Published online Oct 7, 2012. doi: 10.3748/wjg.v18.i37.5283
Revised: June 11, 2012
Accepted: June 15, 2012
Published online: October 7, 2012
AIM: To investigate the preventive effect of N-acetyl-seryl-aspartyl-lysyl-proline (AcSDKP) on bile duct ligation (BDL)-induced liver fibrosis in rats.
METHODS: Liver fibrosis in rats was induced by BDL and AcSDKP was infused subcutaneously for 2 wk via a osmotic minipump (Alzet 2ML4) immediately after BDL operation. After scarifying, serum and liver specimens were collected. Hematoxylin and eosin staining, Sirius red staining, enzyme linked immunosorbent assay, Western blot or real-time polymerase chain reaction were used to determinate liver functions, histological alterations, collagen deposition, mRNA expression of markers for fibroblasts, transforming growth factor-β1 (TGF-β1) and bone morphogenetic protein-7 (BMP-7).
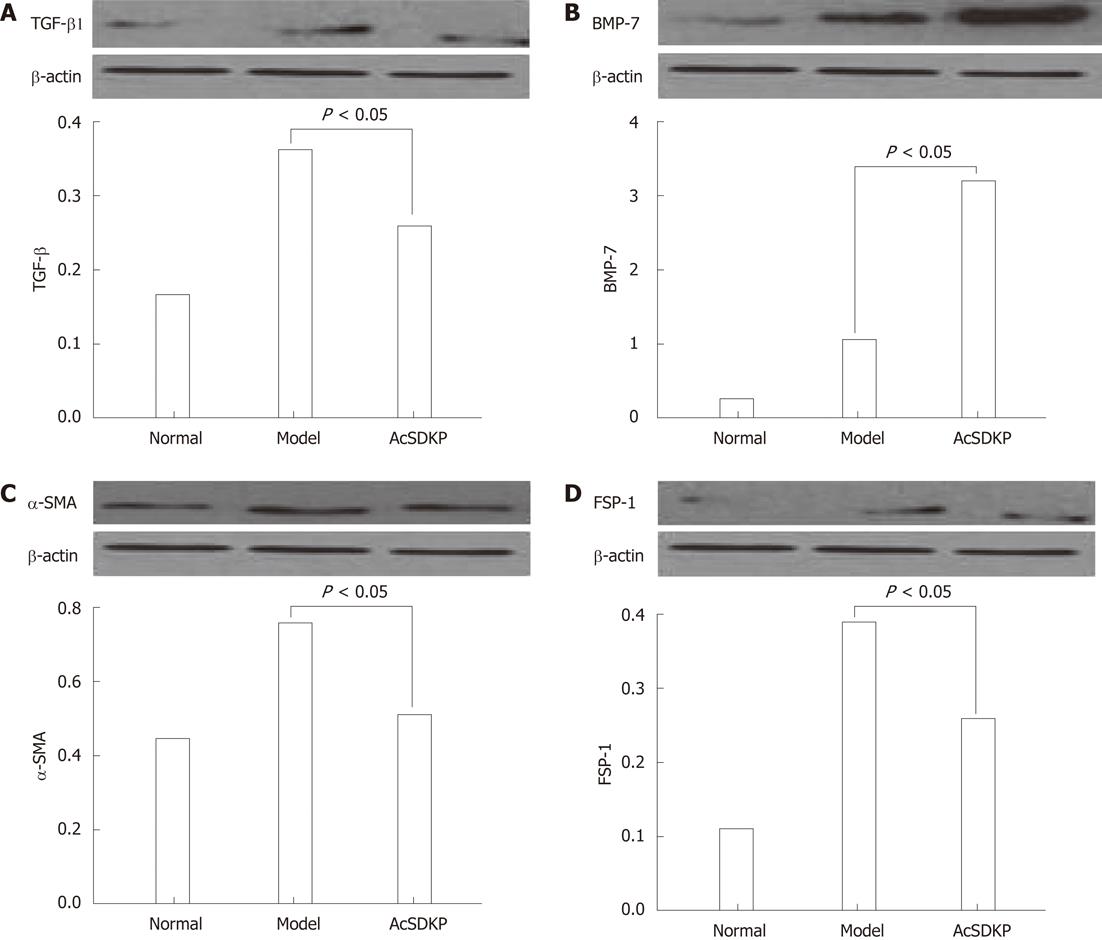
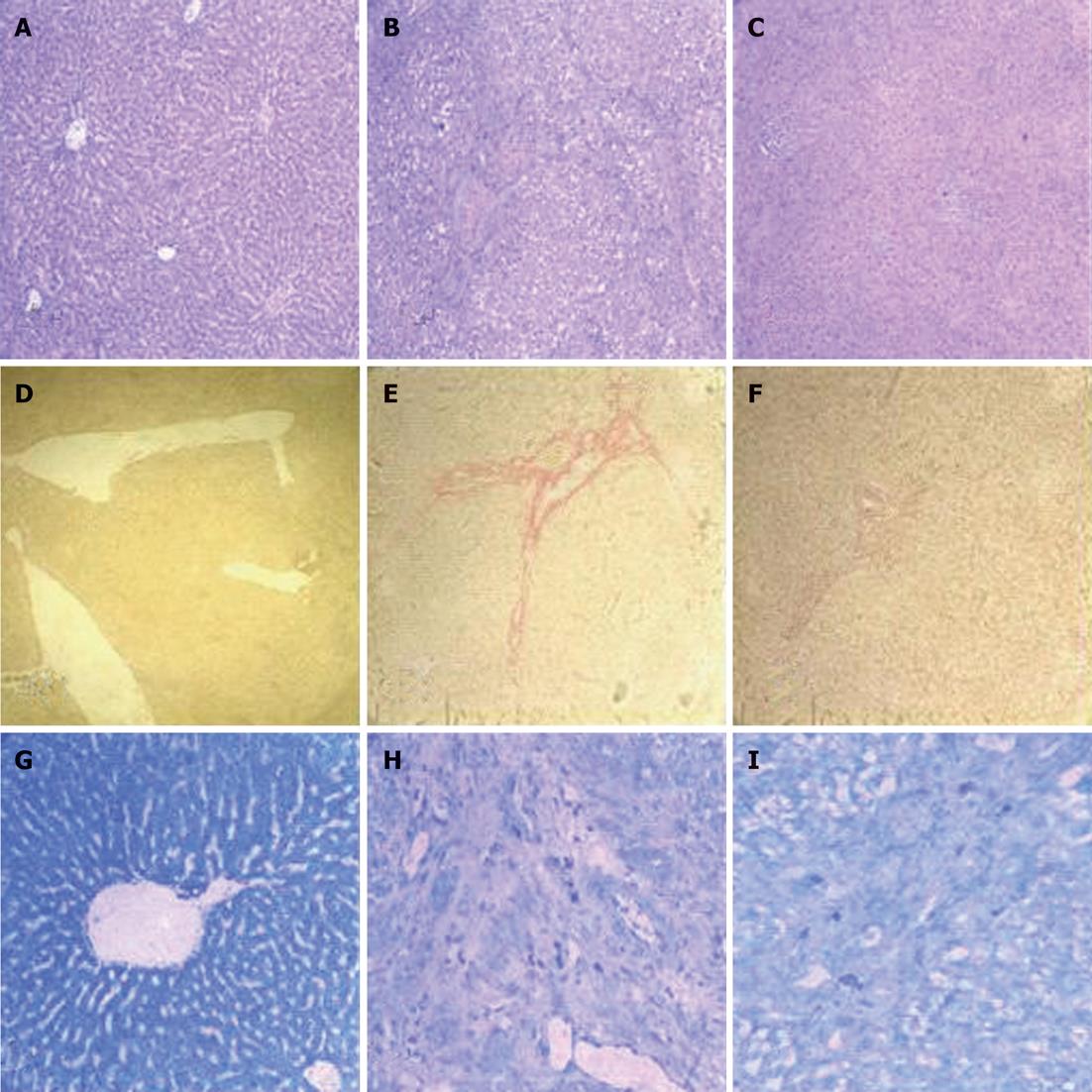
RESULTS: When compared to model rats, chronic exogenous AcSDKP infusion suppressed profibrogenic TGF-β1 signaling, α-smooth muscle actin positivity (α-SMA), fibroblast specific protein-1 (FSP-1) staining and collagen gene expression. Col I, Col III, matrix metalloproteinase-2, tissue inhibitors of metalloproteinase-1 and tissue inhibitors of metalloproteinase-2 mRNA expressions were all significantly downregulated by AcSDKP infusion (2.02 ± 1.10 vs 14.16 ± 6.50, 2.02 ± 0.45 vs 10.00 ± 3.35, 2.91 ± 0.30 vs 7.83 ± 1.10, 4.64 ± 1.25 vs 18.52 ± 7.61, 0.46 ± 0.16 vs 0.34 ± 0.12, respectively, P < 0.05). Chronic exogenous AcSDKP infusion attenuated BDL-induced liver injury, inflammation and fibrosis. BDL caused a remarkable increase in alanine transaminase, aspartate transaminase, total bilirubin, and prothrombin time, all of which were reduced by AcSDKP infusion. Mast cells, collagen accumulation, α-SMA, TGF-β1, FSP-1 and BMP-7 increased. The histological appearance of liver specimens was also improved.
CONCLUSION: Infusion of exogenous AcSDKP attenuated BDL-induced fibrosis in the rat liver. Preservation of AcSDKP may be a useful therapeutic approach in the management of liver fibrosis.
- Citation: Zhang L, Xu LM, Chen YW, Ni QW, Zhou M, Qu CY, Zhang Y. Antifibrotic effect of N-acetyl-seryl-aspartyl-lysyl-proline on bile duct ligation induced liver fibrosis in rats. World J Gastroenterol 2012; 18(37): 5283-5288
- URL: https://www.wjgnet.com/1007-9327/full/v18/i37/5283.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i37.5283
N-acetyl-seryl-aspartyl-lysyl-proline (AcSDKP) is an endogenous tetrapeptide normally present in the plasma and organs of humans and experimental animals[1-3]. It is released locally in tissues from its precursor thymosin-β4 (Tβ4) most likely by prolyl oligopeptidase (POP), a serine proteinase found in mammalian tissues[4,5]. AcSDKP is cleaved to an inactive form by the NH2-terminal catalytic domain of angiotensin converting enzyme (ACE)[6].
Originally described as a natural inbibitor of hematopoietic stem cell proliferation, AcSDKP is now recognized as a critical negative regulator for extracellular matrix (ECM) accumulation in organs under both physiological and pathological conditions. Decreased basal levels of endogenous AcSDKP by ACE over expression or by POP inhibitors promote cardiac fibrosis and/or glomerulosclerosis[7,8]. Exogenous AcSDKP infusion reduces collagen deposition in rats heart and/or kidney under hypertensive and ischemic conditions[9]. AcSDKP also mediates the antifibrogenic effect of ACE inhibitors in the heart[10]. The mechanism of action of AcSDKP includes suppression of inflammation, ECM-producing cell proliferation, collagen production, and more importantly transforming growth factor-β1 (TGF-β1) signaling[7-9,11,12].
Indeed, these key cellular and molecular mechanisms are critical in regulating ECM accumulation in multiple organs, in particular the liver[13]. Moreover, ACE inhibition is beneficial in several liver fibrosis models where there is increased ACE activity and potentially excessive AcSDKP degradation, Tβ4 and significant POP activity are present in the liver, where AcSDKP is produced locally, and may play a role in the regulation of hepatic cell responses in vivo[14,15].
Our previous studies had revealed that AcSDKP ameliorated carbon tetrachloride (CCl4)-induced liver fibrosis and liver functions in the rat liver. The current study was aimed to investigate the preventive effect of AcSDKP on bile duct ligation (BDL)-induced liver fibrosis in rats. The potential mechanisms involved were also examined.
We explored the effects of AcSDKP on liver fibrosis by infusion of exogenous AcSDKP into the BDL rat models. Our results demonstrate that exogenous AcSDKP preserves basal levels of AcSDKP in the liver and significantly reduces the development of liver fibrosis in this model. Based on these findings, we propose that AcSDKP plays an important role in attenuating liver fibrosis. The underlying mechanisms may involve decreased production of profibrotic cytokines and reduced collagen expression and accumulation.
BDL-induced rat liver fibrosis models: all animal handling and experimental procedures were approved by the Animal Care and Use Committee of the Shanghai Jiaotong Uinversity School of Medicine. Male Sprague-Dawley rats (200-250 g) were obtained from the Shanghai Experimental Animal Center (Shanghai, China). The rat model of liver fibrosis was induced by BDL. Upon sacrifice, blood was collected and serum and/or plasma were obtained. Liver tissue was either fixed in 10% neutral buffered formalin, frozen in optimal cutting temperature, or snap frozen in liquid nitrogen and stored at -80 °C.
Experiment: AcSDKP-infused BDL-treated rats and the BDL model were established as above (n = 8-10). AcSDKP-infused BDL-treated rats were infused with AcSDKP at 800 μg/kg per day through a subcutaneous osmotic minipump (Alza Corp, Palo Alto, CA) beginning simultaneously with BDL. Rats were sacrificed at 2 wk. This dosage was used because it increased plasma AcSDKP to a concentration similar to that induced by captopril (100 μg/kg per day, 3- to 5-fold-change), without any adverse effect on the circulatory system[9].
Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin, and albumin in serum and prothrombin time in plasma were measured using an automated analyzer.
Formalin-fixed paraffin sections of the liver were stained with hematoxylin and esosin for pathological analysis or Sirius red for collagen. Collagen was quantified with Image Quant 5.1 software as previously described[16]. Positive cells were enumerated in 10 randomly selected fields at 400 × magnification.
Total RNA was extracted from livers using Trizol and was reverse-transcribed using an iscript cDNA synthesis kit. Real-time polymerase chain reaction (PCR) was performed on an iCycler system using the SYBR green Master Mix. Primer specificity was confirmed by sequencing PCR products. β-actin was the internal control. Data were presented according to the ΔΔCt method.
Frozen liver tissue was homogenized in ice-cold RIPA buffer containing protease and phosphatase inhibitors. A full list of antibodies is available in Supplemental data. Western blot was performed as previously described[17]. Bands were quantified by Scion Image 4.0.3. The loading control was tubulin.
Hydroxyproline content in liver tissue was determined as previously described[18].
Data are expressed as means ± SE. Comparisons were performed using analysis of variance. Least significant difference procedure analyses were performed when > 2 groups were present. P < 0.05 was considered statistically significant.
Chronic exogenous AcSDKP infusion suppressed profibrogenic TGF-β1 signaling,α-SMA, eibroblast specific protein-1 and bone morphogenetic protein-7 staining and collagen gene expression
When compared to model rats, TGF-β1 was significantly downregulated in AcSDKP-infused BDL-treated rats (Figure 1A). In contrast, bone morphogenetic protein-7 (BMP-7) staining in the liver of BDL-treated rats was increased by AcSDKP (Figure 1B). α-SMA, fibroblast specific protein-1 (FSP-1), collagen I, collagen III, tissue inhibitor of metalloproteinase-1 and 2 mRNA all were downregulated by AcSDKP infusion (Figure 1C and D). Collagen I, collagen III, matrix metalloproteinases-2, tissue inhibitors of metalloproteinase-1 and tissue inhibitors of metalloproteinase-2 mRNA expressions were all significantly downregulated by AcSDKP infusion (2.02 ± 1.10 vs 14.16 ± 6.50, 2.02 ± 0.45 vs 10.00 ± 3.35, 2.91 ± 0.30 vs 7.83 ± 1.10, 4.64 ± 1.25 vs 18.52 ± 7.61, 0.46 ± 0.16 vs 0.34 ± 0.12, respectively, P < 0.05). Matrix metalloproteinase-2 expression was increased in BDL-treated rats but suppressed by AcSDKP.
BDL caused a remarkable increase in ALT, AST, total bilirubin, and prothrombin time, all of which were reduced by AcSDKP infusion (Table 1). The histological appearance of liver specimens was also improved (Figure 2A-C). Marked collagen accumulation was observed in AcSDKP-infused BDL-treated vs model rats, which was attenuated by AcSDKP infusion (Figure 2D-F). The reduction in total collagen was further confirmed by decreased hydroxyproline content. When compared to model rats, hyaluronic acid, ammonia terminal procollagen β peptide and hydroxyproline were all significantly decreased by AcSDKP infusion (127.4 ± 31.8 vs 267.2 ± 99.4, 6.9 ± 0.5 vs 35.2 ± 4.3, 162.3 ± 42.4 vs 398.2 ± 60.4, respectively, P < 0.05). Total mast cells decreased in AcSDKP vs model BDL-treated rats (Figure 2G-I).
Here, we demonstrate that in the liver, chronic exogenous AcSDKP infusion preserves basal levels of AcSDKP and attenuates BDL-induced fibrosis. This is supported by other studies showing an important role of AcSDKP in preventing heart[19,20] and kidney[21,22] fibrosis at basal concentrations. Our previous studies had also revealed that AcSDKP ameliorated CCL4-induced liver fibrosis and liver functions in rats.
Attenuation of liver fibrosis by AcSDKP is associated with suppressed inflammation and TGF-β signaling. Our results show that AcSDKP suppressed mast cells infiltration, TGF-β1 signaling and myofibroblasts in vivo.
Recent evidence suggests that epithelial-to-mesenchymal transition (EMT) may also contribute to liver fibrogenesis[23]. TGF-β1 is still generally considered to be the main positive regulator of EMT and ECM accumulation[23]. Indeed, our results show that AcSDKP suppressed TGF-β signaling and reduced the EMT markers α-SMA and FSP-1 in vivo[24,25]. In addition, AcSDKP increased BMP-7. BMP-7 counteracts the effects of TGF-β1 and is a prototypical negative regulator of EMT. Nevertheless, a more sophisticated study is required to fully elucidate the possible role of AcSDKP-induced inhibition of EMT in the attenuation of liver fibrosis.
There are some limitations in this study. We did not include a group of control rats infused with AcSDKP primarily because exogenous infusion of AcSDKP restored the peptide levels to control levels and this dose has been shown to have no adverse effects systemically. Secondly, the cellular mechanisms of AcSDKP action were not fully elucidated in our current study. The presence of an AcSDKP receptor on cells has been suggested[26]. We speculate that AcSDKP may directly affect liver cells by binding and activating its receptor on the cell surface, resulting in suppression of certain profibrogenic intracellular signaling pathways. Further studies to clone the receptor or develop specific receptor antagonists will enable full characterization of the cellular mechanisms involved in the antifibrotic effects of AcSDKP in vivo and in vitro.
In summary, this study shows that chronic exogenous AcSDKP infusion preserves basal levels of AcSDKP and attenuates liver fibrosis induced by BDL in rats. Our study strongly suggests a significant role for AcSDKP in the development of liver fibrosis and potentiates the usefulness of this tetrapeptide in the prevention of this disease. Additional studies are needed to gain further insight into the biological effect of AcSDKP in the liver and further studies are ultimately warranted in the human.
N-acetyl-seryl-aspartyl-lysyl-proline (AcSDKP) is an endogenous tetrapeptide in vivo which has antifibrogenic effects on the heart, the lung and the kidney. The authors’ previous studies had revealed that AcSDKP ameliorated carbon tetrachloride-induced liver fibrosis and liver functions in the rat liver.
Originally described as a natural inhibitor of hematopoietic stem cell proliferation, AcSDKP is now recognized as a critical negative regulator for extracellular matrix accumulation in organs under both physiological and pathological conditions.
This is the first study to investigate the preventive effect of endogenous AcSDKP in bile duct ligation (BDL)-induced fibrosis in the rat liver and the potential mechanisms involved were also examined. The results strongly suggest a significant role for AcSDKP in the development of liver fibrosis and potentiates the usefulness of this tetrapeptide in the prevention of this disease.
Preservation of AcSDKP may be a useful therapeutic approach in the management of liver fibrosis.
This is a good descriptive study in which authors analyze the preventive effect of AcSDKP on BDL-induced liver fibrosis in rats. The results are interesting and suggest that infusion of exogenous AcSDKP attenuated BDL-induced fibrosis in the rat liver. Preservation of AcSDKP may be a useful therapeutic approach in the management of liver fibrosis.
Peer reviewer: Dr. Jeff Butterworth, Shrewsbury and Telford Hospitals NHS Trust, 102 The Mount, Shrewsbury SY3 8PH, United Kingdom
S- Editor Gou SX L- Editor O’Neill M E- Editor Xiong L
| 1. | Dainiak N. Negative regulators of hematopoietic stem cells and progenitors. Exp Hematol. 1992;20:1154-1155. [PubMed] |
| 2. | Pradelles P, Frobert Y, Créminon C, Ivonine H, Frindel E. Distribution of a negative regulator of haematopoietic stem cell proliferation (AcSDKP) and thymosin beta 4 in mouse tissues. FEBS Lett. 1991;289:171-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Liu JM, Garcia-Alvarez MC, Bignon J, Kusinski M, Kuzdak K, Riches A, Wdzieczak-Bakala J. Overexpression of the natural tetrapeptide acetyl-N-ser-asp-lys-pro derived from thymosin beta4 in neoplastic diseases. Ann N Y Acad Sci. 2010;1194:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Cavasin MA, Rhaleb NE, Yang XP, Carretero OA. Prolyl oligopeptidase is involved in release of the antifibrotic peptide Ac-SDKP. Hypertension. 2004;43:1140-1145. [PubMed] |
| 6. | Rousseau A, Michaud A, Chauvet MT, Lenfant M, Corvol P. The hemoregulatory peptide N-acetyl-Ser-Asp-Lys-Pro is a natural and specific substrate of the N-terminal active site of human angiotensin-converting enzyme. J Biol Chem. 1995;270:3656-3661. [PubMed] |
| 7. | Pokharel S, van Geel PP, Sharma UC, Cleutjens JP, Bohnemeier H, Tian XL, Schunkert H, Crijns HJ, Paul M, Pinto YM. Increased myocardial collagen content in transgenic rats overexpressing cardiac angiotensin-converting enzyme is related to enhanced breakdown of N-acetyl-Ser-Asp-Lys-Pro and increased phosphorylation of Smad2/3. Circulation. 2004;110:3129-3135. [PubMed] [DOI] [Full Text] |
| 8. | Cavasin MA, Liao TD, Yang XP, Yang JJ, Carretero OA. Decreased endogenous levels of Ac-SDKP promote organ fibrosis. Hypertension. 2007;50:130-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Liu YH, D'Ambrosio M, Liao TD, Peng H, Rhaleb NE, Sharma U, André S, Gabius HJ, Carretero OA. N-acetyl-seryl-aspartyl-lysyl-proline prevents cardiac remodeling and dysfunction induced by galectin-3, a mammalian adhesion/growth-regulatory lectin. Am J Physiol Heart Circ Physiol. 2009;296:H404-H412. [PubMed] [DOI] [Full Text] |
| 10. | Rasoul S, Carretero OA, Peng H, Cavasin MA, Zhuo J, Sanchez-Mendoza A, Brigstock DR, Rhaleb NE. Antifibrotic effect of Ac-SDKP and angiotensin-converting enzyme inhibition in hypertension. J Hypertens. 2004;22:593-603. [PubMed] |
| 11. | Rhaleb NE, Peng H, Harding P, Tayeh M, LaPointe MC, Carretero OA. Effect of N-acetyl-seryl-aspartyl-lysyl-proline on DNA and collagen synthesis in rat cardiac fibroblasts. Hypertension. 2001;37:827-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 76] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Peng H, Carretero OA, Peterson EL, Rhaleb NE. Ac-SDKP inhibits transforming growth factor-beta1-induced differentiation of human cardiac fibroblasts into myofibroblasts. Am J Physiol Heart Circ Physiol. 2010;298:H1357-H1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Wallace K, Burt AD, Wright MC. Liver fibrosis. Biochem J. 2008;411:1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 243] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 14. | Lin SC, Morrison-Bogorad M. Developmental expression of mRNAs encoding thymosins beta 4 and beta 10 in rat brain and other tissues. J Mol Neurosci. 1990;2:35-44. [PubMed] |
| 15. | Agirregoitia N, Casis L, Gil J, Ruiz F, Irazusta J. Ontogeny of prolyl endopeptidase and pyroglutamyl peptidase I in rat tissues. Regul Pept. 2007;139:52-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Lan L, Chen Y, Sun C, Sun Q, Hu J, Li D. Transplantation of bone marrow-derived hepatocyte stem cells transduced with adenovirus-mediated IL-10 gene reverses liver fibrosis in rats. Transpl Int. 2008;21:581-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Chen YW, Li DG, Wu JX, Chen YW, Lu HM. Tetrandrine inhibits activation of rat hepatic stellate cells stimulated by transforming growth factor-beta in vitro via up-regulation of Smad 7. J Ethnopharmacol. 2005;100:299-305. [PubMed] [DOI] [Full Text] |
| 18. | Zheng J, Chen Y, Pat B, Dell'italia LA, Tillson M, Dillon AR, Powell PC, Shi K, Shah N, Denney T. Microarray identifies extensive downregulation of noncollagen extracellular matrix and profibrotic growth factor genes in chronic isolated mitral regurgitation in the dog. Circulation. 2009;119:2086-2095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Valkova M. Hepatic fibrogenesis. Bratisl Lek Listy. 2002;103:76-85. [PubMed] |
| 20. | Kossakowska AE, Edwards DR, Lee SS, Urbanski LS, Stabbler AL, Zhang CL, Phillips BW, Zhang Y, Urbanski SJ. Altered balance between matrix metalloproteinases and their inhibitors in experimental biliary fibrosis. Am J Pathol. 1998;153:1895-1902. [PubMed] |
| 21. | Benyon RC, Iredale JP, Goddard S, Winwood PJ, Arthur MJ. Expression of tissue inhibitor of metalloproteinases 1 and 2 is increased in fibrotic human liver. Gastroenterology. 1996;110:821-831. [PubMed] |
| 22. | Kanasaki K, Koya D, Sugimoto T, Isono M, Kashiwagi A, Haneda M. N-Acetyl-seryl-aspartyl-lysyl-proline inhibits TGF-beta-mediated plasminogen activator inhibitor-1 expression via inhibition of Smad pathway in human mesangial cells. J Am Soc Nephrol. 2003;14:863-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Choi SS, Diehl AM. Epithelial-to-mesenchymal transitions in the liver. Hepatology. 2009;50:2007-2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 257] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 24. | Choi SS, Omenetti A, Witek RP, Moylan CA, Syn WK, Jung Y, Yang L, Sudan DL, Sicklick JK, Michelotti GA. Hedgehog pathway activation and epithelial-to-mesenchymal transitions during myofibroblastic transformation of rat hepatic cells in culture and cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1093-G1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Zeisberg M, Yang C, Martino M, Duncan MB, Rieder F, Tanjore H, Kalluri R. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem. 2007;282:23337-23347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 587] [Cited by in RCA: 643] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 26. | Zhuo JL, Carretero OA, Peng H, Li XC, Regoli D, Neugebauer W, Rhaleb NE. Characterization and localization of Ac-SDKP receptor binding sites using 125I-labeled Hpp-Aca-SDKP in rat cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2007;292:H984-H993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |