Published online Aug 7, 2012. doi: 10.3748/wjg.v18.i29.3839
Revised: May 22, 2012
Accepted: May 26, 2012
Published online: August 7, 2012
The association between inflammatory bowel disease (IBD) and colorectal cancer (CRC) has been recognised since 1925 and still accounts for 10%-15% of deaths in IBD. IBD-associated CRC (IBD-CRC) affects patients at a younger age than sporadic CRC. The prognosis for sporadic CRC and IBD-CRC is similar, with a 5-year survival of approximately 50%. Identifying at risk patients and implementing appropriate surveillance for these patients is central to managing the CRC risk in IBD. The increased risk of colorectal cancer in association with IBD is thought to be due to genetic and acquired factors. The link between inflammation and cancer is well recognised but the molecular biology, immune pathobiology and genetics of IBD-CRC are areas of much ongoing research. This review examines the literature relating to IBD-CRC, focusing on the incidence of IBD-CRC and examining potential risk factors including age at diagnosis, gender, duration and extent of colitis, severity of inflammation, family history of sporadic CRC and co-existent primary sclerosing cholangitis (PSC). Confirmed risk factors for IBD-CRC are duration, severity and extent of colitis, the presence of co-existent PSC and a family history of CRC. There is insufficient evidence currently to support an increased frequency of surveillance for patients diagnosed with IBD at a younger age. Evidence-based guidelines advise surveillance colonoscopy for patients with colitis 8 to 10 years after diagnosis, with the interval for further surveillance guided by risk factors (extent of disease, family history of CRC, post-inflammatory polyps, concomitant PSC, personal history of colonic dysplasia, colonic strictures). There is a move away from using random colonic biopsies towards targeted biopsies aimed at abnormal areas identified by newer colonoscopic techniques (narrow band imaging, chromoendoscopy, confocal microendoscopy).
- Citation: Dyson JK, Rutter MD. Colorectal cancer in inflammatory bowel disease: What is the real magnitude of the risk? World J Gastroenterol 2012; 18(29): 3839-3848
- URL: https://www.wjgnet.com/1007-9327/full/v18/i29/3839.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i29.3839
Crohn[1] first described colorectal cancer (CRC) in association with inflammatory bowel disease (IBD) in 1925 and colorectal cancer still accounts for 10%-15% of deaths in patients with IBD[2]. The incidence of IBD has two peaks; between 15 and 30 years, and between 50 and 80 years[3]. Due to this younger age peak, the mean age for developing IBD-associated CRC (IBD-CRC) is lower than that seen in sporadic CRC[4]. A meta-analysis of 116 studies found the mean age of IBD-CRC diagnosis to be 43.2 years[5]. Lakatos et al[6] found the average age of IBD-CRC diagnosis to be 10 to 15 years younger than sporadic CRC in Eastern Europe (50.9 years vs 62.2 years). The prognosis for sporadic CRC and IBD-CRC is similar with a 5-year survival of approximately 50%[7]. Identifying at risk patients and implementing appropriate surveillance for these patients is central to managing the CRC risk in IBD.
The increased risk of colorectal cancer in association with IBD is thought to be due to genetic and acquired factors[8]. The link between inflammation and cancer is well recognised but the molecular biology, immune pathobiology and genetics of IBD-CRC are areas of much ongoing research. The role of the immune system for both tumour promotion and prevention is being examined. Studies indicate that immune cells are important in tumour promotion, with leucocyte infiltration and inflammatory mediators found in association with tumours[9,10].
Genome-wide association studies have now identified approximately 99 susceptibility loci/genes relating to IBD. The better understanding of the genetics of IBD gives us more insight into disease pathogenesis. Some of the key themes identified are the role of interleukin (IL)-23/IL-17 signalling in IBD, defective barrier function in ulcerative colitis (UC) and defective processing of intracellular bacteria in Crohn’s disease[11]. Overlap of these loci/genes is seen with many diseases including CRC: E-cadherin (CDH1) has been associated with CRC[12], UC[13], and possibly Crohn’s disease[14].
IBD-CRC and sporadic CRC both have a dysplasia-cancer sequence and require multiple mutations to result in carcinoma[7]. Sporadic CRC usually occurs as the end point of the adenoma-carcinoma sequence. Multiple molecular alterations occur within this sequence: chromosomal instability, microsatellite instability, hypermethylation[15,16]. However, there are often differences in the timing and frequency of these events between sporadic and IBD-CRC. The molecular and genetic alterations occur more rapidly in IBD-CRC and in an unconventional sequence[17]. It is suggested that the inflammation occurring in colitis results in a cascade of abnormal epithelial proliferation in addition to the genetic alterations that occur[18]. In sporadic CRC, loss of the adenomatous polyposis coli gene function occurs early and p53 mutations occur late. The timing of these events is frequently reversed in IBD-CRC. It is also notable that in IBD-CRC, p53 mutations may be found within non-dysplastic mucosa. This is markedly different from sporadic CRC where p53 mutations are seen in morphologically aggressive lesions[19].
The dysplasia-cancer sequence is a useful concept but it is more complex in IBD-CRC. There is not always a clear, stepwise transition from normal, through low and high grade dysplasia (HGD), to cancer[17]. However, low grade dysplasia (LGD) can clearly progress to more advanced lesions and there is varying evidence as to the size of this risk. The 5-year cumulative risk has been shown to be as high as 33%-54% in a number of studies[20-23]. This is in contrast to a 2%-10% risk of CRC over 10 years found by other groups[24,25].
Cancer can occur without preceding detection of dysplasia, and LGD can regress or progress directly to cancer without the intermediate HGD[26]. The high rate of synchronous and metachronous cancers associated with finding high grade dysplasia in biopsies usually leads to proctocolectomy[27]. In a review of 10 prospective trials, 42% of patients undergoing colectomy for HGD had a synchronous CRC[28]. Low grade dysplasia is an independent risk factor for CRC and often found in flat lesions which are difficult to see endoscopically. This is in contrast to sporadic CRC where dysplasia occurs within raised polypoid lesions. The concern is that flat LGD may be accompanied by HGD or cancer elsewhere in the colon. Studies suggest that 16%-27% of patients undergoing colectomy for LGD have synchronous CRC[20,22,28]. Opinions differ as to how LGD should be managed: colectomy versus colonoscopic surveillance. Informed patient choice is important here. If surveillance is pursued colonoscopy should be repeated in 3 to 6 mo. The presence of multifocal LGD is a much stronger argument for colectomy.
It is important to quantify the risk of CRC in association with IBD. The reported risk varies widely between studies. This is partly due to the different methodology used in studies. Data comes from a mixture of tertiary referral centres, district general hospitals and population-based studies. Information from tertiary centres is likely to include patients with severe disease who are at greater risk of IBD-CRC. Early studies included patients who had already been referred with a diagnosis of CRC and those admitted to hospital with IBD, rather than gold-standard population-based studies which have a lower proportion of patients with severe or extensive colitis.
Eaden et al[5] performed a meta-analysis of 116 studies including 54 478 UC patients with 1698 cases of IBD-CRC. The analysis included studies from a wide variety of centres: tertiary referral centres, population-based studies and hospital-based centres, and from different geographical areas. Studies from the United Kingdom and United States found a higher incidence [4 and 5 per 1000 person-years duration (pyd), respectively] than those from Scandinavia (2 per 1000 pyd). They found the overall prevalence of CRC in UC to be 3.7%, increasing to 5.4% in those with pancolitis.
Ekbom et al[29] undertook a population-based cohort study of 3117 patients with UC who were diagnosed between 1922 and 1983. Ninety one patients were found to have IBD-CRC giving a standardised incidence ratio of 5.7 (95% CI: 4.6-7.0) as compared with the expected incidence of CRC in the general population.
Söderlund et al[30] undertook a population-based study of 7607 patients with IBD who were diagnosed between 1954 and 1989 for a total of 198 227 person-years. A total of 196 cases of CRC occurred in 188 patients, giving an overall incidence of 85 (95% CI: 82-109) cases per 100 000 person-years. This corresponds to a standardised incidence ratio of 2.3 (95% CI: 2.0-2.6) as compared with the general population.
Other population-based studies have suggested a much lower risk of IBD-CRC. Palli et al[31] followed 689 patients with UC over 14 years (1978-1992) and found 10 cases of IBD-CRC, equating to an annual crude incidence of 0.13%. A study in Olmsted County followed 378 patients with UC for a total of 5567 person-years (1940-2004) and found 6 cases of IBD-CRC. They calculated the annual crude incidence to be 0.10% and the cumulative risk of CRC at 30 years to be as low as 2%. They found no statistically significant increase in the standardised mortality ratio (SMR) for CRC between IBD and non-IBD populations and concluded that the risk of CRC is only increased in patients with extensive colitis[32]. Bernstein et al[33] retrospectively examined 2672 patients with UC over a total of 19 665 person-years between 1984 and 1997. They found the annual risks to be 0.16% for colon cancer and 0.06% for rectal cancer.
Rutter et al[20] followed 600 patients with extensive UC (as shown by barium enema or colonoscopy) for 5932 person-years as part of a colonoscopic surveillance programme (1970-2001). Data was gathered prospectively. Ninety-one patients (163 episodes) were found to have dysplasia or CRC. They calculated the cumulative probability of IBD-CRC to be 7.6% and a decreasing incidence over the period studied.
A Hungarian population-based study followed 723 UC patients for a total of 8564 person-years (1974-2004) and found 13 cases of IBD-CRC. They calculated the cumulative risks according to disease duration: 0.6% after 10 years, 5.4% after 20 years and 7.5% after 30 years[6]. Hungary has a high rate of sporadic CRC and a low rate of colectomy for non-CRC reasons; factors which might have been expected to result in a higher rate of CRC.
Winther et al[34] followed 1160 patients with UC over 22 290 person-years (1962-1987) and found 13 cases of IBD-CRC, giving an annual crude incidence of 0.06% and cumulative risk of 2.1% at 30 years. They found no statistically significant increase in the SMR for CRC between IBD and non-IBD populations. This study is from Denmark where the colectomy rate is one of the highest in the world: a fact that may affect the results and underestimate the risk of IBD-CRC.
The increased risk of CRC in UC has been long established. More recently, data has shown that Crohn’s colitis carries a similar magnitude of risk for the same disease extent. A Canadian cohort study matched a population-based IBD database to a cancer registry in North America between 1984 and 1997. There were 2857 cases of Crohn’s disease and 2672 of UC. There was an increased incidence of CRC for patients with Crohn’s [risk ratio (RR) 2.64; 95% CI: 1.69-4.12] or UC (RR 2.75; 95% CI: 1.91-3.97) as compared to the general population but no statistically significant difference between the two IBD diagnoses[33]. They found the risk of rectal cancer to be increased in UC (RR 1.90; 95% CI: 1.05-3.43) but not in Crohn’s colitis (RR 1.08; 95% CI: 0.43-2.70). A limitation of this study was the lack of definition of disease site or extent.
Ekbom et al[35] also studied the risk of CRC in Crohn’s disease. In a cohort study of 1655 patients in Sweden, patients with terminal ileal Crohn’s had the same risk of CRC as the general population but those with colonic Crohn’s had a RR of 5.6 (95% CI: 2.1-12.2).
Eaden et al[5] examined how the incidence of IBD-CRC is changing over time by plotting the cancer risk against the mid-point for each study. Although the incidence had increased between 1995 and 2001 the change was not statistically significant (slope 0.003, P = 0.80).
Rutter et al[20] looked at the changing incidence of CRC over time within their 30-year colonoscopic surveillance program. They found a statistically significant decrease in CRC incidence (r = -0.40, P = 0.04), specifically for cancers proximal to the splenic flexure (r = -0.54, P = 0.005) and early cancers since 1975 (Dukes’ A or B; r = -0.4, P = 0.04). However, there was not a statistically significant change in incidence for distal cancers (r = 0.11, P = 0.59) or advanced cancers (Dukes’ C or D; r = -0.05, P = 0.79).
Jess et al[32] examined a population-based cohort from Minnesota diagnosed between 1940 and 2001. They followed 692 patients with IBD for a total of 10 470 person-years. They analysed the incidence of CRC and the year that IBD was diagnosed. All their patients were diagnosed with CRC before 1980. The standardised incidence ratio for CRC diagnosed before 1980 was 1.6 (95% CI: 0.6-3.4) in comparison to 0 for those diagnosed after 1980. However, this difference between calendar periods was not statistically significant. They suggested that use of maintenance therapy and surveillance colonoscopy may be responsible for the absence of CRC seen in this cohort.
Söderlund et al[30] examined the relative risk of CRC across calendar periods in their population-based study of 7607 patients with IBD who were diagnosed between 1954 and 1989. This cohort was followed up until 2004. After adjusting for type and extent of IBD, gender, age, and time since IBD diagnosis, the RRs were 1.7 (95% CI: 0.6-4.4) from 1960 to 1969, 1.3 (95% CI: 0.7-2.6) from 1970 to 1979, 1.2 (95% CI: 0.7-2.2) from 1980 to 1989, 1.1 (95% CI: 0.7-1.8) from 1990 to 1999, and 1.0 from 2000-2004. Although there was a trend towards decreasing incidence of CRC this did not reach statistical significance.
A longer duration of colitis is associated with an increased risk of IBD-CRC; it is relatively rare before 8 years of colitis. However, Lutgens et al[36] conducted a retrospective, nationwide database search to identify patients with IBD-CRC in the Netherlands between 1990 and 2006. For the 149 patients identified, they calculated the time interval between the diagnoses of IBD and cancer. They found that 22%-28% of patients developed cancer before the starting points for surveillance (8-10 years for extensive colitis and 15-20 years for left-sided disease). When the time interval from onset of symptoms to CRC diagnosis was used, 17%-22% would have developed cancer prior to surveillance endoscopy.
Identifying when the risk increases is central to guiding timing of surveillance strategies. Patients may also have colitis for a time before the diagnosis is made. This means they are exposed to cancer risk for a longer period if diagnosis is delayed.
In the meta-analysis by Eaden et al[5], 41 studies reported colitis duration and 19 of these stratified duration into 10 year intervals. The overall incidence of IBD-CRC in any patient with UC was 3 per 1000 pyd. The cumulative incidence of IBD-CRC in patients with UC was 2% at 10 years, 8% at 20 years, and 18% after 30 years of disease. In the 61 studies that reported duration of colitis at the time of IBD-CRC diagnosis the mean was 16.3 years (95% CI: 15.0-17.6).
Lakatos et al[6] retrospectively examined data relating to 723 patients within their 30-year database of IBD patients. They found 13 cases of IBD-CRC in 8564 person-years. They examined the incidence of CRC according to disease duration and found the cumulative risk to be 0.6% at 10 years (95% CI: 0.2-1.0), 5.4% at 20 years (95% CI: 3.7-7.1), and 7.5% at 30 years (95% CI: 4.8-10.2).
The prospective surveillance data from Rutter et al[20] found the median duration of UC at diagnosis of CRC to be 23.5 years (range 11-48 years). The cumulative incidence of CRC was 0% at 10 years, 2.5% at 20 years, 7.6% at 30 years, 10.8% at 40 years, and 13.5% at 45 years (from onset of colitis). This lower incidence of IBD-CRC is particularly interesting given that the data comes from a specialist centre with a low colectomy rate and might have been expected to show a higher incidence. Data being obtained from a surveillance programme may in itself be responsible for the lower incidence of CRC, and regular colonic examination should prompt treatment measures to gain disease control.
There is conflicting evidence as to whether younger age at diagnosis of IBD is an independent risk factor for IBD-CRC. This evidence is not easy to evaluate as children tend to have more extensive and severe colitis than those diagnosed as adults, and younger people have the potential for longer colitis duration, which is itself a risk factor.
An important question is whether patients studied actually develop colitis at a younger age or whether they are diagnosed late after a period of undiagnosed inflammation. Eaden et al[5] included 21 studies in their meta-analysis that examined age at onset of UC (over 20 years old). They excluded studies that reported the age at diagnosis of UC due to potential delay in diagnosis. They found a negative trend between younger age at onset (in adulthood) and increased risk of CRC but this was not statistically significant (z = -1.61, P = 0.11). They also analysed 12 studies looking at the incidence of IBD-CRC in children with UC (average age at onset of UC was 10 years old), only 5 of which commented on the duration of follow up. They found the overall incidence to be 6 per 1000 pyd and the cumulative risk of CRC to be 5.5% at 10 years, 10.8% at 20 years, and 15.7% at 30 years; higher than the corresponding rates for adults. However, these studies did not report incidence according to 10 yearly intervals so they assumed the log incidence rate to be constant. The small number of studies in children necessitates cautious interpretation of results.
Ekbom et al[29] conducted a population-based cohort study of 3117 patients who were diagnosed with UC between 1922 and 1983 in Sweden. CRC was identified in 91 patients. They found age at diagnosis to be an independent risk factor for CRC. After adjusting for the extent of disease, they found the relative risk of CRC decreased by about half (adjusted standardized incidence ratio = 0.51; 95% CI: 0.46-0.56) for each increase in age group at diagnosis (under 15 years, 15-29 years, 30-39 years, 40-49 years, 50-59 years, and over 60 years). For those with extensive disease, after 35 years of disease, the cumulative risk for IBD-CRC was 40% if diagnosed under 15 years and 25% if diagnosed between 15 and 39 years of age.
Other studies have not confirmed this association. Greenstein et al[37] found that the CRC risk was higher in patients diagnosed with IBD above 30-40 years of age compared with those diagnosed below 20 years old. Data from the 30-year study of Rutter et al[20] showed that patients who developed CRC had a higher median age of onset of disease than those not developing cancer. This suggests that early onset is not an independent risk factor for IBD-CRC. Winther et al[34] found the time between onset of colitis and the development of IBD-CRC to be the same in young and old patients.
Karvellas et al[38] undertook a retrospective audit of adult patients with UC who were diagnosed with CRC between 1991 and 2002 in Edmonton, Alberta. They found that patients diagnosed with UC over the age of 40 years developed CRC more quickly than younger patients. The median disease duration at the time of CRC diagnosis was 22 years in patients under 40 years, and 10 years for those over 40 years [odds ratio (OR): 11.5; 95% CI: 2.41-20.16; P = 0.00029].
The greater the disease extent, the greater the risk of CRC. Patients with proctitis and proctosigmoiditis are at the lowest risk, left-sided colitis carries moderate risk and those with pan-colitis are at the highest risk of CRC[39,40].
How we measure the extent of disease is important; macroscopic versus radiological versus histological. Ekbom et al[29] assessed IBD-CRC risk in UC according to extent of disease; proctitis versus left-sided colitis versus pancolitis. The extent was determined from barium enema and colonoscopy reports. The relative risk for CRC was 1.7 for proctitis, 2.8 for left-sided colitis, and 14.8 for pancolitis, as compared with the general population.
Söderlund et al[30] examined the risk of CRC according to extent of colitis in their population-based study. They found the relative risks of CRC to be 2.7 for all patients with UC (95% CI: 2.3-3.2), 5.6 for pancolitis (95% CI: 4.0-4.7), 2.1 for Crohn’s colitis (95% CI: 1.2-3.4) and 1.7 for proctitis (95% CI: 1.2-2.4).
IBD-CRC is thought to occur in the context of inflammation and anti-inflammatory treatments are felt to decrease the risk of CRC. Recent studies have focused on this association.
Rutter et al[20] conducted a case-control study of patients with colorectal neoplasia identified within a surveillance program between 1988 and 2002 (n = 68). They found a significant correlation between both colonoscopic (OR: 2.5; P < 0.001) and histological (OR: 5.1; P < 0.001) inflammation and the risk of neoplasia. When multivariate analysis was performed, histological inflammation remained a significant risk factor (OR: 4.7; P < 0.001). In a follow-up study from this work, Rutter et al[41] found that mucosal healing may decrease the risk of neoplasia; macroscopically normal mucosa appears to return the CRC risk to that of the general population.
Gupta et al[42] retrospectively studied a cohort of 418 patients undergoing colonoscopic surveillance for UC. They found a significant relationship between histological inflammation over time and progression to advanced neoplasia (hazard ratio 3.0; 95% CI: 1.4-6.3) which remained an independent risk factor in multivariate analysis. This is in contrast to other studies showing that those with quiescent disease have a similar risk of developing CRC as those with active disease[6,43].
Post-inflammatory polyps have been associated with an increased risk of CRC. They do not in themselves have malignant potential[44]. It is possible that multiple post-inflammatory polyps increase the miss rate of dysplastic lesions or that their presence is evidence of more severe previous inflammation. A retrospective study using the Mayo Clinic centralised diagnostic index identified 188 patients with UC-associated CRC and matched them to 1528 gender and disease-extent matched controls. The presence of post-inflammatory polyps remained statistically associated with CRC even after adjusting for surveillance and anti-inflammatory treatments[45]. In the follow-up case-control study by Rutter et al[41], cases of CRC were significantly more likely to have post-inflammatory polyps than the controls (OR: 2.14; 95% CI: 1.24-3.70).
In their population-based study, Söderlund et al[30] looked specifically at the gender-related risk of IBD-CRC by identifying those diagnosed with CRC between 1960 and 2004. They compared their results with the general population using standardised incidence ratios and data obtained from national health and census registers. There were 196 cases of IBD-CRC giving an overall incidence of 110 cases per 100 000 person-years. The relative risk in males was 2.6 (95% CI: 2.2-3.1) and in females 1.9 (95% CI: 1.5-2.4) compared to the general population. The cumulative incidence at 40 years after the diagnosis of IBD was 8.3% in males and 3.5% in females.
Ekbom et al[29,35] found a similar relative risk for IBD-CRC in men and women whether they have UC (5.6 in men and 5.9 in women) or Crohn’s disease (2.8 in men and 2.1 in women).
Patients with IBD who have a family history of sporadic CRC are at increased risk. The magnitude of the risk has been found to be a 2-3 fold increase in both case control and population-based studies. Askling et al[46] undertook a population-based cohort study of 19 876 patients with UC or Crohn’s disease born between 1941 and 1995. They found that a family history of CRC was associated with a more than 2-fold risk of IBD-CRC (adjusted RR 2.5; 95% CI: 1.4-4.4) and those with a 1st-degree relative diagnosed with CRC before 50 years of age had a higher risk (RR 9.2; 95% CI: 3.7-23). In a retrospective cohort study, Velayos et al[45] also found family history of CRC to be an important risk factor for IBD-CRC in patients with UC (OR: 3.7; 95% CI: 1.0-13.2)[47].
Smith et al[43] first identified a link between UC and primary sclerosing cholangitis (PSC) in 1965. The association between Crohn’s disease and PSC was identified by Atkinson and Carroll in 1964[48]. It has since been shown that the risk of IBD-CRC is greater in the presence of co-existent PSC. The adjusted relative risk for dysplasia or cancer is reported as 3.15 (95% CI: 1.37-7.27) for patients with PSC and UC as compared to those with UC alone[49]. Kornfeld et al[50] found the cumulative risk was 33% at 20 years and 40% at 30 years from the diagnosis of UC. Causative theories include that a high concentration of bile acids in the lumen of the colon may contribute to the increased risk[51]. Lundqvist et al[52] suggest that patients with PSC often have a longer duration of colitis and this in itself may explain the increased risk of CRC. However, patients with PSC seem to have more quiescent colitis[53]. Sokol et al[53], also found that patients with co-existent IBD and PSC receive more 5-aminosalicylic acid (5-ASA) treatment than patients with IBD alone. Given the importance of the severity of inflammation in the aetiology of IBD-CRC this points to the existence of PSC itself having a carcinogenic effect.
Soetikno et al[54] conducted a meta-analysis of 11 studies to establish whether the risk of CRC is increased in patients with concomitant UC and PSC. They found that patients with UC-PSC are at increased risk of colorectal dysplasia and carcinoma compared with patients with UC alone (OR: 4.79; 95% CI: 3.58-6.41) and the risk is still increased if CRC is considered alone (OR: 4.09; 95% CI: 2.89-5.76). Broomé et al[55] studied 120 patients in Stockholm; 40 with UC-PSC and 80 with UC alone. They found that those with concomitant UC and PSC have a significantly higher risk of developing CRC.
As with all pathology, prevention is better than cure. By focussing on the risk factors predisposing people to IBD-CRC it is hoped we can reduce the incidence of dysplasia and cancer. Any chemoprevention strategy must be acceptable to patients and physicians, in terms of safety, efficacy and cost. Ongoing colonic inflammation has been accepted as one of the causative factors behind IBD-CRC. Studies have focussed on using maintenance anti-inflammatory medications to prevent the development of dysplasia and cancer.
5-ASA compounds are used as maintenance therapy in colitis. In vitro studies found that they inhibit the nuclear factor kappa B pathway which is involved in tumour survival and sustaining chronic inflammation[4,56,57]. Due to the widespread use of 5-ASA compounds for maintenance therapy, prospective randomised trials are lacking with respect to chemoprevention. Velayos et al[58] undertook a meta-analysis of 9 studies which included a total of 1932 patients with UC. They found a protective effect of 5-ASA against colorectal cancer (OR: 0.51; 95% CI: 0.37-0.69) and colorectal cancer/dysplasia as a combined end point (OR: 0.51; 95% CI: 0.38-0.69). They did not find an association between 5-ASA use and a lower risk of dysplasia (OR: 1.18; 95% CI: 0.41-3.43) although they make the point that only 2 of the studies looked at this endpoint.
Terdiman et al[59] have reached a different conclusion with their observational study looking at the association between 5-ASA and IBD-CRC. They found that exposure to 5-ASA compounds during the 12 mo prior to the diagnosis of IBD-CRC was not associated with a decreased cancer risk (OR: 0.97; 95% CI: 0.77-1.23). They also found a non-statistically significant trend between a lower risk of IBD-CRC and an increased number of 5-ASA prescriptions in the previous year. Their conclusion was that 5-ASA compounds to do not have a protective effect against IBD-CRC when assessed over a short period. The important factor here seems to be the time period studied. Any preventative strategy has to be seen as a long term management approach. By using chemoprevention we are trying to prevent the genetic alterations which occur in the context of inflamed mucosa. The relationship between 5-ASA compounds and any protective effect must surely be assessed over a longer time period in order to discount it. The design of this study is too short and the results should be interpreted with caution.
The link between IBD-CRC and co-existent PSC is well established. The presence of additional bile acids in the colonic lumen is felt to contribute to this increased risk. Ursodeoxycholic acid (UDCA) decreases the amount of bile acids and studies have suggested treatment with UDCA may reduce the risk. Tung et al[60] found a strong relationship between UDCA and a decreased risk of colonic dysplasia (OR: 0.18; 95% CI: 0.05-0.61). The relationship remained after adjusting for gender, age at diagnosis of colitis, duration of colitis, duration of PSC, severity of liver disease, and sulfasalazine use. Pardi et al[61] also found a relative risk of 0.26 for colorectal dysplasia or cancer using UDCA when compared to placebo. These studies are in patients with colitis and PSC. The potential for using UDCA for patients with colitis only has not been explored. There are concerns about the side effects of using the medication in patients without PSC.
Given the potential benefit with suppression of inflammation, interest has been shown in other anti-inflammatory treatments. The role of steroids as chemopreventive agents has been explored. Evidence suggests a reduction in CRC risk with systemic and topical steroids[45,62]. However, the significant complications that occur with long-term steroid treatment make this strategy unacceptable. No chemopreventive effect has been shown with azathioprine or 6-mercaptopurine[63].
Other suggested therapies include folic acid and statins. Patients with IBD are at risk of folate deficiency. Studies have shown a protective trend against CRC but the effect is not statistically significant[64,65]. Potack et al[66] suggest that given that folate is safe and inexpensive, supplementation should be considered for risk reduction. A study in Israel suggested statin therapy is associated with a risk reduction in sporadic CRC and a 94% risk reduction in patients with IBD[67]. This potential benefit needs further investigation.
As described above, the quoted risk of developing cancer in colitis varies greatly from study to study. More recent population-based studies suggest a much lower risk than the earlier cohort studies. Methodological differences, the more recent widespread use of anti-inflammatory treatments and the advent of surveillance colonoscopy programmes may account for these differences. There is insufficient evidence to support the discontinuation of surveillance programmes and IBD is still believed to expose patients to an increased risk of CRC.
The aim of any screening or surveillance programme must be to identify early lesions to enable treatment and prevention before the development of invasive cancer. Prophylactic proctocolectomy eliminates the risk of CRC but this strategy is not acceptable to most patients or physicians. A surveillance programme must be acceptable to patients and practically possible to implement. The success of such programmes relies on patients engaging with follow up; they must understand the risks of not being tested and also that no surveillance strategy is without a miss rate.
Equally, physicians must implement the guidelines effectively. Many current guidelines advocate random quadrantic biopsies every 10 cm throughout the entire colon. This approach only visualises less than 1% of colonic mucosa. Rubin et al[68] found the probability of detecting dysplasia was 90% if 33 and 95% if 56 biopsies were taken. Studies have shown that clinicians do not take sufficient biopsies[69,70].
The distinct differences between sporadic CRC and IBD-CRC are important for surveillance strategies. Bowel cancer screening in the general population relies on identification of adenomatous lesions which can be resected before they transform into carcinoma. IBD-CRC poses different challenges: dysplastic lesions do not follow the adenoma-carcinoma sequence, they can be difficult to see (flat lesions), difficult to resect completely, and multifocal. A meta-analysis of 1225 patients with UC found the likelihood of finding concurrent cancer at the time of colectomy for high or low grade dysplasia was 42% and 19%, respectively[28].
Due to the increased risk, patients diagnosed with PSC who are not previously known to have IBD should have a screening colonoscopy. For patients diagnosed with UC already, yearly surveillance colonoscopy should be performed from the point of diagnosis with PSC.
There has been increased focus on targeted biopsies and methods to improve identification of dysplastic lesions. Chromoendoscopy involves spraying dye (indigo carmine or methylene blue) on to the colonic mucosa to enable more detailed examination. Rutter et al[71] compared consecutive, random and indigo carmine targeted biopsies. Chromoendoscopy found 7 dysplastic lesions in 157 targeted biopsies, compared to no dysplasia in 2904 non-targeted biopsies. Hurlstone et al[72] conducted a prospective case-controlled study of 700 patients and also found a higher yield of dysplasia using indigo carmine chromoendoscopy as compared to conventional colonoscopy with random biopsies. They found 69 dysplastic lesions using chromoendoscopy and only 24 lesions with random biopsies (P < 0.001). These results are supported by Kiesslich et al[73], who found a statistically significantly higher rate of neoplasia detection with methylene blue chromoendoscopy.
Narrow band imaging (NBI) is available on most colonoscopes. It uses optical filter technology to improve the visibility of vessels, pit pattern and other soft tissue structures. Dekker et al[74] performed a prospective, randomised trial to compare NBI and conventional colonoscopy. They did not find a statistically significant difference between the two methods; a similar number of dysplastic lesions were identified and missed using both methods.
Confocal laser endomicroscopy visualises the histology of the mucosa in real time. This is useful for characterising lesions rather than finding the lesion in the first place. Kiesslich et al[75] compared confocal chromoscopic endomicroscopy with conventional colonoscopy with random biopsies in a randomised controlled trial. They found the yield of neoplasia was increased 4.75 times using the new approach (P = 0.005) using 50% less biopsies. Hurlstone et al[76] compared confocal chromoscopic endomicroscopy to chromoendoscopy alone in a prospective, randomised controlled trial. They found that endomicroscopy increased the diagnostic yield of neoplasia 2.5 times.
However, it is already well known that there is significant inter-observer variability between even expert gastrointestinal pathologists when interpreting dysplasia. The use of confocal endomicroscopy requires the endoscopist to have the ability to interpret histological findings. For this modality to be widely used endoscopists would require extensive training to enable accuracy.
The best technique for surveillance is evolving. There is a move away from using random colonic biopsies towards targeted biopsies aimed at abnormal areas identified by newer colonoscopic techniques (chromoendoscopy, confocal microendoscopy). There has been concern regarding the specialist training needed for endoscopists to use chromoendoscopy effectively. Nevertheless, the 2010 guidelines from the British Society of Gastroenterology (BSG) recommend the use of chromoendoscopy with targeted biopsies for colitis surveillance[77].
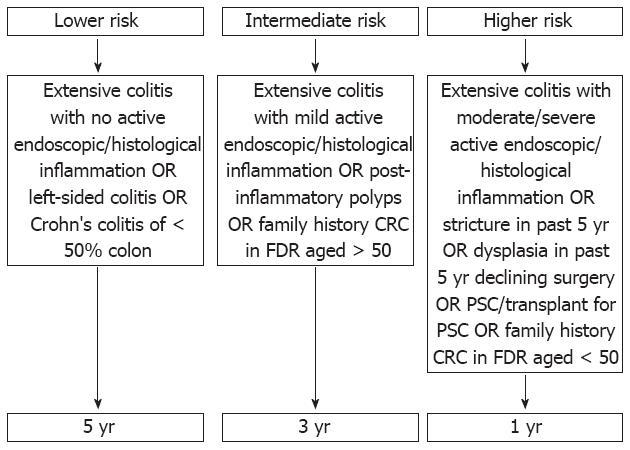
Current guidance from the BSG advises all patients with IBD should have a screening colonoscopy approximately 10 years from symptom onset (ideally when in remission) with pancolonic dye spraying and targeted biopsies of abnormal areas. The risk of IBD-CRC is estimated based on duration and extent of disease, co-existent risk factors (PSC, family history of sporadic CRC), and the endoscopic and histological findings at colonoscopy. The surveillance intervals are based on this assessment of risk (Figure 1)[77].
In conclusion, confirmed risk factors for IBD-CRC are duration, severity and extent of colitis, the presence of co-existent PSC and a family history of CRC. There is insufficient evidence currently to support an increased frequency of surveillance for patients diagnosed with IBD at a younger age. Evidence-based guidelines advise surveillance colonoscopy for patients with colitis 8 to 10 years after diagnosis, with the interval for further surveillance guided by risk factors (extent of disease, family history of CRC, post-inflammatory polyps, concomitant PSC, personal history of colonic dysplasia, colonic strictures)[78-80].
Peer reviewers: Boris Kirshtein, MD, Department of Surgery “A”, Soroka Medical Center, Ben Gurion University of the Negev, PO Box 151, Beer Sheva 84101, Israel; Takayuki Yamamoto, MD, Inflammatory Bowel Disease Center, Yokkaichi Social Insurance Hospital, 10-8 Hazuyamacho, Yokkaichi 510-0016, Japan
S- Editor Lv S L- Editor A E- Editor Li JY
| 1. | Crohn BB. The sigmoidoscopic picture of chronic ulcerative colitis (non-specific). Amer J Med Sci. 1925;170:220-228. [RCA] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 200] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Munkholm P. Review article: the incidence and prevalence of colorectal cancer in inflammatory bowel disease. Aliment Pharmacol Ther. 2003;18 Suppl 2:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 395] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 3. | Calkins BM, Lilienfeld AM, Garland CF, Mendeloff AI. Trends in incidence rates of ulcerative colitis and Crohn's disease. Dig Dis Sci. 1984;29:913-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 152] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Munkholm P, Loftus EV, Reinacher-Schick A, Kornbluth A, Mittmann U, Esendal B. Prevention of colorectal cancer in inflammatory bowel disease: value of screening and 5-aminosalicylates. Digestion. 2006;73:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1985] [Cited by in RCA: 2074] [Article Influence: 86.4] [Reference Citation Analysis (1)] |
| 6. | Lakatos L, Mester G, Erdelyi Z, David G, Pandur T, Balogh M, Fischer S, Vargha P, Lakatos PL. Risk factors for ulcerative colitis-associated colorectal cancer in a Hungarian cohort of patients with ulcerative colitis: results of a population-based study. Inflamm Bowel Dis. 2006;12:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 155] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 7. | Rhodes JM, Campbell BJ. Inflammation and colorectal cancer: IBD-associated and sporadic cancer compared. Trends Mol Med. 2002;8:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 241] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 8. | Askling J, Dickman PW, Karlén P, Broström O, Lapidus A, Löfberg R, Ekbom A. Family history as a risk factor for colorectal cancer in inflammatory bowel disease. Gastroenterology. 2001;120:1356-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 283] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 9. | Danese S, Mantovani A. Inflammatory bowel disease and intestinal cancer: a paradigm of the Yin-Yang interplay between inflammation and cancer. Oncogene. 2010;29:3313-3323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 10. | Mantovani A. Cancer: Inflaming metastasis. Nature. 2009;457:36-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 491] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 11. | Lees CW, Barrett JC, Parkes M, Satsangi J. New IBD genetics: common pathways with other diseases. Gut. 2011;60:1739-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 454] [Article Influence: 32.4] [Reference Citation Analysis (3)] |
| 12. | Houlston RS, Webb E, Broderick P, Pittman AM, Di Bernardo MC, Lubbe S, Chandler I, Vijayakrishnan J, Sullivan K, Penegar S. Meta-analysis of genome-wide association data identifies four new susceptibility loci for colorectal cancer. Nat Genet. 2008;40:1426-1435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 440] [Cited by in RCA: 445] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 13. | Barrett JC, Lee JC, Lees CW, Prescott NJ, Anderson CA, Phillips A, Wesley E, Parnell K, Zhang H, Drummond H. Genome-wide association study of ulcerative colitis identifies three new susceptibility loci, including the HNF4A region. Nat Genet. 2009;41:1330-1334. [PubMed] |
| 14. | Muise AM, Walters TD, Glowacka WK, Griffiths AM, Ngan BY, Lan H, Xu W, Silverberg MS, Rotin D. Polymorphisms in E-cadherin (CDH1) result in a mis-localised cytoplasmic protein that is associated with Crohn's disease. Gut. 2009;58:1121-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 15. | van Dieren JM, Wink JC, Vissers KJ, van Marion R, Hoogmans MM, Dinjens WN, Schouten WR, Tanke HJ, Szuhai K, Kuipers EJ. Chromosomal and microsatellite instability of adenocarcinomas and dysplastic lesions (DALM) in ulcerative colitis. Diagn Mol Pathol. 2006;15:216-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Tahara T, Inoue N, Hisamatsu T, Kashiwagi K, Takaishi H, Kanai T, Watanabe M, Ishii H, Hibi T. Clinical significance of microsatellite instability in the inflamed mucosa for the prediction of colonic neoplasms in patients with ulcerative colitis. J Gastroenterol Hepatol. 2005;20:710-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Zisman TL, Rubin DT. Colorectal cancer and dysplasia in inflammatory bowel disease. World J Gastroenterol. 2008;14:2662-2669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 102] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 18. | Murthy S, Flanigan A, Clearfield H. Colorectal cancer in inflammatory bowel disease: molecular and clinical features. Gastroenterol Clin North Am. 2002;31:551-564, x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Xie J, Itzkowitz SH. Cancer in inflammatory bowel disease. World J Gastroenterol. 2008;14:378-389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 215] [Cited by in RCA: 224] [Article Influence: 13.2] [Reference Citation Analysis (1)] |
| 20. | Rutter MD, Saunders BP, Wilkinson KH, Rumbles S, Schofield G, Kamm MA, Williams CB, Price AB, Talbot IC, Forbes A. Thirty-year analysis of a colonoscopic surveillance program for neoplasia in ulcerative colitis. Gastroenterology. 2006;130:1030-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 450] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 21. | Connell WR, Lennard-Jones JE, Williams CB, Talbot IC, Price AB, Wilkinson KH. Factors affecting the outcome of endoscopic surveillance for cancer in ulcerative colitis. Gastroenterology. 1994;107:934-944. [PubMed] |
| 22. | Ullman T, Croog V, Harpaz N, Sachar D, Itzkowitz S. Progression of flat low-grade dysplasia to advanced neoplasia in patients with ulcerative colitis. Gastroenterology. 2003;125:1311-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 246] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 23. | Ullman TA, Loftus EV, Kakar S, Burgart LJ, Sandborn WJ, Tremaine WJ. The fate of low grade dysplasia in ulcerative colitis. Am J Gastroenterol. 2002;97:922-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 101] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Befrits R, Ljung T, Jaramillo E, Rubio C. Low-grade dysplasia in extensive, long-standing inflammatory bowel disease: a follow-up study. Dis Colon Rectum. 2002;45:615-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 120] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 25. | Lim CH, Dixon MF, Vail A, Forman D, Lynch DA, Axon AT. Ten year follow up of ulcerative colitis patients with and without low grade dysplasia. Gut. 2003;52:1127-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 145] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 26. | Itzkowitz SH. Molecular biology of dysplasia and cancer in inflammatory bowel disease. Gastroenterol Clin North Am. 2006;35:553-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 125] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 27. | Itzkowitz SH, Present DH. Consensus conference: Colorectal cancer screening and surveillance in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:314-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 395] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 28. | Bernstein CN, Shanahan F, Weinstein WM. Are we telling patients the truth about surveillance colonoscopy in ulcerative colitis? Lancet. 1994;343:71-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 362] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 29. | Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1294] [Cited by in RCA: 1198] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 30. | Söderlund S, Brandt L, Lapidus A, Karlén P, Broström O, Löfberg R, Ekbom A, Askling J. Decreasing time-trends of colorectal cancer in a large cohort of patients with inflammatory bowel disease. Gastroenterology. 2009;136:1561-1567; quiz 1818-1819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 173] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 31. | Palli D, Trallori G, Bagnoli S, Saieva C, Tarantino O, Ceroti M, d'Albasio G, Pacini F, Amorosi A, Masala G. Hodgkin's disease risk is increased in patients with ulcerative colitis. Gastroenterology. 2000;119:647-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 94] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Jess T, Loftus EV, Velayos FS, Harmsen WS, Zinsmeister AR, Smyrk TC, Schleck CD, Tremaine WJ, Melton LJ, Munkholm P. Risk of intestinal cancer in inflammatory bowel disease: a population-based study from olmsted county, Minnesota. Gastroenterology. 2006;130:1039-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 321] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 33. | Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91:854-862. [PubMed] |
| 34. | Winther KV, Jess T, Langholz E, Munkholm P, Binder V. Long-term risk of cancer in ulcerative colitis: a population-based cohort study from Copenhagen County. Clin Gastroenterol Hepatol. 2004;2:1088-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 209] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 35. | Ekbom A, Helmick C, Zack M, Adami HO. Increased risk of large-bowel cancer in Crohn's disease with colonic involvement. Lancet. 1990;336:357-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 448] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 36. | Lutgens MW, Vleggaar FP, Schipper ME, Stokkers PC, van der Woude CJ, Hommes DW, de Jong DJ, Dijkstra G, van Bodegraven AA, Oldenburg B. High frequency of early colorectal cancer in inflammatory bowel disease. Gut. 2008;57:1246-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 148] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 37. | Greenstein AJ, Sachar DB, Smith H, Pucillo A, Papatestas AE, Kreel I, Geller SA, Janowitz HD, Aufses AH. Cancer in universal and left-sided ulcerative colitis: factors determining risk. Gastroenterology. 1979;77:290-294. [PubMed] |
| 38. | Karvellas CJ, Fedorak RN, Hanson J, Wong CK. Increased risk of colorectal cancer in ulcerative colitis patients diagnosed after 40 years of age. Can J Gastroenterol. 2007;21:443-446. [PubMed] |
| 39. | Levin B. Inflammatory bowel disease and colon cancer. Cancer. 1992;70:1313-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 40. | Gyde SN, Prior P, Allan RN, Stevens A, Jewell DP, Truelove SC, Lofberg R, Brostrom O, Hellers G. Colorectal cancer in ulcerative colitis: a cohort study of primary referrals from three centres. Gut. 1988;29:206-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 324] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 41. | Rutter MD, Saunders BP, Wilkinson KH, Rumbles S, Schofield G, Kamm MA, Williams CB, Price AB, Talbot IC, Forbes A. Cancer surveillance in longstanding ulcerative colitis: endoscopic appearances help predict cancer risk. Gut. 2004;53:1813-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 298] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 42. | Gupta RB, Harpaz N, Itzkowitz S, Hossain S, Matula S, Kornbluth A, Bodian C, Ullman T. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology. 2007;133:1099-1105; quiz 1340-1341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 617] [Cited by in RCA: 570] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 43. | Smith MP, Loe RH. Sclerosing cholangitis. Review of recent case reports and associated diseases and four new cases. Am J Surg. 1965;110:239-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 44. | Kelly JK, Gabos S. The pathogenesis of inflammatory polyps. Dis Colon Rectum. 1987;30:251-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 45. | Velayos FS, Loftus EV, Jess T, Harmsen WS, Bida J, Zinsmeister AR, Tremaine WJ, Sandborn WJ. Predictive and protective factors associated with colorectal cancer in ulcerative colitis: A case-control study. Gastroenterology. 2006;130:1941-1949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 254] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 46. | Askling J, Dickman PW, Karlén P, Broström O, Lapidus A, Löfberg R, Ekbom A. Colorectal cancer rates among first-degree relatives of patients with inflammatory bowel disease: a population-based cohort study. Lancet. 2001;357:262-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 47. | Loftus EV. Epidemiology and risk factors for colorectal dysplasia and cancer in ulcerative colitis. Gastroenterol Clin North Am. 2006;35:517-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 48. | Atkinson AJ, Carroll WW. Sclerosing cholangitis. Association with regional enteritis. JAMA. 1964;188:183-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 16] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 49. | Shetty K, Rybicki L, Brzezinski A, Carey WD, Lashner BA. The risk for cancer or dysplasia in ulcerative colitis patients with primary sclerosing cholangitis. Am J Gastroenterol. 1999;94:1643-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 167] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 50. | Kornfeld D, Ekbom A, Ihre T. Is there an excess risk for colorectal cancer in patients with ulcerative colitis and concomitant primary sclerosing cholangitis? A population based study. Gut. 1997;41:522-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 160] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 51. | Lakatos PL, Lakatos L. Risk for colorectal cancer in ulcerative colitis: changes, causes and management strategies. World J Gastroenterol. 2008;14:3937-3947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 339] [Cited by in RCA: 321] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 52. | Lundqvist K, Broomé U. Differences in colonic disease activity in patients with ulcerative colitis with and without primary sclerosing cholangitis: a case control study. Dis Colon Rectum. 1997;40:451-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 101] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 53. | Sokol H, Cosnes J, Chazouilleres O, Beaugerie L, Tiret E, Poupon R, Seksik P. Disease activity and cancer risk in inflammatory bowel disease associated with primary sclerosing cholangitis. World J Gastroenterol. 2008;14:3497-3503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 64] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 54. | Soetikno RM, Lin OS, Heidenreich PA, Young HS, Blackstone MO. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: a meta-analysis. Gastrointest Endosc. 2002;56:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 383] [Article Influence: 16.7] [Reference Citation Analysis (1)] |
| 55. | Broomé U, Löfberg R, Veress B, Eriksson LS. Primary sclerosing cholangitis and ulcerative colitis: evidence for increased neoplastic potential. Hepatology. 1995;22:1404-1408. [PubMed] |
| 56. | Bantel H, Berg C, Vieth M, Stolte M, Kruis W, Schulze-Osthoff K. Mesalazine inhibits activation of transcription factor NF-kappaB in inflamed mucosa of patients with ulcerative colitis. Am J Gastroenterol. 2000;95:3452-3457. [PubMed] |
| 57. | Kaiser GC, Yan F, Polk DB. Mesalamine blocks tumor necrosis factor growth inhibition and nuclear factor kappaB activation in mouse colonocytes. Gastroenterology. 1999;116:602-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 174] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 58. | Velayos FS, Terdiman JP, Walsh JM. Effect of 5-aminosalicylate use on colorectal cancer and dysplasia risk: a systematic review and metaanalysis of observational studies. Am J Gastroenterol. 2005;100:1345-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 379] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 59. | Terdiman JP, Steinbuch M, Blumentals WA, Ullman TA, Rubin DT. 5-Aminosalicylic acid therapy and the risk of colorectal cancer among patients with inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:367-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 60. | Tung BY, Emond MJ, Haggitt RC, Bronner MP, Kimmey MB, Kowdley KV, Brentnall TA. Ursodiol use is associated with lower prevalence of colonic neoplasia in patients with ulcerative colitis and primary sclerosing cholangitis. Ann Intern Med. 2001;134:89-95. [PubMed] |
| 61. | Pardi DS, Loftus EV, Kremers WK, Keach J, Lindor KD. Ursodeoxycholic acid as a chemopreventive agent in patients with ulcerative colitis and primary sclerosing cholangitis. Gastroenterology. 2003;124:889-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 355] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 62. | Eaden J, Abrams K, Ekbom A, Jackson E, Mayberry J. Colorectal cancer prevention in ulcerative colitis: a case-control study. Aliment Pharmacol Ther. 2000;14:145-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 378] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 63. | Matula S, Croog V, Itzkowitz S, Harpaz N, Bodian C, Hossain S, Ullman T. Chemoprevention of colorectal neoplasia in ulcerative colitis: the effect of 6-mercaptopurine. Clin Gastroenterol Hepatol. 2005;3:1015-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 64. | Lashner BA, Heidenreich PA, Su GL, Kane SV, Hanauer SB. Effect of folate supplementation on the incidence of dysplasia and cancer in chronic ulcerative colitis. A case-control study. Gastroenterology. 1989;97:255-259. [PubMed] |
| 65. | Lashner BA, Provencher KS, Seidner DL, Knesebeck A, Brzezinski A. The effect of folic acid supplementation on the risk for cancer or dysplasia in ulcerative colitis. Gastroenterology. 1997;112:29-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 179] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 66. | Potack J, Itzkowitz SH. Colorectal cancer in inflammatory bowel disease. Gut Liver. 2008;2:61-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 67. | Poynter JN, Gruber SB, Higgins PD, Almog R, Bonner JD, Rennert HS, Low M, Greenson JK, Rennert G. Statins and the risk of colorectal cancer. N Engl J Med. 2005;352:2184-2192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 595] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 68. | Rubin CE, Haggitt RC, Burmer GC, Brentnall TA, Stevens AC, Levine DS, Dean PJ, Kimmey M, Perera DR, Rabinovitch PS. DNA aneuploidy in colonic biopsies predicts future development of dysplasia in ulcerative colitis. Gastroenterology. 1992;103:1611-1620. [PubMed] |
| 69. | Bernstein CN, Weinstein WM, Levine DS, Shanahan F. Physicians' perceptions of dysplasia and approaches to surveillance colonoscopy in ulcerative colitis. Am J Gastroenterol. 1995;90:2106-2114. [PubMed] |
| 70. | Eaden JA, Ward BA, Mayberry JF. How gastroenterologists screen for colonic cancer in ulcerative colitis: an analysis of performance. Gastrointest Endosc. 2000;51:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 148] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 71. | Rutter MD, Saunders BP, Schofield G, Forbes A, Price AB, Talbot IC. Pancolonic indigo carmine dye spraying for the detection of dysplasia in ulcerative colitis. Gut. 2004;53:256-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 353] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 72. | Hurlstone DP, Sanders DS, Lobo AJ, McAlindon ME, Cross SS. Indigo carmine-assisted high-magnification chromoscopic colonoscopy for the detection and characterisation of intraepithelial neoplasia in ulcerative colitis: a prospective evaluation. Endoscopy. 2005;37:1186-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 185] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 73. | Kiesslich R, Fritsch J, Holtmann M, Koehler HH, Stolte M, Kanzler S, Nafe B, Jung M, Galle PR, Neurath MF. Methylene blue-aided chromoendoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology. 2003;124:880-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 557] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 74. | Dekker E, van den Broek FJ, Reitsma JB, Hardwick JC, Offerhaus GJ, van Deventer SJ, Hommes DW, Fockens P. Narrow-band imaging compared with conventional colonoscopy for the detection of dysplasia in patients with longstanding ulcerative colitis. Endoscopy. 2007;39:216-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 202] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 75. | Kiesslich R, Hoffman A, Neurath MF. Colonoscopy, tumors, and inflammatory bowel disease - new diagnostic methods. Endoscopy. 2006;38:5-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 76. | Hurlstone DP, Kiesslich R, Thomson M, Atkinson R, Cross SS. Confocal chromoscopic endomicroscopy is superior to chromoscopy alone for the detection and characterisation of intraepithelial neoplasia in chronic ulcerative colitis. Gut. 2008;57:196-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 77. | Cairns SR, Scholefield JH, Steele RJ, Dunlop MG, Thomas HJ, Evans GD, Eaden JA, Rutter MD, Atkin WP, Saunders BP. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut. 2010;59:666-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 807] [Article Influence: 53.8] [Reference Citation Analysis (2)] |
| 78. | Centre for Clinical Practice at National Institute for Health and Clinical Excellence. Colonoscopic Surveillance for Prevention of Colorectal Cancer in People with Ulcerative Colitis, Crohn's Disease or Adenomas. London: National Institute for Health and Clinical Excellence 2011; . [PubMed] |
| 79. | Carter MJ, Lobo AJ, Travis SP. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2004;53 Suppl 5:V1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 772] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 80. | Davila RE, Rajan E, Baron TH, Adler DG, Egan JV, Faigel DO, Gan SI, Hirota WK, Leighton JA, Lichtenstein D. ASGE guideline: colorectal cancer screening and surveillance. Gastrointest Endosc. 2006;63:546-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 225] [Article Influence: 11.8] [Reference Citation Analysis (0)] |