Published online Jul 7, 2012. doi: 10.3748/wjg.v18.i25.3296
Revised: November 15, 2011
Accepted: March 19, 2012
Published online: July 7, 2012
AIM: To investigate the expression and significance of caudal-related homeobox transcription factor (Cdx2) in gastric carcinoma (GC) and precancerous lesions.
METHODS: The expression of Cdx2 in GC, precancerous lesions and normal gastric mucosa were detected using immunohistochemical method. Hematoxylin and eosin staining, alcian blue/periodic acid-schiff and high iron diamine/alcian blue staining were used to classify intestinal metaplasia (IM) and GC.
RESULTS: Cdx2 was not detected in normal gastric mucosa. Cdx2 expression was detected in 87.1% (101/116) of IM, 50% (36/72) of dysplasia and 48.2% (41/85) of GC. The Cdx2-expressing cells in IM were more prevalent than in dysplasia and carcinoma (P < 0.05). There was no relationship between Cdx2 expression and the classification of IM or the degree of dysplasia. Expression of Cdx2 was significantly higher in intestinal-type carcinoma than in diffuse and mixed-type carcinoma (P < 0.05). Positive expression of Cdx2 was mainly found in moderately to well differentiated GC. There was a negative association between nuclear Cdx2 expression and lymph node metastasis and tumor, nodes, metastasis stage of GC (P < 0.05). The patients with Cdx2-positive expression showed a higher survival rate than those with Cdx2-negative expression (P = 0.038). Multivariate analysis revealed that the expression of Cdx2 and lymph node metastasis were independent prognostic indicators of GC (P < 0.05).
CONCLUSION: Cdx2 may be closely related to IM and the intestinal-type GC and implicate better biological behavior and outcome. Cdx2 is useful for predicting the prognosis of GC.
- Citation: Qin R, Wang NN, Chu J, Wang X. Expression and significance of homeodomain protein Cdx2 in gastric carcinoma and precancerous lesions. World J Gastroenterol 2012; 18(25): 3296-3302
- URL: https://www.wjgnet.com/1007-9327/full/v18/i25/3296.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i25.3296
Gastric carcinoma (GC) is one of the most common malignant diseases and is the second most common cause of cancer-related death in China and in the world[1]. The pathogenesis of GC is still not very clear. Despite wide acceptance of the gastritis-metaplasia-dysplasia-carcinoma sequence, the precise molecular alterations underlying this progression pathway remain to be delineated[2].
Caudal-related homeobox transcription factor (Cdx2), which is a member of the caudal-related homeobox gene family, plays an important role in mammalian early intestinal development and the maintenance of intestinal epithelia through its regulation of intestine-specific gene transcription[3,4]. Normally, Cdx2 is expressed in small intestinal and colonic epithelia, but not in gastric epithelium[5]. Although Cdx2 expression is restricted to the intestinal epithelium under normal conditions, an ectopic expression has been reported in the gastric mucosa, both in intestine-like metaplasia and in a subset of GCs[6,7]. Recently, Mutoh et al[8] and Almeida et al[4] reported that the ectopic expression of Cdx2 in the gastric mucosa of transgenic mice is related to the transdifferentiation of gastric mucosal glands into intestinal-like mucosa, and claimed that Cdx2 gene causes intestinalization in the gastric mucosa. In humans, Cdx2 has been reported to be associated with intestinal metaplasia (IM) in the stomach[6], in which ectopic expression of Cdx2 is speculated to cause the gastric epithelial cells to transdifferentiate into the intestinal phenotype. Several reports have also suggested a tumor suppressor role for Cdx2 in human colorectal carcinogenesis[9-11], and this might also be true for gastric cancers. But the question as to whether the ectopic expression of Cdx2 has any influence on cancer initiation and/or progression in the stomach remains unanswered.
GC is a markedly heterogeneous disease in histologic feature and biological characters, especially in the advanced stages. The clinical evidence showed that the biological behavior and prognosis could be significantly different among the patients with the same stage, histological type, or differentiation grade. Therefore, searching for the biomarkers to indicate the biological characters, and predicting the outcome of patients with GC, is the major focus of research on GC. A number of biomarkers have been found to be involved in the development and progression of GC. Although expression of Cdx2 has been detected in some GCs, few studies reported the relationship between Cdx2 expression and prognosis of GC[12,13].
To better understand the mechanisms underlying malignant transformation and its relationship with developmental processes, we studied and compared the expression of the intestine-specific homeodomain protein Cdx2 in metaplasia, dysplasia and GCs, and the morphologic appearance. Furthermore, in the present study, we analyzed the association between Cdx2 and Lauren’s classification, lymph node metastasis, invasion depth, distant metastasis, vascular invasion, tumor size, as well as tumor, nodes, metastasis (TNM) stages, to evaluate the clinical significance of this marker in the histological classification and the prognosis assessment of GC.
The present study consisted of 85 cases with surgically resected gastric specimens and 228 cases with endoscopic biopsies were obtained from the Department of Pathology, the First Affiliated Hospital of Anhui Medical University of China from 2000 to 2005, under a protocol approved by the Institutional Review Board. Slides of GC were reviewed to analyze pathologic parameters, including tumor size, histological grading, depth of invasion, and the presence of nodal metastasis. The 85 patients with GCs (aged 20-87 years, mean 61.75 years; 25 females and 60 males) included 20 early cases and 65 advanced cases. Among them, 10 were classified as well-differentiated adenocarcinoma, 34 as moderately differentiated, and 30 as poorly differentiated adenocarcinoma, and 11 as mucinous cell type. Based on Lauren’s classification system, all GCs were categorized into three histological types: intestinal, diffuse, and mixed[14]. Forty-three cases were classified as intestinal, 35 as diffuse and 7 as mixed. TNM staging was assessed according to the system established by the American Joint Committee on Cancer (AJCC, 19 at pTNM stage I and II, and 66 at pTNM stage III and IV). All patients were followed up until January 2010 for a minimum of 5 years. No patient had received chemotherapy or radiation therapy before surgery. In addition, 228 cases of gastric endoscopic biopsies included 10 cases of normal gastric mucosa, 30 cases of chronic superficial gastritis, 116 cases of gastric IM, and 72 cases of gastric dysplasia (39 cases of mild dysplasia, 20 cases of moderate dysplasia and 13 cases of severe dysplasia). The study was approved by the Research Ethics Committee of Anhui Medical University, China. Informed consent was obtained from all patients. All specimens were handled anonymously according to the ethical and legal requirements.
The samples were fixed with 10% neutral-buffered formalin and embedded in paraffin. Paraffin-embedded samples were serially sectioned at 4 μm and mounted on slides. IM was classified into complete type and incomplete type, using Alcian Blue (pH 2.5)/Periodic Acid-Schiff staining and Alcian blue/high-iron diamine (AB/HID) staining (Baso Diagnostics Inc, China). After deparaffinization and rehydration, the sections were incubated with Alcian Blue pH 2.5 for 30 min, followed by 0.5% periodic acid for 10 min and Schiff solution for 10 min. The sections were then counterstained with modified Mayer’s hematoxylin for 5 min, dehydrated with graded ethanols and mounted with coverslip. In complete IM, only goblet cells were stained blue, while in incomplete IM, both the goblet cells and the inter-mediate columnar cells appeared blue.
After routine deparaffinization and rehydration of the slides, antigen retrieval was done by incubation in modified citrate buffer at 121 °C for 20 min. The sections were treated with 0.03% hydrogen peroxide for 5min to block the endogenous peroxidase activity, followed by incubation with anti-Cdx2 monoclonal antibody (1:100, Biogenex, San Ramon, CA, United States) at 4 °C overnight. After washing with 1 × phosphate buffered saline (0.01mol/L, pH 7.4), the sections were subsequently incubated with the biotinylated secondary antibody (Dako, Denmark). The sections were then stained using the UltraSensitive™ Immunohistochemical Staining Kit (Maixin Bio, Fuzhou, China) according to the manufacturer’s instructions. After development with 0.05% 3,3’-diaminobenzidine, the sections were counter-stained with hematoxylin, dehydrated with graded ethanols and xylene and then mounted with coverslip. In negative control samples, the primary antibody was omitted and 5% goat serum was used as the primary antibody. Known positive tissue sections served as positive control samples.
Double staining of immunohistochemistry and AB/HID staining were applied to detect the Cdx2 expression in different subtypes of IM. Compared to either IHC or AB/HID, combination of both staining was able to detect the protein level and subtypes of IM at the same time.
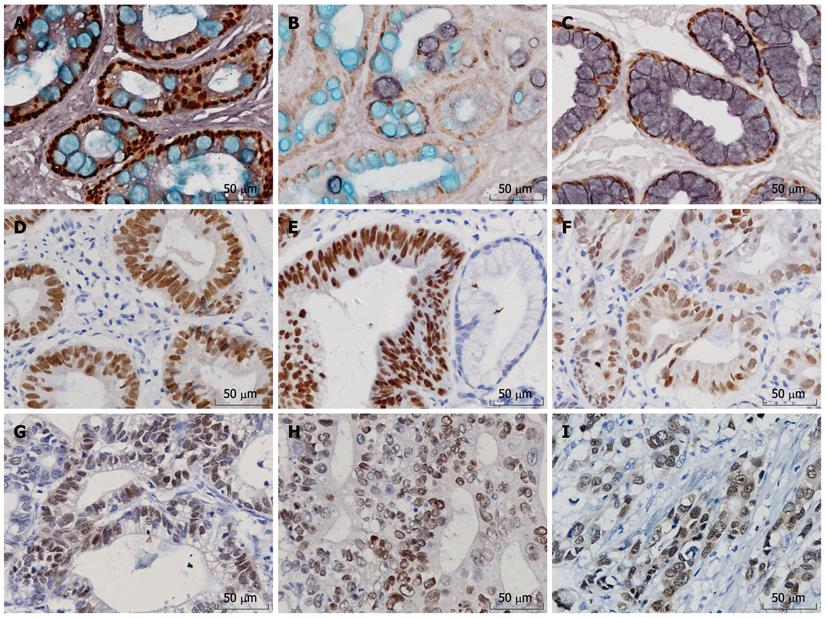
Semiquantitative scores were given as the score of the percentage of positive cells plus the score of the staining intensity. The scoring criteria of the percentage of positive nuclei (Figure 1A-C) were: score 0: 0%-5% positive cancer cells; score 1: 6%-25% positive cancer cells; score 2: 26%-50% positive cancer cells; score 3: 51%-75% positive cancer cells; and score 4: 76%-100% positive cancer cells. The intensity was scored as follows: score 0, no staining; score 1, mild staining; score 2, moderate staining; and score 3, strong staining. The final scores were from 0 to 7. Specimens with a Cdx2 score of 0-2 were considered negative, whereas specimens with a score of 3-7 were considered positive for Cdx2 expression.
Two experienced investigators independently examined the staining who blinded to the clinicopathological data. Different scores between the two investigators were observed in 15% of the cases, and a consensus was achieved in all the cases after discussion.
All data were analyzed using SPSS 13.0 software. Fisher’s exact test or χ2 test was used to calculate the association of Cdx2 expression with various clinicopathological features. Cumulative survival was estimated by the Kaplan-Meier method and differences between survival curves were analyzed by the log-rank test. The influence of each variable on survival was analyzed by the multivariate Logistic regression analysis. Differences were assumed to be statistically significant when P < 0.05.
Cdx2 protein expressed in the nuclei of goblet cells of intestinal mucosa, some columnar epithelial cells and some gastric cancer cells. Representative pictures of immunohistochemical staining of Cdx2 are shown in Figure 1.
Gastric IM has been classified as complete (small intestine) or incomplete (colonic) using histochemical staining techniques and into three types based on the staining pattern of its mucins: I (complete), and II and III (incomplete). Type I is the most common type: paneth cells are present and goblet cells secrete sialomucins. Types II and III are characterized by the presence of columnar cells and goblet cells secreting sialomucins and/or sulfomucins; the columnar cells secrete sialomucins in type II and sulfomucins in type III. Cdx2 protein can not be detected in normal gastric mucosa and chronic superficial gastritis. The expression rates of Cdx2 were 87.1% in IM (101/116, Figure 1A-C), 50% in dysplasia tissues (36/72, Figure 1D-F) and 48.2% (41/85, Figure 1G-I) in gastric cancer, respectively. The positive rates of IM, dysplasia and GC were higher than that of normal mucosa (P < 0.05). The expression of Cdx2 in IM was much higher than that in dysplasia and carcinoma (P < 0.05). There was no significant difference between dysplasia and carcinoma (P > 0.05). The expression rates of Cdx2 were 90%, 85% and 75%, respectively in type I, II and III IM, but the difference was not statistically significant (P > 0.05). Cases of gastric epithelial dysplasia were graded as mild, moderate and severe dysplasia according to the previously established criteria that included the degree of architectural complexity and cytological atypia[15]. The positive rates of Cdx2 were 43.5%, 55% and 61.5% in mild, moderate and severe dysplasia, showing an increasing trend. But there was no statistically significant correlation between Cdx2 expression and grade of dysplasia (P > 0.05, Table 1).
| Type | Cases | Cdx2 positive cases | % | χ2 | P value |
| Different subtypes of intestinal metaplasia | 116 | 101 | 87.1 | 33.826 | 0.000 |
| IM (type I) | 80 | 72 | 90 | 31.400 | 0.000 |
| IM (type II) | 20 | 17 | 85 | 7.426 | 0.003 |
| IM (type III) | 16 | 12 | 75 | 5.365 | 0.012 |
| Dysplasia | 72 | 36 | 50 | 0.004 | 0.873 |
| Mild | 39 | 17 | 43.5 | 0.083 | 0.700 |
| Moderate | 20 | 11 | 55 | 0.088 | 0.637 |
| Severe | 13 | 8 | 61.5 | 0.355 | 0.553 |
| Gastric carcinoma | 85 | 41 | 48.2 |
The correlation of Cdx2 expression with clinicopathological parameters in gastric cancer patients is depicted in Table 2. Cdx2 expression was associated with direct Lauren classification, differentiation, lymph node metastasis and clinical stage. The positive rate of Cdx2 in intestinal gastric cancer (67.4%) was significantly higher than that in diffuse and mixed-type gastric cancer (25.7%, 42.9%, P = 0.001). The positive rate of Cdx2 in well and moderately differentiated adenocarcinoma (65.9%) was significantly higher than that in the poorly differentiated and mucinous adenocarcinoma (29.3%, P = 0.001). In addition, the positive rate of Cdx2 in the group without lymph node metastasis (63.9%) was significantly higher than that with lymph node metastasis (36.7%, P = 0.017). The positive rate of Cdx2 of pTNM stage III and IV (40.9%) was also reduced compared with that of pTNM stage I and II (73.7%, P = 0.018). Cdx2 expression was not associated with the remaining clinicopathological parameters evaluated, including depth of invasion, sex, and age of patients.
| Pathological parameters | n | Cdx2positive cases | Positiverate (%) | χ2 | P value |
| Gender | 0.071 | 0.642 | |||
| Male | 60 | 30 | 50 | ||
| Female | 25 | 11 | 44 | ||
| Age (yr) | 0.000 | 1.000 | |||
| ≥ 55 | 57 | 28 | 49.1 | ||
| < 55 | 28 | 13 | 46.4 | ||
| Lauren classification | 13.544 | 0.001 | |||
| Intestinal | 43 | 29 | 67.4 | ||
| Diffuse | 35 | 9 | 25.7 | ||
| Mixed type | 7 | 3 | 42.9 | ||
| Differentiation | 9.991 | 0.001 | |||
| Well and moderately differentiated | 44 | 29 | 65.9 | ||
| Poorly differentiated and mucinous adenocarcinoma | 41 | 12 | 29.3 | ||
| Depth of invasion | 5.630 | 0.131 | |||
| T1 | 9 | 7 | 77.8 | ||
| T2 | 11 | 5 | 45.5 | ||
| T3 | 25 | 14 | 56 | ||
| T4 | 40 | 15 | 37.5 | ||
| Lymph node metastasis | 5.089 | 0.017 | |||
| No | 36 | 23 | 63.9 | ||
| Yes | 49 | 18 | 36.7 | ||
| Clinical stages | 5.102 | 0.018 | |||
| I + II | 19 | 14 | 73.7 | ||
| III + IV | 66 | 27 | 40.9 |
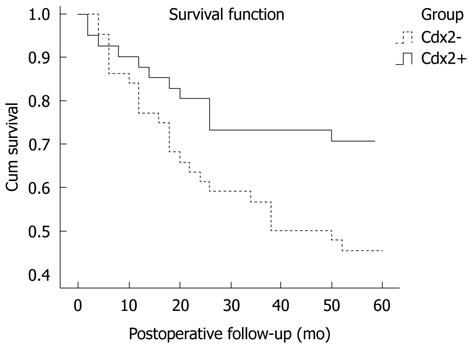
The association between the 5-year survival rate and the expression of Cdx2 was analyzed using the Kaplane Meier method. The results are shown in Figure 2. The patients with nuclear Cdx2 expression showed a significantly higher 5-year survival rate than patients without Cdx2 expression (P = 0.038). Based on the Cox regression analysis in the 85 patients, Cdx2 expression and lymph node metastasis seemed to be independent prognostic indicators (P = 0.001, P = 0.019, respectively, Table 3).
| Variables | B | SE | Walt | Exp (B) | P value |
| Cdx2 | -1.290 | 0.397 | 10.586 | 0.275 | 0.001 |
| Lymph node metastasis | 0.808 | 0.344 | 5.501 | 2.243 | 0.019 |
It was proposed by Correa[16] that human gastric carcinogenesis is a multistep process that progresses from chronic gastritis, atrophy, IM, dysplasia and finally leads to gastric cancer. This model, although challenged by a few, is widely accepted, especially for the intestinal type of gastric cancer. IM is most frequently recognized as a pathological condition in the human stomach, where it often develops from progression of chronic gastritis, and has been extensively studied as a putative preneoplastic lesion of intestinal-type GC. Cdx2 was found to be intensively involved in intestinal metaplastic differentiation[17]. During mouse small intestinal development, Cdx2 is expressed much earlier than Cdx1 in the hindgut[18], and in human IM, expression of Cdx2 precedes that of Cdx1 during the progression of IM, implying that the expression of Cdx2 may trigger the initiation and development of IM[19,20]. Cdx2 may stimulate intestinal proliferation and differentiation by transcriptional activation of intestine-specific proteins (MUC2, sucrase-isomaltase, carbonic anhydrase I). Aberrant expression of Cdx2 is prominent in intestinal-type gastric adenocarcinoma and Cdx-2 may therefore play an important role in gastric carcinogenesis, especially in the intestinal type[12]. In this study, we found that Cdx2 was not expressed in normal gastric mucosa and superficial gastritis, but was expressed in 87.1% of IM. The expression of Cdx2 was also higher in intestinal-type than in diffuse-type GC. Cdx2 has been shown to be a key molecule associated with IM and intestinal or differentiated type GC.
IM can be classified into different subtypes by several classification systems. The most widely accepted one is to classify IM into complete type and incomplete type, with the latter carrying a higher risk of gastric cancer. The complete type is characterized by the presence of absorptive cells, paneth cells and goblet cells secreting sialomucins, similar to the small intestinal phenotype. The incomplete type is characterized by the presence of columnar and goblet cells secreting sialomucins and/or sulphomucins, similar to the colonic phenotype. Using alcian-blue/periodic acid Schiff and high-iron diamine-alcian blue technique, Jass et al[21] classified IM into three subtypes. Type I corresponded to the complete type, while type II and type III were classified as the incomplete type according to the mucins secreted by the columnar cells: sialomucins in type II and sulphomucins in type III. Several studies claimed that only type III IM is associated with an increased risk of gastric cancer, but other reports cast doubt on it. In this study, staining of both immunohistochemistry and AB/HID was applied to detect the Cdx2 protein level and subtypes of IM at the same time. The expression rates of Cdx2 were 90%, 85% and 75% respectively in type I, II and III IM. There was no difference of Cdx2 expression among type I, II and III IM. But Liu et al[22] demonstrated that the expression of Cdx2 was significantly decreased in incomplete IM compared with complete IM. This may be explained by the different antibodies and different staining protocols.
So far, there have been a few reports about Cdx2 expression in gastric epithelial dysplasia. Similar to Woodland’s finding[23], we found no relationship between the grade of gastric epithelial dysplasia and Cdx2 expression. But Kim et al[24] demonstrated a positive correlation between Cdx2 expression and the increasing grade of dysplasia.
Our data showed that Cdx2 expression was decreased in gastric cancers, when compared with IM and dysplasia. This collective experience may suggest a potential tumor suppressor role for Cdx2, in view of its sequential decrease in expression along with the stepwise gastric carcinogenesis (IM, epithelial dysplasia and gastric cancer). This opinion is shared by Liu et al[22] and Xin et al[25], who showed that Cdx2 expression is progressively decreased in gastric IM, dysplasia, and cancer. In our study, Cdx2-positive expressing tumors had tendencies towards less invasiveness and fewer lymph node metastases. The Cdx2-positive patients were found to have a better outcome than Cdx2-negative patients. Multivariate analysis revealed that Cdx2 represents an independent prognostic indicator. The consequences are consistent with the studies by Seno et al[12] and Mizoshita et al[13]. We can speculate that, while ectopically expressed in gastric cancers, the Cdx2 gene may play a tumor suppressor role in slowing down cancer progression in the stomach, similar to what happens in the colon. Previous studies suggest that Cdx2 is a tumor-suppressor gene with regard to colorectal carcinogenesis. Several reports[26,27] have revealed that Cdx2 could promote differentiation, inhibit proliferation, and increase sensitivity to apoptosis of intestinal epithelial cells, and colon cancer derived cells. Moreover, Cdx2 was documented to up-regulate transcription of p21/WAF1/CIP1[28,29] and PTEN[30], which plays a critical role in differentiation and tumor suppression, and promotes intestinal differentiation as a cyclin-dependent kinase inhibitor, leading to cell-cycle arrest. The role of Cdx2 in gastric cancer still remains unclear and Cdx2 may have different roles depending on cancer type.
In conclusion, our findings indicated that Cdx2 is a special and sensitive marker for IM. We revealed that Cdx2 might be closely related to the intestinal-type GC and implicate better biological behavior and outcome. Cdx2 might be useful in predicting the prognosis of GC. Further studies are necessary to confirm our findings.
Intestinal metaplasia (IM) of the stomach is a preneoplastic lesion that confers an increased risk for the development of gastric carcinoma (GC), which remains the second leading cause of cancer death worldwide. Caudal-related homeobox transcription factor (Cdx2) is an intestinal transcription factor responsible for regulating the proliferation and differentiation of intestinal epithelial cells. The role of Cdx-2 in GCs is not clearly understood.
Human Cdx2 is known as a caudal-related homeodomain transcription factor that is expressed in the intestinal epithelium and is important in differentiation and maintenance of the intestinal epithelial cells. Although Cdx2 expression is restricted to the intestinal epithelium under normal conditions, an ectopic expression has been reported in the gastric mucosa, both in intestine-like metaplasia and in a subset of GCs. Although expression of Cdx2 has been detected in some GCs, few studies reported the relationship between Cdx2 expression and prognosis of GC.
This paper evaluated the immunohistochemical expression of the intestine-specific transcription factor Cdx2 in a large number of GC cases, compared with that in gastric IM, gastric dysplasia, superficial gastritis and normal gastric mucosa. The Cdx2-expressing cells in IM were more prevalent than in dysplasia and carcinoma. In GC, expression of Cdx2 was significantly higher in intestinal-type carcinoma than in diffuse and mixed-type carcinoma. Cdx2 might be closely related to IM and the intestinal-type GC and implicate better biological behavior and outcome. Cdx2 might be useful in predicting the prognosis for GC.
Cdx2 expression in GC may serve as a useful marker for diagnosis, to judge the degree of malignancy and to predict the prognosis of the patients.
The Cdx2, located on chromosome 13 in humans and chromosome 5 in mice, is a member of the ParaHox cluster. Cdx2 is from a class of genes encoding transcription factors that constitute mammalian homologues of the Drosophila gene Caudal. Caudal homologues have been identified in a number of organisms including humans and mice and play essential roles in intestinal development and maintenance of the epithelia. In mice and humans, there are 3 caudal homologues: Cdx1, Cdx2 and Cdx4, of which only Cdx2 plays a role in development of the gastrointestinal tract.
This is an interesting, well written paper evaluating the immunohistochemical expression of the intestine-specific transcription factor Cdx2 in a large number of GC cases, compared with that in gastric IM, gastric dysplasia, superficial gastritis and normal gastric mucosa. It is the first study indicating a prognostic role of Cdx2 expression in GC.
Peer reviewer: Guida Portela-Gomes, Professor, University of Lisbon, Rua Domingos Sequeira-128, Estoril 2765-525, Portugal
S- Editor Gou SX L- Editor Ma JY E- Editor Zheng XM
| 1. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 2. | Yuasa Y. Control of gut differentiation and intestinal-type gastric carcinogenesis. Nat Rev Cancer. 2003;3:592-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 249] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 3. | Silberg DG, Swain GP, Suh ER, Traber PG. Cdx1 and cdx2 expression during intestinal development. Gastroenterology. 2000;119:961-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 464] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 4. | Almeida R, Silva E, Santos-Silva F, Silberg DG, Wang J, De Bolós C, David L. Expression of intestine-specific transcription factors, CDX1 and CDX2, in intestinal metaplasia and gastric carcinomas. J Pathol. 2003;199:36-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 210] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 5. | Freund JN, Domon-Dell C, Kedinger M, Duluc I. The Cdx-1 and Cdx-2 homeobox genes in the intestine. Biochem Cell Biol. 1998;76:957-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 66] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Satoh K, Mutoh H, Eda A, Yanaka I, Osawa H, Honda S, Kawata H, Kihira K, Sugano K. Aberrant expression of CDX2 in the gastric mucosa with and without intestinal metaplasia: effect of eradication of Helicobacter pylori. Helicobacter. 2002;7:192-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Park do Y, Srivastava A, Kim GH, Mino-Kenudson M, Deshpande V, Zukerberg LR, Song GA, Lauwers GY. CDX2 expression in the intestinal-type gastric epithelial neoplasia: frequency and significance. Mod Pathol. 2010;23:54-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Mutoh H, Sakurai S, Satoh K, Tamada K, Kita H, Osawa H, Tomiyama T, Sato Y, Yamamoto H, Isoda N. Development of gastric carcinoma from intestinal metaplasia in Cdx2-transgenic mice. Cancer Res. 2004;64:7740-7747. [PubMed] |
| 9. | Baba Y, Nosho K, Shima K, Freed E, Irahara N, Philips J, Meyerhardt JA, Hornick JL, Shivdasani RA, Fuchs CS. Relationship of CDX2 loss with molecular features and prognosis in colorectal cancer. Clin Cancer Res. 2009;15:4665-4673. [PubMed] |
| 10. | Mallo GV, Rechreche H, Frigerio JM, Rocha D, Zweibaum A, Lacasa M, Jordan BR, Dusetti NJ, Dagorn JC, Iovanna JL. Molecular cloning, sequencing and expression of the mRNA encoding human Cdx1 and Cdx2 homeobox. Down-regulation of Cdx1 and Cdx2 mRNA expression during colorectal carcinogenesis. Int J Cancer. 1997;74:35-44. [PubMed] |
| 11. | Vider BZ, Zimber A, Hirsch D, Estlein D, Chastre E, Prevot S, Gespach C, Yaniv A, Gazit A. Human colorectal carcinogenesis is associated with deregulation of homeobox gene expression. Biochem Biophys Res Commun. 1997;232:742-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Seno H, Oshima M, Taniguchi MA, Usami K, Ishikawa TO, Chiba T, Taketo MM. CDX2 expression in the stomach with intestinal metaplasia and intestinal-type cancer: Prognostic implications. Int J Oncol. 2002;21:769-774. [PubMed] |
| 13. | Mizoshita T, Tsukamoto T, Nakanishi H, Inada K, Ogasawara N, Joh T, Itoh M, Yamamura Y, Tatematsu M. Expression of Cdx2 and the phenotype of advanced gastric cancers: relationship with prognosis. J Cancer Res Clin Oncol. 2003;129:727-734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 105] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [PubMed] |
| 15. | Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, Dixon MF, Fenoglio-Preiser CM, Fléjou JF, Geboes K. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1463] [Cited by in RCA: 1548] [Article Influence: 61.9] [Reference Citation Analysis (0)] |
| 16. | Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735-6740. [PubMed] |
| 17. | Mizoshita T, Inada K, Tsukamoto T, Kodera Y, Yamamura Y, Hirai T, Kato T, Joh T, Itoh M, Tatematsu M. Expression of Cdx1 and Cdx2 mRNAs and relevance of this expression to differentiation in human gastrointestinal mucosa--with special emphasis on participation in intestinal metaplasia of the human stomach. Gastric Cancer. 2001;4:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Eda A, Osawa H, Yanaka I, Satoh K, Mutoh H, Kihira K, Sugano K. Expression of homeobox gene CDX2 precedes that of CDX1 during the progression of intestinal metaplasia. J Gastroenterol. 2002;37:94-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 126] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Silberg DG, Sullivan J, Kang E, Swain GP, Moffett J, Sund NJ, Sackett SD, Kaestner KH. Cdx2 ectopic expression induces gastric intestinal metaplasia in transgenic mice. Gastroenterology. 2002;122:689-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 354] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 20. | Kang JM, Lee BH, Kim N, Lee HS, Lee HE, Park JH, Kim JS, Jung HC, Song IS. CDX1 and CDX2 expression in intestinal metaplasia, dysplasia and gastric cancer. J Korean Med Sci. 2011;26:647-653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Jass JR, Filipe MI. The mucin profiles of normal gastric mucosa, intestinal metaplasia and its variants and gastric carcinoma. Histochem J. 1981;13:931-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 124] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Liu Q, Teh M, Ito K, Shah N, Ito Y, Yeoh KG. CDX2 expression is progressively decreased in human gastric intestinal metaplasia, dysplasia and cancer. Mod Pathol. 2007;20:1286-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | Woodland JG. CDX-2 and MIB-1 expression in the colorectum: correlation with morphological features of adenomatous lesions. Br J Biomed Sci. 2006;63:68-73. [PubMed] |
| 24. | Kim HS, Lee JS, Freund JN, Min KW, Lee JS, Kim W, Juhng SW, Park CS. CDX-2 homeobox gene expression in human gastric carcinoma and precursor lesions. J Gastroenterol Hepatol. 2006;21:438-442. [PubMed] [DOI] [Full Text] |
| 25. | Xin S, Huixin C, Benchang S, Aiping B, Jinhui W, Xiaoyan L, Yu WB, Minhu C. Expression of Cdx2 and claudin-2 in the multistage tissue of gastric carcinogenesis. Oncology. 2007;73:357-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Mallo GV, Soubeyran P, Lissitzky JC, André F, Farnarier C, Marvaldi J, Dagorn JC, Iovanna JL. Expression of the Cdx1 and Cdx2 homeotic genes leads to reduced malignancy in colon cancer-derived cells. J Biol Chem. 1998;273:14030-14036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 131] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Gross I, Duluc I, Benameur T, Calon A, Martin E, Brabletz T, Kedinger M, Domon-Dell C, Freund JN. The intestine-specific homeobox gene Cdx2 decreases mobility and antagonizes dissemination of colon cancer cells. Oncogene. 2008;27:107-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Bai YQ, Miyake S, Iwai T, Yuasa Y. CDX2, a homeobox transcription factor, upregulates transcription of the p21/WAF1/CIP1 gene. Oncogene. 2003;22:7942-7949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Saegusa M, Hashimura M, Kuwata T, Hamano M, Wani Y, Okayasu I. A functional role of Cdx2 in beta-catenin signaling during transdifferentiation in endometrial carcinomas. Carcinogenesis. 2007;28:1885-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Semba S, Satake S, Matsushita M, Yokozaki H. Phosphatase activity of nuclear PTEN is required for CDX2-mediated intestinal differentiation of gastric carcinoma. Cancer Lett. 2009;274:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |