiogenesis, release of inflammatory cytokines and/or chemokines, and recruitment of immune cells in the tumor microenvironment. Apart from some infectious agents such as hepatitis B virus and Helicobacter pylori, inflammation-related molecules and their related inflammatory networks play important roles in the development of this group of cancers. However, it is clear that a single or even a few molecules are unable to represent the inflammatory predisposition of a given cancer. In the inflammation-related cancers, inflammatory molecules do not function in isolation, but interact with one another to form inflammatory networks. A small number of highly connected protein nodes (known as hubs) in the inflammatory network may have high probabilities of engaging in essential biological functions. In our previous work on screening hub genes associated with liver metastasis of gastric cancer, we identified NR4A2, one of the hub genes related to gastric cancer malignant phenotype[6]. Here, we summarize the role of NR4A2 in inflammation and cancer.
NR4A2 (also known as NOT, TINUR, RNR-1, HZF-3 and Nurr1) maps to chromosome 2q22-23 and is a transcription factor belonging to the steroid nuclear hormone receptor superfamily. This superfamily also includes two other members, NR4A1 (also known as HMR, N10, TR3, NP10, GFRP1, NAK-1, NGFIβ and Nur77) and NR4A3 (also known as CHN, TEC, CSMF, MINOR and NOR1)[7]. Their ligand-binding pockets are hidden by bulky amino acids, therefore, the three nuclear factors belong to an orphan receptor subfamily. All three subfamily members bind to a consensus sequence AAAGGTCA as monomers or to palindromic DNA binding motif sequence TDATATTTX6AAATGCCA as homodimers. Expression of each family member alone is sufficient to activate the above sequence-directed transcriptional activities, indicating that the family members are constitutive orphan steroid receptors that do not require ligands for activation[8]. The three nuclear receptors have also been implicated in cell cycle regulation, energy metabolism, apoptosis, inflammation and carcinogenesis. They are important for apoptosis in lymphocytes and other cell types[9-12] and for the differentiation of dopaminergic neurons[13]. NR4A1 and NR4A3 have been shown to be involved in apoptosis-related pathways. NR4A1 has been reported to play either an antiapoptotic or proapoptotic function depending on cell types. NR4A3 has been reported to play a partly redundant functional role with NR4A1 in inducing T-cell apoptosis[9]. Knocking out both NR4A1 and NR4A3 in mice leads to rapid postnatal development of acute myeloid leukemia (AML), indicating that they function as potent tumor suppressors[14,15]. Meanwhile, the three NR4A receptors are transcriptional regulators of hepatic glucose metabolism and lipid metabolism. NR4As function as novel branches of cyclic adenosine monophosphate (cAMP)-dependent regulators of hepatic glucose production under healthy and diabetic conditions. Activated NR4A1 and NR4A3 elevate the production of blood glucose[16]. NR4As inhibits adipocyte differentiation, leading to repressed adipogenesis[17]. NR4A1 and NR4A2 can form heterodimers with retinoic acid receptor and influence retinoid signaling and Th17 cell differentiation[18,19].
NR4A2 has been previously linked to neurological diseases because of its essential role in the generation and maintenance of dopaminergic neurons in the brain. Gene encoding NR4A2 may be susceptibility gene for neurological diseases such as Parkinson’s disease, schizophrenia, and manic depression. Homozygous NR4A2 knockout mice have severe impairments in midbrain neuronal development and dopamine expression, and die soon after birth[20]. However, the horizontal and vertical movement of the heterozygous NR4A2 knockout mice is impaired by the reduction in brain dopamine[21]. The most important event in downregulating NR4A2 expression in brain tissues is mutations, such as missense mutations in exon 3, and point mutations in exon 1 of NR4A2 have been reported in disorders related to dopaminergic dysfunction such as schizophrenia, manic depression, and familial Parkinson’s disease[22-24]. In recent years, however, more researches have focused on the relationship between NR4A2 and inflammation.
Immune cells are essential in controlling the inflammatory response. T and B lymphocytes, macrophages, neutrophils, and mast cells are all important in the maintenance of chronic inflammation and play active roles in the initiation and progression of inflammation-related cancers. Cytotoxic CD8+ T lymphocytes and CD4+ T helper lymphocyte subpopulations [Th1, Th2, Th17, and regulatory T (Treg) cells] play key roles in balancing cancer-promoting inflammation and antitumor immunity in the tumor microenvironment. Imbalances in Th1/Th2, neutrophil/CD8+ T cells, and CD8+ T cell/Treg cell in tumors or adjacent tissues, high density of intratumoral macrophage infiltration, as well as high circulating neutrophil-to-lymphocyte ratio are associated with the prognosis of cancer patients[25]. Recently, NR4A2 has been found to function as a critical regulator in T lymphocytes, macrophages, mast cells and even fibroblasts.
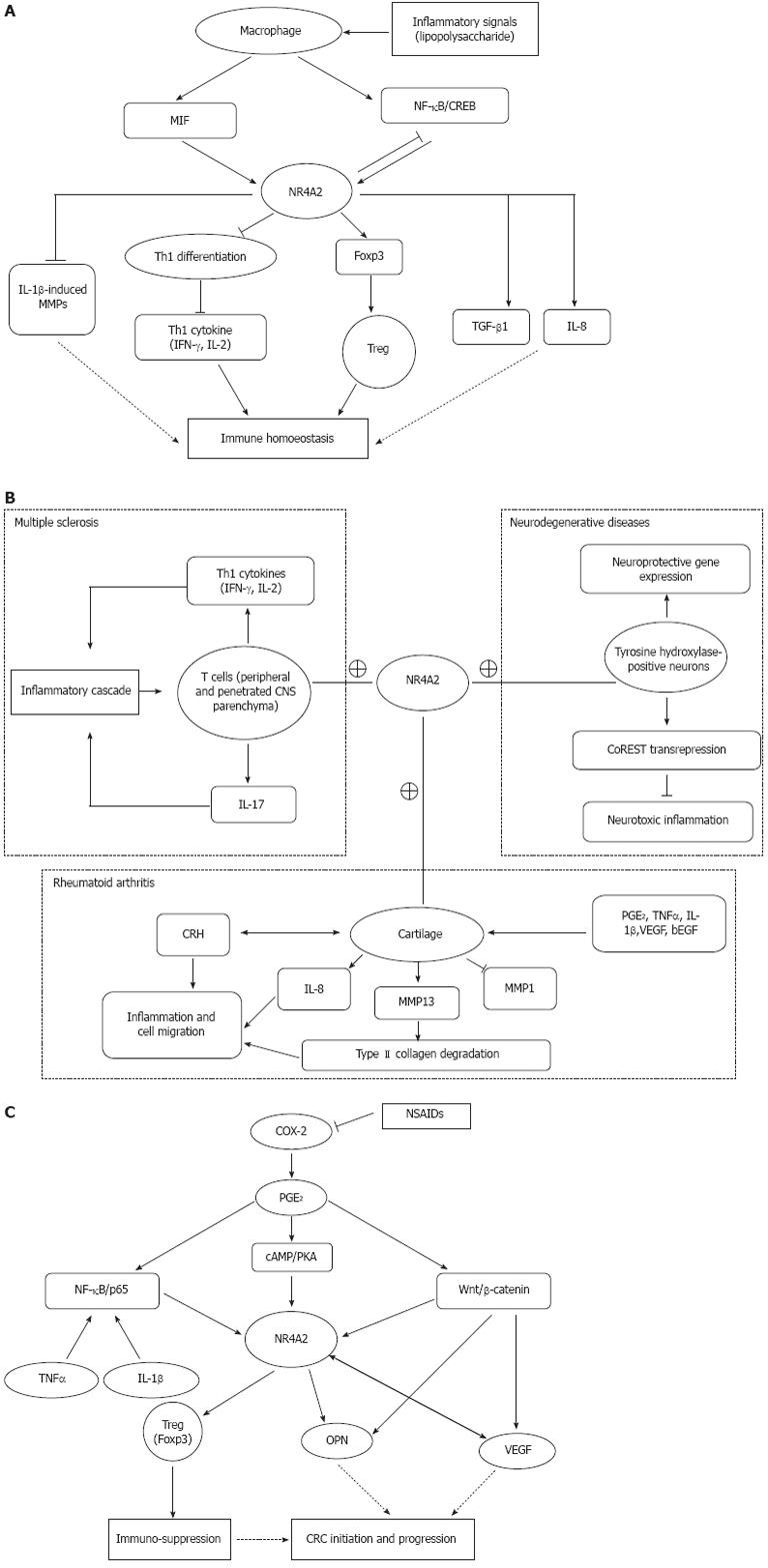
Treg cells (CD4+CD25+Foxp3+) inhibit immune responses by suppressing CD8+ T cell or Th1 cell function, playing an important role in maintaining immune homoeostasis. Foxp3, a hallmark of Treg cells, is specifically expressed in Treg cells and plays a critical role in the differentiation, maintenance and effector functions of these cells. Foxp3 has recently been identified as a direct target of NR4A2. The -209 to +12 promoter region of Foxp3 encoding region has been confirmed to be the minimal responsive region to NR4A2. NR4A2 regulate CD4+ T cells by inducing Foxp3 and strongly repressing Th1 cytokine production, such as interleukin (IL)-2 and interferon (IFN)-γ. NR4A2 in T cell is pivotal for Treg cell induction and suppression of aberrant induction of Th1 under physiological and pathological conditions. Deletion of NR4A2 in T cells attenuates the induction of Treg cells and causes aberrant induction of Th1, leading to exacerbation of colitis. Both IFN-γ and IL-17 are apparently repressed under Th1 and Th17 conditions, respectively, and Foxp3 can be induced under Th1 and Th17 conditions by NR4A2[26]. Thus, NR4A2 is crucial in lineage maintenance and effector functions of Treg cells and in regulation of the Th1/Treg balance, contributing to immune homoeostasis (Figure 1A).
Macrophages can be divided into two main classes: M1 and M2. M1 macrophages (classically activated) originate upon encounter with IFN-γ and microbial stimuli and are characterized by IL-12 production and consequent activation of a polarized type I T cell response, fighting against tumors, producing high amounts of inflammatory cytokines, and activating adoptive immunity. M2 macrophages are responsible for angiogenesis, remodeling and repair of wounded tissues, and promote carcinogenesis and downregulate M1 function. NR4As are highly inducible in macrophages by diverse inflammatory stimuli. Treatment of macrophages with lipopolysaccharide, cytokines, or oxidized lipids triggers the transcriptional induction of NR4A expression. NR4A2 is an intermediate maker for macrophage activation[27]. The nuclear factor (NF)-κB signaling pathway is a principal mediator of inducible NR4A expression in macrophages[28]. NR4A2 is a target of macrophage migration inhibitory factor (MIF) signaling and plays an active role in regulating mitogen-activated protein kinase (MAPK) phosphatase 1, a critical MAPK signaling inhibitor[29]. NR4A2 may serve as a potential transcriptional mediator of inflammatory signals in activated macrophages. However, it is necessary to define the difference in NR4A2 expression between M1 and M2; the two functionally different macrophages.
NR4A2 plays either a proinflammatory or anti-inflammatory role. The conflicting roles of NR4A2 depend on the type of immune disorders. Multiple sclerosis (MS) is an autoimmune disease mediated by Th17 and Th1 cells in the central nervous system (CNS). In an animal model of MS, NR4A2 is selectively upregulated in T cells isolated from the CNS, while forced expression of NR4A2 augments promoter activities of IL-17 and IFN-γ genes, leading to excess production of these cytokines[30]. In contrast, NR4A2 can also exert anti-inflammatory and neuroprotective effects by docking to NF-κB/p65 on target inflammatory gene promoters in an NR4A2/CoREST transrepression pathway in microglia and astrocytes, leading to protection of dopaminergic neurons from inflammation-induced death[31]. Furthermore, NR4A2 is a key downstream mediator of cAMP response element-binding protein (CREB)-induced neuroprotection after insults leading to excitotoxic and oxidative stress[32].
Chronic inflammation is a hallmark of rheumatoid arthritis and osteoarthritis. The arthritis is a well-established disease model to study the mechanism by which NR4A2 is involved in the process of chronic inflammation. NR4A2 expression is markedly higher in synovial tissue of patients with rheumatoid arthritis compared with normal subjects. The high expression of NR4A2 is caused by the stimulation of some proinflammatory cytokines, such as tumor necrosis factor (TNF)-α, IL-1β and prostaglandin E2 (PGE2). Ectopic expression of NR4A2 induced by TNF-α in normal synoviocytes significantly increases cell proliferation and survival, promotes anchorage-independent growth, and induces migration and invasion[33]. The trans-activation of NR4A2 by these cytokines and molecules depends on distinctive subunit binding to the NR4A2 promoter. IL-1β and TNF-α trans-activate the NR4A2 via a proximal promoter region that contains a consensus NF-κB DNA-binding motif. IL-1β- and TNF-α-induced NF-κB binding to this site is due predominantly to p65-p50 heterodimer and p50 homodimer complexes[34]. NR4A2 robustly promotes IL-8 expression via co-operating with the NF-κB/p65 subunit in the presence of TNF-α in human inflammatory disease[35]. NR4A2 and TNF-α also synergistically induce matrix metalloproteinase (MMP)-13 protein which is a critical enzyme for the degradation of type II collagen molecules[36]. NR4A2 trans-activates MMP-13 by directly targeting the proximal region of the MMP-13 promoter[33]. PGE2 signaling leads to the phosphorylation of CREB transcription factors, which can, in turn, bind to the NR4A2 promoter region -171/-163 and activate transcription. PGE2 can repress IL-1β-induced MMP-1 and activate NR4A2 expression. Meanwhile, NR4A2 antagonizes the progression of IL-1β-induced MMP-1 in osteoarthritic cartilage[37]. In human synovial tissue, corticotropin-releasing hormone (CRH) also can induce the expression of NR4A2, which in turn upregulates CRH gene expression. NR4A2 is a downstream effector molecule in the modulation of endothelial function by CRH signaling[38]. Thus, NR4A2 as a mediator of an autocrine inflammatory cascade to amplify the inflammatory response participate in the CRH-receptor-mediated signaling in synovial tissue via the cAMP/CREB pathway. Figure 1B depicts the potential role of NR4A2 on the development of autoimmune diseases (MS and rheumatoid arthritis) and neurodegenerative diseases like Parkinson’s disease.
NR4A2 and endothelin-1 are expressed at a significantly higher level in the CD133+ fraction than the CD133- fraction of colon cancer cells. The overexpression of CD133, NR4A2, and endothelin-1 is also evident in human colon cancer specimens compared to normal tissues. Furthermore, effective knockdown of CD133 protein is associated with a parallel reduction of NR4A2 and endothelin-1 protein, indicating the existence of a functional relationship between CD133, NR4A2 and endothelin-1 expression in colon cancer cells[39]. NR4A2 is involved in anchorage-independent growth of cancer cells and plays a significant role in mediating thromboxane A2 receptor induced cell proliferation and transformation in several cancer cell lines[11,40]. NR4A2 also plays a key role as a transcriptional integration point between the eicosanoid and fatty acid metabolic pathways involving in energy utilization via fatty acid oxidation, thus facilitating CRC cell survival and growth[41]. These results indicate that NR4A2 expressed in cancer cells might play an active role in promoting the initiation and progression of CRC.
CRC is closely related to inflammation, because regular use of nonsteroidal anti-inflammatory drugs (NSAIDs) reduces relative risk of developing CRC by 30%[42]. NSAIDs inhibit cyclooxygenase (COX) enzymes and prostaglandin synthases, which are key components in the arachidonic acid metabolism pathway. The main mechanism of action of NSAIDs on CRC is to inhibit COX-2, the inducible isoform of the COX enzyme. COX-2 is upregulated in inflammation and cancer, resulting in the production of PGE2 which binds to and activates G-protein-coupled prostaglandin E receptor 1 (EP1)[43]. COX-2-derived PGE2 is produced at high levels in CRC. Some evidence indicates that PGE2 plays a pro-oncogenic role in CRC progression. PGE2 stimulation of the human EP1 receptor upregulates the expression of NR4A2 by a mechanism involving the sequential activation of the Rho, protein kinase A (PKA), CREB and NF-κB signaling pathways[44]. NR4A2 is an immediate early gene induced by PGE2 in a cAMP/PKA-dependent manner in CRC cells. NR4A2 can stimulate progression of CRC downstream from COX-2-derived PGE2[45]. The common pathway of NR4A2-related inflammation and cancer is the cAMP/PKA signaling pathway. The cAMP/PKA signaling pathway plays a critical role not only in inflammation but also in carcinogenesis[46-48]. It has been proven that cAMP/PKA can activate CREB-dependent reporter gene expression in gastric cancer cell lines[49]. Through cAMP/PKA activation, PGE2 stabilizes the complex of PI3K/Ras to inhibit cell apoptosis in colon cancer cell lines[50]. The proximal promoter region of NR4A2 contains a CREB-binding site by which this transcriptional factor participates in cAMP-mediated induction[32]. However, the cAMP/PKA pathway is not the only way by which NR4A2 participate in the progression of CRC. PGE2 either modulates the β-catenin signaling axis, a key pathway for colorectal tumorigenesis, or act via NR4A2[51]. PGE2 can induce the expression of NR4A2 and two target genes of Wnt/β-catenin: vascular endothelial growth factor (VEGF) and cyclin D1. NR4A2 protein can be regulated by Wnt signaling[52]. Meanwhile, NR4A2 and Wnt signaling are sequentially repressed after blockade of COX-2 activity, with NR4A2 inhibition occurring in the first few hours of the treatment, whereas repression of the Wnt/β-catenin pathway happens a few days later. This change might be associated with COX-2 inhibitor-induced downregulation of osteopontin (OPN) whose high expression level significantly correlates with advancing tumor stage in human CRC[53]. OPN, a marker of colon cancer progression, is a direct target of Wnt/β-catenin pathway. Furthermore, NR4A2 trans-activates OPN by directly binding to the NR4A2 response element at the OPN promoter[54]. OPN is involved in the development of various inflammatory conditions[55] and also play a pivotal role in carcinogenesis and metastasis of digestive system cancer, such as gastric cancer and hepatocellular carcinoma. High expression of OPN in tumors is associated with tumor invasion, metastasis, and poor prognosis in gastric cancer and CRC[53,56]. Downregulation of OPN, probably through blockade of NR4A2 and Wnt signaling, is an important component of the antitumor activity of COX-2 inhibitors[57]. VEGF potently and rapidly induces expression of NR4A2 mRNA, protein and its promoter activity in endothelial cells. Deletion of the putative CREB site in the proximal region of the NR4A2 promoter markedly reduces VEGF-induced promoter activity. VEGF also stimulates the binding of nuclear CREB protein to its site in the NR4A2 promoter. Knockdown of endogenous NR4A2 expression attenuates VEGF-induced endothelial cell proliferation, migration and in vivo angiogenesis[58]. The growth and metastasis of cancer cells rely on angiogenesis, and VEGF is an important angiogenic factor. It has been reported that VEGF is highly expressed in gastrointestinal cancers and plays a pivotal role in tumor angiogenesis, tumor growth, and metastasis[59-61]. The correlation among the expression levels of OPN, COX-2 and VEGF in gastric cancer indicates that OPN, COX-2, and VEGF synergistically promote angiogenesis and metastasis[62]. The three molecules are all closely related to NR4A2. The NF-κB signaling pathway serves as a principal regulator of inducible NR4A expression in some chronic inflammation, while NF-κB pathway plays a major function in CRC development and progress[63]. These results indicate that aberrant expression of NR4A2 provokes several inflammation-related signaling pathways and promotes the development and progression of gastrointestinal cancers. Figure 1C depicts the potential role of NR4A2 on the development of CRC.
Foxp3+ Treg cells are significantly increased in gastric mucosa of patients with gastritis, peptic ulcer, and those with gastric cancer, as compared with healthy controls[64]. An imbalance of colitogenic Th1 cells and Treg cells facilitates the development of aggravated chronic enterocolitis[65]. Although NR4A2 has not been implicated in predicting CRC prognosis, the NR4A2-related Th lymphocytes are closely linked to adverse outcome of CRC patients. Foxp3+ and CD3+ T-cell densities are increased in CRC tissues compared with autologous normal mucosa. Furthermore, a low CD3+/Foxp3+ cell ratio and low numbers of CD3+ T cells in tumors predict shorter disease-free survival and are stronger prognostic variables than tumor stage or number of lymph node metastases[66]. The density of Foxp3+ Treg cells in tumor draining lymph nodes (TDLNs) is dramatically higher than that in peripheral blood lymphocytes, but significantly lower than that in tumor-infiltrating lymphocytes. Foxp3+ Treg cells in TDLNs are more correlated with disease progression and potentially influence CD8+ T-cell functions[67]. A new Treg cell population, CD8+CD25+Foxp3+cells, has been found in CRC tissues. IL-6 and transforming growth factor-β1 can synergistically induce the generation of these new Treg cells that may contribute to tumor immune escape and disease progression[68]. The CRC patients with high expression of the Th17 cluster have a poor prognosis, whereas patients with high expression of the Th1 cluster have prolonged disease-free survival, thus functional Th1 and Th17 clusters yield opposite effects on patient survival in CRC[69]. Th17 and Treg cells accumulate in the tumor microenvironment of early gastric cancer and then infiltration of Treg cells gradually increases according to disease progression, in contrast to Th17 cells[70]. Significantly more Foxp3+ Treg cells accumulate in gastric tumors. The elevated Foxp3 expression in tumor-infiltrating Treg cells correlates with expression of COX-2 and PGE2 and is associated with the TNM stage in gastric cancer patients. Tumor-infiltrating Treg cells with increased Foxp3 expression can mediate immune suppression via COX-2/PGE2 production in the gastric cancer microenvironment[71]. NR4A2 trans-activates the transcription factor Foxp3, while Foxp3 plays a key role in Treg cell function. Furthermore, NR4A2 is highly expressed in the CD133+ CRC cells and also pivotal for Treg cell induction and suppression of aberrant induction of Th1 cells[26,39]. NR4A2 is a target of MIF/MAPK signaling, while MIF expression in tumors is inversely associated with the prognosis of hepatocellular carcinoma[25,29]. Thus, it can be inferred that NR4A2 expression in lymphocytes and/or tumor cells might promote gastrointestinal inflammation and carcinogenesis and also indicate poor prognosis of CRC and gastric cancer.
There are conflicting data concerning the oncogenic or tumor suppressive function of the three NR4A family members. Several lines of evidence mentioned above indicate that NR4A2 plays an important role in promoting inflammation and gastrointestinal cancers. Downregulation of NR4A2 results in reduced anchorage-independent growth that is largely attributable to increased anoikis, furthermore, downregulation of NR4A2 as well as NR4A1 promotes intrinsic apoptosis in several other experimental cancer cells such as cervical cancer[11]. Thus, NR4A family members exhibit oncogenic functions with regard to cell proliferation and anti-apoptosis. However, knocking out both NR4A1 and NR4A3 in mice leads to rapid postnatal development of AML, indicating they function as critical tumor suppressors[14]. NR4A2 also exhibits tumor suppressor function in bladder cancer. Chemical-induced activation of NR4A2 results in bladder cancer cell apoptosis and suppresses bladder cancer growth, as reported by the research group of Kamat[72]. However, this group also reported that cytoplasmic dislocation of NR4A2 in bladder cancer was associated with poor prognosis, and silencing of endogenous NR4A2 attenuated the migration of bladder cancer cells, indicating that NR4A2 functions as a tumor-promoting factor[73]. NR4A2 is a nuclear factor, therefore, cytoplasmic dislocation might indicate loss of function of NR4A2. Thus, NR4A2 is more likely to function as a tumor suppressor in bladder cancer. These conflicting data can be explained by the hypothesis that NR4A2 functions differently in different tissues. More studies using cancer of different origins are necessary to elucidate the functions of NR4A2 in human cancers.
In summary, NR4A2 plays a pivotal role in some inflammatory diseases and cancers. NR4A2 functions as an inflammatory mediator primarily via at least two distinct signaling pathways, cAMP/PKA and NF-κB, suggesting an important common role for this transcription factor in mediating multiple inflammatory signals. NR4A2 trans-activates the transcription factor Foxp3, while Foxp3 plays a key role in Treg cell function. Treg cells contribute significantly to tumoral immune escape and disease progression. NR4A2 trans-activates OPN while OPN is a direct target of Wnt/β-catenin pathway. There is a crosstalk between NR4A2 and Wnt/β-catenin signaling pathways in human gastrointestinal cancers such as CRC. Taken together, the aberrant expression of NR4A2 in the tumors and tumor-infiltrating lymphocytes might facilitate gastrointestinal inflammation, carcinogenesis and cancer metastasis. NR4A2 emerges as an important nuclear factor linking gastrointestinal inflammation and cancers, especially CRC, and might serve as a candidate therapeutic target for the inflammation-related gastrointestinal cancers.
Peer reviewer: Flavio Maina, PhD, Developmental Biology Institute of Marseille-Luminy, CNRS UMR 6216, Campus de Luminy-case 907, 13288 Marseille, France
S- Editor Lv S L- Editor Kerr C E- Editor Zhang DN
| 1. | Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5245] [Cited by in RCA: 5762] [Article Influence: 240.1] [Reference Citation Analysis (0)] |
| 2. | Ferrone C, Dranoff G. Dual roles for immunity in gastrointestinal cancers. J Clin Oncol. 2010;28:4045-4051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 192] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 3. | Vannella L, Lahner E, Annibale B. Risk for gastric neoplasias in patients with chronic atrophic gastritis: a critical reappraisal. World J Gastroenterol. 2012;18:1279-1285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 111] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (3)] |
| 4. | Rizzo A, Pallone F, Monteleone G, Fantini MC. Intestinal inflammation and colorectal cancer: a double-edged sword? World J Gastroenterol. 2011;17:3092-3100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 56] [Reference Citation Analysis (0)] |
| 5. | Han YF, Zhao J, Ma LY, Yin JH, Chang WJ, Zhang HW, Cao GW. Factors predicting occurrence and prognosis of hepatitis-B-virus-related hepatocellular carcinoma. World J Gastroenterol. 2011;17:4258-4270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | Chang W, Ma L, Lin L, Gu L, Liu X, Cai H, Yu Y, Tan X, Zhai Y, Xu X. Identification of novel hub genes associated with liver metastasis of gastric cancer. Int J Cancer. 2009;125:2844-2853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Li QX, Ke N, Sundaram R, Wong-Staal F. NR4A1, 2, 3--an orphan nuclear hormone receptor family involved in cell apoptosis and carcinogenesis. Histol Histopathol. 2006;21:533-540. [PubMed] |
| 8. | Winoto A, Littman DR. Nuclear hormone receptors in T lymphocytes. Cell. 2002;109 Suppl:S57-S66. [PubMed] |
| 9. | Cheng LE, Chan FK, Cado D, Winoto A. Functional redundancy of the Nur77 and Nor-1 orphan steroid receptors in T-cell apoptosis. EMBO J. 1997;16:1865-1875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 251] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | Li H, Kolluri SK, Gu J, Dawson MI, Cao X, Hobbs PD, Lin B, Chen G, Lu J, Lin F. Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3. Science. 2000;289:1159-1164. [PubMed] |
| 11. | Ke N, Claassen G, Yu DH, Albers A, Fan W, Tan P, Grifman M, Hu X, Defife K, Nguy V. Nuclear hormone receptor NR4A2 is involved in cell transformation and apoptosis. Cancer Res. 2004;64:8208-8212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 12. | Lin B, Kolluri SK, Lin F, Liu W, Han YH, Cao X, Dawson MI, Reed JC, Zhang XK. Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell. 2004;116:527-540. [PubMed] |
| 13. | Sacchetti P, Carpentier R, Ségard P, Olivé-Cren C, Lefebvre P. Multiple signaling pathways regulate the transcriptional activity of the orphan nuclear receptor NURR1. Nucleic Acids Res. 2006;34:5515-5527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Mullican SE, Zhang S, Konopleva M, Ruvolo V, Andreeff M, Milbrandt J, Conneely OM. Abrogation of nuclear receptors Nr4a3 and Nr4a1 leads to development of acute myeloid leukemia. Nat Med. 2007;13:730-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 255] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 15. | Ramirez-Herrick AM, Mullican SE, Sheehan AM, Conneely OM. Reduced NR4A gene dosage leads to mixed myelodysplastic/myeloproliferative neoplasms in mice. Blood. 2011;117:2681-2690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Pei L, Waki H, Vaitheesvaran B, Wilpitz DC, Kurland IJ, Tontonoz P. NR4A orphan nuclear receptors are transcriptional regulators of hepatic glucose metabolism. Nat Med. 2006;12:1048-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 258] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 17. | Chao LC, Bensinger SJ, Villanueva CJ, Wroblewski K, Tontonoz P. Inhibition of adipocyte differentiation by Nur77, Nurr1, and Nor1. Mol Endocrinol. 2008;22:2596-2608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Volakakis N, Joodmardi E, Perlmann T. NR4A orphan nuclear receptors influence retinoic acid and docosahexaenoic acid signaling via up-regulation of fatty acid binding protein 5. Biochem Biophys Res Commun. 2009;390:1186-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1505] [Cited by in RCA: 1590] [Article Influence: 88.3] [Reference Citation Analysis (0)] |
| 20. | Zetterström RH, Solomin L, Jansson L, Hoffer BJ, Olson L, Perlmann T. Dopamine neuron agenesis in Nurr1-deficient mice. Science. 1997;276:248-250. [PubMed] |
| 21. | Bensinger SJ, Tontonoz P. A Nurr1 pathway for neuroprotection. Cell. 2009;137:26-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Chen YH, Tsai MT, Shaw CK, Chen CH. Mutation analysis of the human NR4A2 gene, an essential gene for midbrain dopaminergic neurogenesis, in schizophrenic patients. Am J Med Genet. 2001;105:753-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Buervenich S, Carmine A, Arvidsson M, Xiang F, Zhang Z, Sydow O, Jönsson EG, Sedvall GC, Leonard S, Ross RG. NURR1 mutations in cases of schizophrenia and manic-depressive disorder. Am J Med Genet. 2000;96:808-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Le WD, Xu P, Jankovic J, Jiang H, Appel SH, Smith RG, Vassilatis DK. Mutations in NR4A2 associated with familial Parkinson disease. Nat Genet. 2003;33:85-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 347] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 25. | Chen L, Zhang Q, Chang W, Du Y, Zhang H, Cao G. Viral and host inflammation-related factors that can predict the prognosis of hepatocellular carcinoma. Eur J Cancer. 2012;48:1977-1987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 26. | Sekiya T, Kashiwagi I, Inoue N, Morita R, Hori S, Waldmann H, Rudensky AY, Ichinose H, Metzger D, Chambon P. The nuclear orphan receptor Nr4a2 induces Foxp3 and regulates differentiation of CD4+ T cells. Nat Commun. 2011;2:269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 174] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 27. | Barish GD, Downes M, Alaynick WA, Yu RT, Ocampo CB, Bookout AL, Mangelsdorf DJ, Evans RM. A Nuclear Receptor Atlas: macrophage activation. Mol Endocrinol. 2005;19:2466-2477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 196] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 28. | Pei L, Castrillo A, Chen M, Hoffmann A, Tontonoz P. Induction of NR4A orphan nuclear receptor expression in macrophages in response to inflammatory stimuli. J Biol Chem. 2005;280:29256-29262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 226] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 29. | Ralph JA, Ahmed AU, Santos LL, Clark AR, McMorrow J, Murphy EP, Morand EF. Identification of NURR1 as a mediator of MIF signaling during chronic arthritis: effects on glucocorticoid-induced MKP1. Am J Pathol. 2010;177:2366-2378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Doi Y, Oki S, Ozawa T, Hohjoh H, Miyake S, Yamamura T. Orphan nuclear receptor NR4A2 expressed in T cells from multiple sclerosis mediates production of inflammatory cytokines. Proc Natl Acad Sci USA. 2008;105:8381-8386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 31. | Saijo K, Winner B, Carson CT, Collier JG, Boyer L, Rosenfeld MG, Gage FH, Glass CK. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell. 2009;137:47-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 792] [Cited by in RCA: 750] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 32. | Volakakis N, Kadkhodaei B, Joodmardi E, Wallis K, Panman L, Silvaggi J, Spiegelman BM, Perlmann T. NR4A orphan nuclear receptors as mediators of CREB-dependent neuroprotection. Proc Natl Acad Sci USA. 2010;107:12317-12322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 33. | Mix KS, McMahon K, McMorrow JP, Walkenhorst DE, Smyth AM, Petrella BL, Gogarty M, Fearon U, Veale D, Attur MG. Orphan nuclear receptor NR4A2 induces synoviocyte proliferation, invasion, and matrix metalloproteinase 13 transcription. Arthritis Rheum. 2012;64:2126-2136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | McEvoy AN, Murphy EA, Ponnio T, Conneely OM, Bresnihan B, FitzGerald O, Murphy EP. Activation of nuclear orphan receptor NURR1 transcription by NF-kappa B and cyclic adenosine 5'-monophosphate response element-binding protein in rheumatoid arthritis synovial tissue. J Immunol. 2002;168:2979-2987. [PubMed] |
| 35. | Aherne CM, McMorrow J, Kane D, FitzGerald O, Mix KS, Murphy EP. Identification of NR4A2 as a transcriptional activator of IL-8 expression in human inflammatory arthritis. Mol Immunol. 2009;46:3345-3357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 36. | Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006;11:529-543. [PubMed] |
| 37. | Mix KS, Attur MG, Al-Mussawir H, Abramson SB, Brinckerhoff CE, Murphy EP. Transcriptional repression of matrix metalloproteinase gene expression by the orphan nuclear receptor NURR1 in cartilage. J Biol Chem. 2007;282:9492-9504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 38. | Ralph JA, Zocco D, Bresnihan B, Fitzgerald O, McEvoy AN, Murphy EP. A role for type 1alpha corticotropin-releasing hormone receptors in mediating local changes in chronically inflamed tissue. Am J Pathol. 2007;170:1121-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Puglisi MA, Barba M, Corbi M, Errico MF, Giorda E, Saulnier N, Boninsegna A, Piscaglia AC, Carsetti R, Cittadini A. Identification of Endothelin-1 and NR4A2 as CD133-regulated genes in colon cancer cells. J Pathol. 2011;225:305-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Li X, Tai HH. Activation of thromboxane A(2) receptors induces orphan nuclear receptor Nurr1 expression and stimulates cell proliferation in human lung cancer cells. Carcinogenesis. 2009;30:1606-1613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 41. | Holla VR, Wu H, Shi Q, Menter DG, DuBois RN. Nuclear orphan receptor NR4A2 modulates fatty acid oxidation pathways in colorectal cancer. J Biol Chem. 2011;286:30003-30009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 42. | Shadman M, Newcomb PA, Hampton JM, Wernli KJ, Trentham-Dietz A. Non-steroidal anti-inflammatory drugs and statins in relation to colorectal cancer risk. World J Gastroenterol. 2009;15:2336-2339. [PubMed] |
| 43. | Menter DG, Dubois RN. Prostaglandins in cancer cell adhesion, migration, and invasion. Int J Cell Biol. 2012;2012:723419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 44. | Ji R, Sanchez CM, Chou CL, Chen XB, Woodward DF, Regan JW. Prostanoid EP₁ receptors mediate up-regulation of the orphan nuclear receptor Nurr1 by cAMP-independent activation of protein kinase A, CREB and NF-κB. Br J Pharmacol. 2012;166:1033-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Holla VR, Mann JR, Shi Q, DuBois RN. Prostaglandin E2 regulates the nuclear receptor NR4A2 in colorectal cancer. J Biol Chem. 2006;281:2676-2682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 46. | Ji H, Zhang Y, Shen X, Gao F, Huang CY, Abad C, Busuttil RW, Waschek JA, Kupiec-Weglinski JW. Neuropeptide PACAP in mouse liver ischemia and reperfusion injury: immunomodulation via cAMP-PKA pathway. Hepatology. 2012;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 47. | Palorini R, De Rasmo D, Gaviraghi M, Danna LS, Signorile A, Cirulli C, Chiaradonna F, Alberghina L, Papa S. Oncogenic K-ras expression is associated with derangement of the cAMP/PKA pathway and forskolin-reversible alterations of mitochondrial dynamics and respiration. Oncogene. 2012;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 48. | Jones SE, Palmer TM. Protein kinase A-mediated phosphorylation of RhoA on serine 188 triggers the rapid induction of a neuroendocrine-like phenotype in prostate cancer epithelial cells. Cell Signal. 2012;24:1504-1514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 49. | Li Y, Chen Y, Tao Y, Wang Y, Chen Y, Xu W. Fibronectin increases RhoA activity through inhibition of PKA in the human gastric cancer cell line SGC-7901. Mol Med Report. 2011;4:65-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 50. | Leone V, di Palma A, Ricchi P, Acquaviva F, Giannouli M, Di Prisco AM, Iuliano F, Acquaviva AM. PGE2 inhibits apoptosis in human adenocarcinoma Caco-2 cell line through Ras-PI3K association and cAMP-dependent kinase A activation. Am J Physiol Gastrointest Liver Physiol. 2007;293:G673-G681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 51. | Chan TA. Prostaglandins and the colon cancer connection. Trends Mol Med. 2006;12:240-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 52. | Kitagawa H, Ray WJ, Glantschnig H, Nantermet PV, Yu Y, Leu CT, Harada S, Kato S, Freedman LP. A regulatory circuit mediating convergence between Nurr1 transcriptional regulation and Wnt signaling. Mol Cell Biol. 2007;27:7486-7496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 53. | Likui W, Hong W, Shuwen Z. Clinical significance of the upregulated osteopontin mRNA expression in human colorectal cancer. J Gastrointest Surg. 2010;14:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 54. | Lammi J, Huppunen J, Aarnisalo P. Regulation of the osteopontin gene by the orphan nuclear receptor NURR1 in osteoblasts. Mol Endocrinol. 2004;18:1546-1557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 55. | Uede T. Osteopontin, intrinsic tissue regulator of intractable inflammatory diseases. Pathol Int. 2011;61:265-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 149] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 56. | Dai N, Bao Q, Lu A, Li J. Protein expression of osteopontin in tumor tissues is an independent prognostic indicator in gastric cancer. Oncology. 2007;72:89-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 57. | Zagani R, Hamzaoui N, Cacheux W, de Reyniès A, Terris B, Chaussade S, Romagnolo B, Perret C, Lamarque D. Cyclooxygenase-2 inhibitors down-regulate osteopontin and Nr4A2-new therapeutic targets for colorectal cancers. Gastroenterology. 2009;137:1358-66.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 58. | Zhao D, Desai S, Zeng H. VEGF stimulates PKD-mediated CREB-dependent orphan nuclear receptor Nurr1 expression: role in VEGF-induced angiogenesis. Int J Cancer. 2011;128:2602-2612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 59. | Tayama M, Furuhata T, Inafuku Y, Okita K, Nishidate T, Mizuguchi T, Kimura Y, Hirata K. Vascular endothelial growth factor 165b expression in stromal cells and colorectal cancer. World J Gastroenterol. 2011;17:4867-4874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 60. | Yin Y, Cao LY, Wu WQ, Li H, Jiang Y, Zhang HF. Blocking effects of siRNA on VEGF expression in human colorectal cancer cells. World J Gastroenterol. 2010;16:1086-1092. [PubMed] |
| 61. | Kamiyama T, Takahashi M, Nakanishi K, Yokoo H, Kamachi H, Kobayashi N, Ozaki M, Todo S. α-fetoprotein, vascular endothelial growth factor receptor-1 and early recurrence of hepatoma. World J Gastroenterol. 2012;18:340-348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 62. | Tang H, Wang J, Bai F, Zhai H, Gao J, Hong L, Xie H, Zhang F, Lan M, Yao W. Positive correlation of osteopontin, cyclooxygenase-2 and vascular endothelial growth factor in gastric cancer. Cancer Invest. 2008;26:60-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 63. | Coghill AE, Newcomb PA, Poole EM, Hutter CM, Makar KW, Duggan D, Potter JD, Ulrich CM. Genetic variation in inflammatory pathways is related to colorectal cancer survival. Clin Cancer Res. 2011;17:7139-7147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 64. | Cheng HH, Tseng GY, Yang HB, Wang HJ, Lin HJ, Wang WC. Increased numbers of Foxp3-positive regulatory T cells in gastritis, peptic ulcer and gastric adenocarcinoma. World J Gastroenterol. 2012;18:34-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 65. | Liao G, Detre C, Berger SB, Engel P, de Waal Malefyt R, Herzog RW, Bhan AK, Terhorst C. Glucocorticoid-induced tumor necrosis factor receptor family-related protein regulates CD4(+)T cell-mediated colitis in mice. Gastroenterology. 2012;142:582-591.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 66. | Sinicrope FA, Rego RL, Ansell SM, Knutson KL, Foster NR, Sargent DJ. Intraepithelial effector (CD3+)/regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology. 2009;137:1270-1279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 261] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 67. | Deng L, Zhang H, Luan Y, Zhang J, Xing Q, Dong S, Wu X, Liu M, Wang S. Accumulation of foxp3+ T regulatory cells in draining lymph nodes correlates with disease progression and immune suppression in colorectal cancer patients. Clin Cancer Res. 2010;16:4105-4112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 68. | Chaput N, Louafi S, Bardier A, Charlotte F, Vaillant JC, Ménégaux F, Rosenzwajg M, Lemoine F, Klatzmann D, Taieb J. Identification of CD8+CD25+Foxp3+ suppressive T cells in colorectal cancer tissue. Gut. 2009;58:520-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 206] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 69. | Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH, Pagès F. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71:1263-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 774] [Cited by in RCA: 896] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 70. | Maruyama T, Kono K, Mizukami Y, Kawaguchi Y, Mimura K, Watanabe M, Izawa S, Fujii H. Distribution of Th17 cells and FoxP3(+) regulatory T cells in tumor-infiltrating lymphocytes, tumor-draining lymph nodes and peripheral blood lymphocytes in patients with gastric cancer. Cancer Sci. 2010;101:1947-1954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 71. | Yuan XL, Chen L, Li MX, Dong P, Xue J, Wang J, Zhang TT, Wang XA, Zhang FM, Ge HL. Elevated expression of Foxp3 in tumor-infiltrating Treg cells suppresses T-cell proliferation and contributes to gastric cancer progression in a COX-2-dependent manner. Clin Immunol. 2010;134:277-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 72. | Inamoto T, Papineni S, Chintharlapalli S, Cho SD, Safe S, Kamat AM. 1,1-Bis(3'-indolyl)-1-(p-chlorophenyl)methane activates the orphan nuclear receptor Nurr1 and inhibits bladder cancer growth. Mol Cancer Ther. 2008;7:3825-3833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 73. | Inamoto T, Czerniak BA, Dinney CP, Kamat AM. Cytoplasmic mislocalization of the orphan nuclear receptor Nurr1 is a prognostic factor in bladder cancer. Cancer. 2010;116:340-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |