Published online Dec 14, 2012. doi: 10.3748/wjg.v18.i46.6861
Revised: September 11, 2012
Accepted: September 19, 2012
Published online: December 14, 2012
Hepatocellular carcinoma (HCC) is difficult to eradicate due to its resilient nature. Portal vein is often involved in tumors of large size, which exclude the patient from surgical resection and local ablative therapy, such as percutaneous ethanol injection (PEI) and radiofrequency ablation (RFA) because they were considered neither effective nor safe. Currently, there is almost no effective treatment for HCC of such condition. As a unique antitumor agent in form of lipophilic fluid for local injection, para-toluenesulfonamide (PTS) produces mild side effects while necrotizing the tumor tissues quickly and efficiently. Being largely different from both PEI and RFA therapies, PTS can disseminate itself in tumors more easily than other caustic agents, such as alcohol. So PTS may offer additional benefit to HCCs with vascular involvement. We herein describe a 70-year-old HCC patient who was treated with the combination of PTS injection and transcatheter arterial chemoembolization, resulting in a significantly improved clinical prognosis.
- Citation: He Q, Kuang AR, Guan YS, Liu YQ. Puncture injection of para-toluenesulfonamide combined with chemoembolization for advanced hepatocellular carcinoma. World J Gastroenterol 2012; 18(46): 6861-6864
- URL: https://www.wjgnet.com/1007-9327/full/v18/i46/6861.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i46.6861
Transcatheter arterial chemoembolization (TACE) has become the standard treatment for unresectable hepatocellular carcinoma (HCC). Nonetheless, clinical outcomes are often unsatisfactory, especially for recurrent cases. As a novel anticancer agent, para-toluenesulfonamide (PTS) is completely different from genetic, classical chemical and molecular targeted drugs, and has shown amazing antitumor effect in animal HCC experiment[1,2]. Primary pharmacological studies suggest that PTS inhibits tumor growth by acting as a tumor necrotizing agent[1,2]. PTS may strengthen the effect of TACE in advanced HCC. We herein report a patient with refractory HCC who was treated with PTS injection after TACE, which resulted in a very good clinical prognosis.
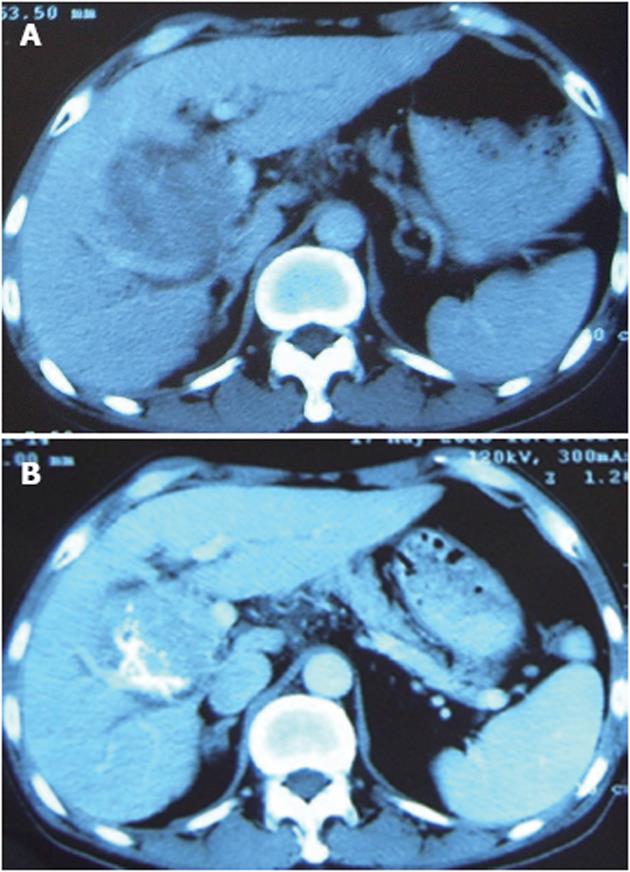
The patient was a 70-year-old man. A mass was found in his right lobe of the liver in May 2004. He refused to receive further diagnosis and treatment until January 2006 when he began to suffer from anorexia and dull abdominal pain. Computer tomography (CT) detected a large mass (about 9.5 cm × 8.5 cm × 8.5 cm) in the right lobe of the liver with significant enhancement (Figure 1A). Alpha-fetoprotein (AFP) level was > 1210 ng/mL. He was diagnosed with a histologically proven HCC. Due to the large tumor size and invasion to the portal vein, the patient underwent two rounds of TACE with an interval of one month. The drugs used were 5fluoro2deoxyuridine (1.0 g), epirubicin (40 mg) and lipiodol (10 mL). In May 2006, three months after the second TACE, a routine postoperative CT scan found a minimal decrease in tumor size with necrosis < 25% (Figure 1B), while the AFP level was still > 1210 ng/mL. The second angiography revealed hepatic artery-portal vein fistula and hepatic arteriovenous fistula. Although additional gelfoam pieces were used and the fistula disappeared after embolization, a poor prognosis was still predicted. Consequently, PTS (Beijing Vision Drugs Development Ltd., Beijing, China) was used to enhance the effect of TACE after informed consent was obtained from the patient.
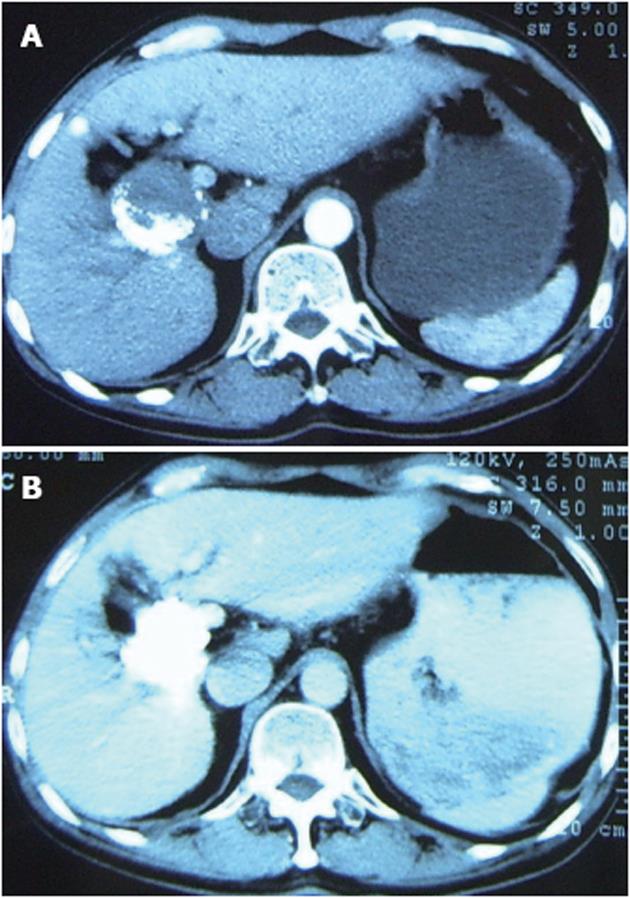
The mass was punctured percutaneously with a fine needle under CT guidance. After the tip of the needle was manipulated into the mass, about 10 mL of PTS was injected intratumorally in a multi-point fashion. This injection was repeated four times. The interval between each procedure was between three and seven days according to the patient’s clinical condition. When all five injections were completed, the AFP level dropped to 1106 ng/mL. Two months later, a routine follow-up CT showed moderate improvement, no blood being supplied to the tumor (Figure 2A), and the AFP level dropped further down to 185 ng/mL. In an attempt to investigate the increased efficacy, PTS injections were repeated three more times. Following a routine check two months later, a CT examination of the abdomen demonstrated complete deposit of oil and no signs of recurrence or tumor embolism (Figure 2B). Furthermore, the AFP was within the normal reference range (4.44 ng/mL).
The only side effect of this therapy was abdominal pain, which occurred after the first two procedures, but subsided shortly thereafter in approximately 10 min. No other discomfort was noted post-procedurally. Four years following the last PTS treatment, the patient exhibited no evidence of recurrence and no other abnormal liver function serum values, and he had a normal serum AFP level. The AFP level changes in the course of the treatment are summarized in Table 1.
| Time (yr-mo) | APF (ng/mL) | TACE | PTS injections |
| 2005-4 | 533.8 | ||
| 2006-1 | > 1210 | Yes | |
| 2006-2 | > 1210 | Yes | |
| 2006-5 | > 1210 | No | 5 times |
| 2006-6 | 1106 | No | |
| 2006-8 | 185 | No | 3 times |
| 2006-10 | 4.44 | No | |
| 2008-6 | 2.69 | No | |
| 2009-3 | 3.84 | No | |
| 2010-4 | 3.86 | No |
Despite the fact that TACE has become the standard treatment for unresectable HCC, it is frequently unsuccessful. Rate of local recurrence following tumor resection is also unacceptably high[3-6]. In addition, TACE alone fails to control the tumor completely, often leading to a poor prognosis. In this report, the patient had received two sessions of TACE and embolized the fistula successfully before admission. Although little effect was got on HCC, the TACE did restrict the tumor’s growth and metastasis that made it possible for PTS to eradicate the tumor later. Due to the large tumor size and involvement of the portal vein, this lesion was inoperable, and other local ablative therapies, such as percutaneous ethanol injection, microwave coagulation therapy and radiofrequency ablation (RFA) were considered neither effective nor safe. Complications caused by these modalities may result in a high mortality rate. Therefore, we attempted to treat the patient with the combined administration of PTS and TACE. Consequently, the patient’s condition was significantly improved with satisfactory tumor control and without severe complications.
Para-toluenesulfonamide (P-TSA) is the active ingredient in PTS. The P-TSA is a white, odorless, crystalline substance that has a very low solubility in water. The molecular formula is C7H9NO2S. The injection solution is a clear, colorless, oil liquid with a characteristic odor and contains 330 mg/mL P-TSA. PTS has been approved for clinical trial injection in both 3 mL ampoule and 5 mL ampoule. Recommended storage temperature is 25 °C (77°F), although a range of 10-35 °C (50-90°F) is acceptable. Long-term exposure to light should be avoided.
PTS produces mild side effects while necrotizing the tumor tissues effectively and thoroughly. However, PTS is still in the phase of clinical trial, and the mechanism of the antitumor activity of PTS is still unclear. Primary pharmacological studies suggest that PTS inhibits tumor growth by acting as a tumor necrotizing agent[1,2].
It has been shown that PTS does not cause serious side effects that have been observed frequently in conventional chemotherapy and locoregional therapy (e.g., RFA, alcohol injection, etc.), such as fever, bone marrow suppression, stomach discomfort, hemorrhage, needle-track seeding, lesion abscess , liver failure, biloma, biliary stricture, portal vein thrombosis, and hemothorax[7-9]. Because most anticancer drugs are corrosive and extremely toxic, they will destroy both cancer cells and normal cells when given locally at high concentrations. PTS is a local therapeutic drug that is injected directly into the tumors and has been shown to cause selectively necrosis in a variety of cancers with minimal damage to normal tissues[2,10].
Local ablative therapies share similar difficulties with surgical resection. The size, site and number of tumors, vascular and extrahepatic involvement as well as liver function of the patient pose a relatively minor effect on the usage of PTS[8,9]. PTS is a more readily available alternative to the local ablative therapies.
PTS, in form of lipophilic fluid, kills tumor cells by a rodent mode. Local and intratumoral injection is the optimal route of PTS delivery. Being largely different from both alcohol and RFA therapies, PTS can disseminate itself in tumors more easily than other caustic agents, such as alcohol. Therefore, a successful PTS administration is to approach to the anatomically dangerous or hard-to-reach areas and diffuse to the target area and induce injury to the tumor tissues. This might be the mechanism as to why PTS combined with TACE could effectively treat the HCC with vascular invasion. As a locoregional antitumor agent, PTS is safe[1,2,10]. But up to date, PTS is still only a locally-used antitumor agent. It is intended mainly for the treatment of a limited number of detectable tumors. PTS is not suitable to be used alone for the treatment of multifocal HCCs.
This case report demonstrated that PTS is effective in treating liver cancers by intratumoral injection, which was hypothesized to enhance the effect of TACE. This combined therapy may prove to be useful in the treatment of patients with refractory and recurrent HCC. Therefore, subsequent large, multi-center, randomized controlled studies are needed to facilitate the introduction of PTS as a novel modality for the treatment of cancers.
Peer reviewers: Alessandro Cucchetti, MD, Liver and Multiorgan Transplant Unit, Policlinico S.Orsola-Malpighi, University of Bologna, PAD 25., Via Massarenti 9, 40138 Bologna, Italy; Zenichi Morise, MD, PhD, Professor, Chairman, Department of Surgery Banbuntane Houtokukai Hospital, Fujita Health University School of Medicine, 3-6-10 Otobashi Nakagawa-ku, Nagoya, Aichi 454-8509, Japan
S- Editor Gou SX L- Editor A E- Editor Xiong L
| 1. | Li MY, Meng H, Zhu WL, Zhou SZ, Li HY, Zhang JR. [Dose-effect relationship of para-toluenesulfonamide for treatment of hepatocellular carcinoma in rats]. Nanfang Yike Daxue Xuebao. 2008;28:249-251. [PubMed] |
| 2. | Meng H, Li MY, Zhu WL, Zhang JR. [Therapeutic effect of para-toluenesulfonamide on transplanted hepatocarcinoma in nude mice]. Nanfang Yike Daxue Xuebao. 2009;29:1024-1025. [PubMed] |
| 3. | Qu XD, Chen CS, Wang JH, Yan ZP, Chen JM, Gong GQ, Liu QX, Luo JJ, Liu LX, Liu R. The efficacy of TACE combined sorafenib in advanced stages hepatocellullar carcinoma. BMC Cancer. 2012;12:263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 4. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2502] [Cited by in RCA: 2611] [Article Influence: 113.5] [Reference Citation Analysis (0)] |
| 5. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2207] [Cited by in RCA: 2270] [Article Influence: 103.2] [Reference Citation Analysis (0)] |
| 6. | He Q, Liu Y, Zou Q, Guan YS. Transarterial injection of H101 in combination with chemoembolization overcomes recurrent hepatocellular carcinoma. World J Gastroenterol. 2011;17:2353-2355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Minami Y, Kudo M. Radiofrequency ablation of hepatocellular carcinoma: a literature review. Int J Hepatol. 2011;2011:104685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Ansari D, Andersson R. Radiofrequency ablation or percutaneous ethanol injection for the treatment of liver tumors. World J Gastroenterol. 2012;18:1003-1008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Signoriello S, Annunziata A, Lama N, Signoriello G, Chiodini P, De Sio I, Daniele B, Di Costanzo GG, Calise F, Olivieri G. Survival after Locoregional Treatments for Hepatocellular Carcinoma: A Cohort Study in Real-World Patients. Scientific World Journal. 2012;2012:564-706. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Zhou JQ, Tang ZQ, Zhang JN, Tang JC. Metabolism and effect of para-toluene-sulfonamide on rat liver microsomal cytochrome P450 from in vivo and in vitro studies. Acta Pharmacol Sin. 2006;27:635-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |