Published online Jun 7, 2012. doi: 10.3748/wjg.v18.i21.2727
Revised: August 13, 2011
Accepted: March 9, 2012
Published online: June 7, 2012
Pediatric Menetrier’s disease (MD) is an uncommon, acute, self-limited hypertrophic gastropathy characterized by enlarged gastric folds associated with epithelial hyperplasia and usually accompanied by protein losing gastropathy. Gastric cytomegalovirus infection is found in one third of MD children and its treatment is often associated with remission. Diagnosis often requires full-thickness biopsy due to inability to detect typical histological findings with conventional endoscopic biopsy. We report an uncommon case of non self-limited pediatric MD needing endoscopic mucosal resection for diagnosis which was then successfully treated with octreotide long-acting release (LAR). To the best of our knowledge, this is the first pediatric MD case successfully treated with octreotide LAR. Our experience suggests octreotide LAR as treatment for refractory MD before gastrectomy.
- Citation: Nardo GD, Oliva S, Aloi M, Ferrari F, Frediani S, Marcheggiano A, Cucchiara S. A pediatric non-protein losing Menetrier's disease successfully treated with octreotide long acting release. World J Gastroenterol 2012; 18(21): 2727-2729
- URL: https://www.wjgnet.com/1007-9327/full/v18/i21/2727.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i21.2727
Menetrier’s disease (MD) is a rare hypertrophic gastropathy characterized by enlarged gastric rugal folds and epithelial hyperplasia, accompanied by protein losing gastropathy[1]. Primarily seen in adults, MD can have a long clinical course and is associated with considerable morbidity and even mortality, which are related to surgical resection and potential risk for malignant transformation[2-4]. Pediatric MD often presents as oedema and hypoalbuminemia due to protein loss through the abnormal gastric mucosa and usually has a benign self-limited course with symptoms resolving within 5 wk[4,5]. Diagnosis often requires full-thickness biopsy in conjunction with endoscopic view of enlarged gastric folds[6] while histology includes foveolar hyperplasia with cystic dilation of pits, accompanied by glandular atrophy of the gastric body[1,2]. Gastric cytomegalovirus (CMV) infection is found in one third of MD children and its treatment is often associated with remission[7,8]. We describe the first case of non self-limited and non protein losing MD, diagnosed with endoscopic mucosal resection (EMR) and successfully treated with octreotide long-acting release (LAR).
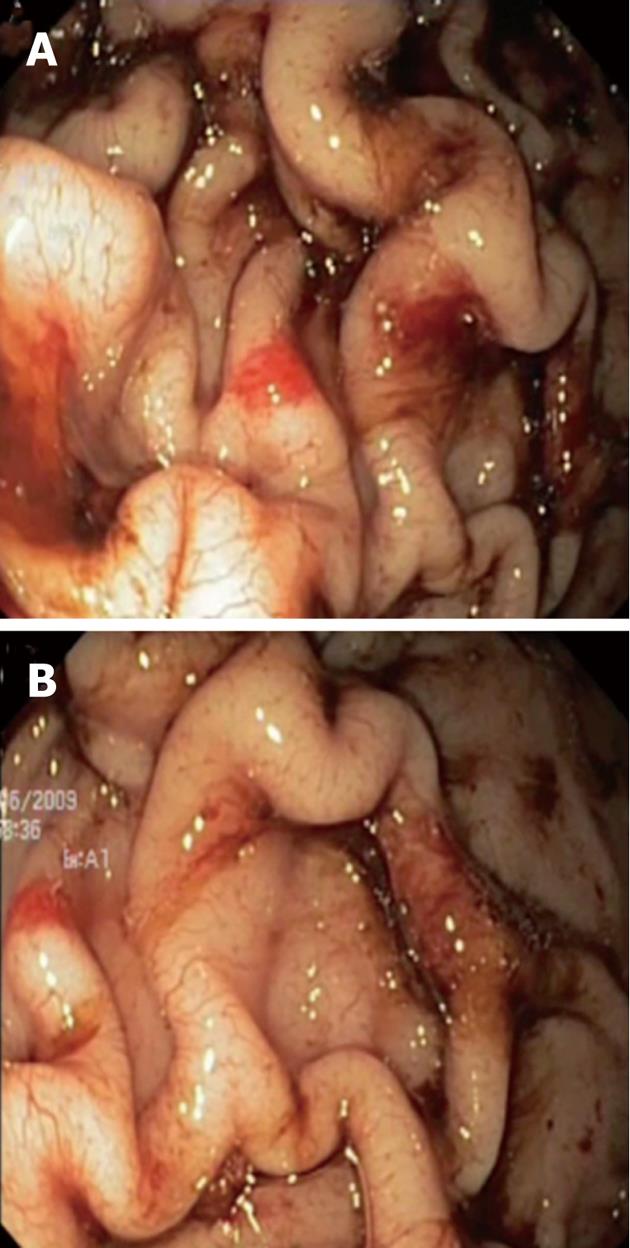
A 4-year-old boy was referred to our Unit for severe iron deficiency anemia (haemoglobin level: 4.7 g/dL and iron studies consistent with iron-deficiency anemia) already treated with blood transfusion in a territorial hospital. The child had a normal physical development with regular growth curve. Liver and spleen diseases were excluded on a clinical and biochemical basis. A grandfather underwent total gastrectomy due to MD. At upper gastrointestinal endoscopy there were marked thickened gastric folds with overlying erosions and exudate involving the corpus and fundus, whereas the antrum and pylorus were normal (Figure 1). Mucosal biopsies showed mild foveolar hyperplasia, dilation of some glands and were negative for Helicobacter pylori and CMV. The latter was also negative in the gastric juice, serum and urine. There was no clinical and laboratory evidence of protein-losing enteropathy and serum gastrin level was normal.
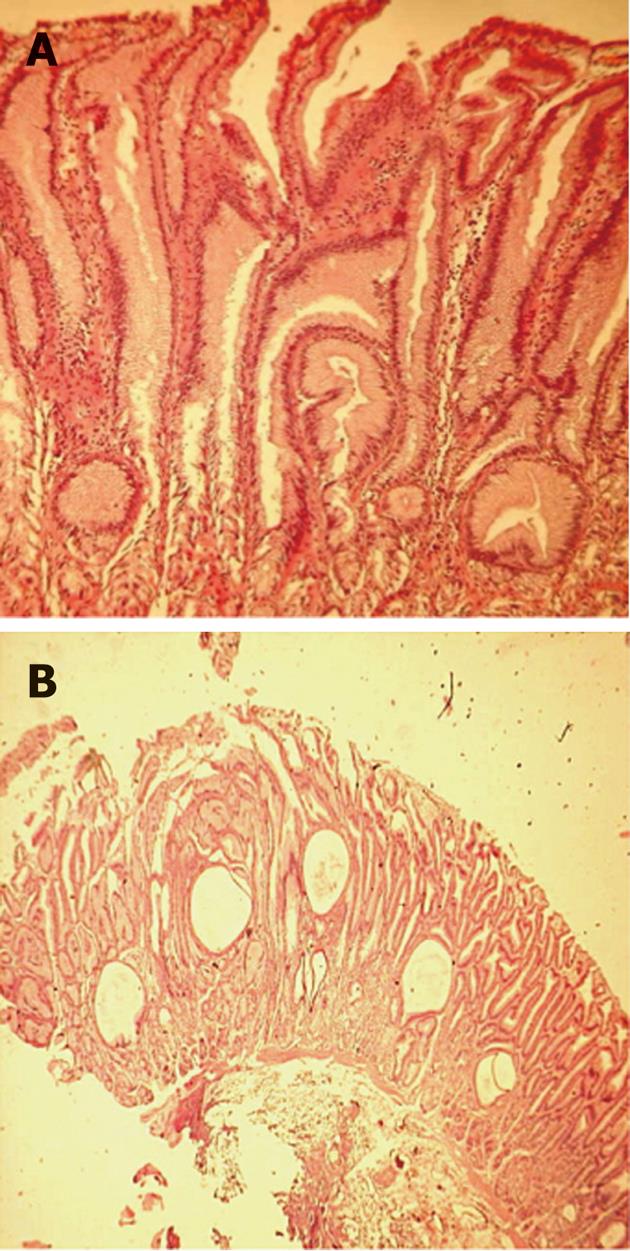
After 2 mo of unsuccessful treatment with omeprazole at 2 mg/kg per day, an EMR was done in the area of greatest gastric folds thickening to obtain a wider sample for a histological diagnosis. Thus, a diagnosis of MD was made. based on the presence of marked epithelial hyperplasia with tortuous and cystically dilated foveolar glands, discontinuous atrophy of stomach glands and significant reduction of parietal cells; a strong small vessel congestion with a diffuse edema was also observed within the lamina propria (Figure 2).
Following a lack of response to conventional therapies and due to preliminary reports on successful use of octreotide in adult MD[9-13], the patient was given ten doses of octreotide (50 μg subcutaneously two times a day), which was well tolerated. He then began octreotide LAR (5 mg intramuscularly every 28 d). Two months later haemoglobin and iron levels were normal. Six months later therapy was stopped and a rapid reduction of the haemoglobin level ensued. Thus, we continued octreotide with long-term normalization of the haemoglobin levels. Fifteen months later gastric folds were less prominent and no erosions at endoscopic follow-up were seen; however the histology was unchanged.
MD is characterized by hypertrophic folds in the body of the stomach, foveolar hyperplasia and hypoproteinemia[1]. While spontaneous remission is rare in adults and gastrectomy is not uncommonly required in refractory disease[2,3], pediatric MD is commonly an acute, self-limited, protein-losing gastropathy[5]. However, a hyperplastic hypersecretory variant of MD with normal or increased acid secretion and no protein loss is reported: it often requires full-thickness biopsy due to an inability to detect typical histological findings with conventional endoscopic biopsy[6].
Therapy in children is supportive and includes adequate hydration, antisecretory agents (histamine 2 receptor antagonists and proton pump inhibitors) and albumin replacement[3-5]. Treatment of CMV, when detected, is usually associated with remission[7,8].
Interestingly, transforming growth factor α, one of the ligands for the epidermal growth factor receptor (EGF-R), has been implicated in the mechanisms underlying MD[14]: it causes dose-dependent in vitro proliferation of gastric epithelial cells and reduction of acid secretion, which are hallmarks of the disease[15,16].
Treatment with an experimental monoclonal antibody against EGF-R has been successful in 3 severe MD cases[17,18]. There is also evidence that somatostatin decreases the number of EGF binding sites at the cell surface[19]; thus, octreotide may modulate EGF-R signalling at several levels[20,21]. These mechanisms and preliminary reports on successful use of octreotide in adults with MD[9-13] prompted us to use this agent in our patient.
In conclusion, we describe an uncommon case of pediatric MD with atypical presentation (only anemia) and chronic unremitting course. MD should be considered in all children with thickened gastric folds at endoscopy, even in the absence of typical clinical and laboratory manifestations. EMR is a good alternative to full thickness biopsy for diagnosing disorders associated with gastric wall thickening such as MD.
To the best of our knowledge, this is the first pediatric MD case successfully treated with octreotide LAR. Our experience suggests octreotide LAR as treatment for refractory MD before gastrectomy.
Peer reviewers: Seyed Mohsen Dehghani, MD, Associate Professor, Department of Pediatrics, Nemazee Hospital, Shiraz University of Medical Sciences, Shiraz 71037-11351, Iran; Jae J Kim, MD, PhD, Associate Professor, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, 50, Irwon-dong, Gangnam-gu, Seoul 135-710, South Korea
S- Editor Shi ZF L- Editor O’Neill M E- Editor Xiong L
| 1. | Lee EL, Feldman M. Gastritis and Gastropathies. Feldman: Sleisenger and Fordtran's Gastrointestinal and Liver Disease. 8th ed. Philadelphia: Saunders; 2006; 1068-1083. |
| 2. | Sundt TM, Compton CC, Malt RA. Ménétrier’s disease. A trivalent gastropathy. Ann Surg. 1988;208:694-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Scharschmidt BF. The natural history of hypertrophic gastrophy (Menetrier’s disease). Report of a case with 16 year follow-up and review of 120 cases from the literature. Am J Med. 1977;63:644-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 94] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Wolfsen HC, Carpenter HA, Talley NJ. Menetrier’s disease: a form of hypertrophic gastropathy or gastritis? Gastroenterology. 1993;104:1310-1319. [PubMed] |
| 5. | Blackstone MM, Mittal MK. The edematous toddler: a case of pediatric Ménétrier disease. Pediatr Emerg Care. 2008;24:682-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Gleeson FC, Mangan TF, Levy MJ. Endoscopic ultrasound and endoscopic mucosal resection features of a non-protein losing form of Ménétrier’s disease. Clin Gastroenterol Hepatol. 2008;6:e24-e25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Eisenstat DD, Griffiths AM, Cutz E, Petric M, Drumm B. Acute cytomegalovirus infection in a child with Ménétrier’s disease. Gastroenterology. 1995;109:592-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Xiao SY, Hart J. Marked gastric foveolar hyperplasia associated with active cytomegalovirus infection. Am J Gastroenterol. 2001;96:223-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Yeaton P, Frierson HF. Octreotide reduces enteral protein losses in Ménétrier’s disease. Am J Gastroenterol. 1993;88:95-98. [PubMed] |
| 10. | Ojeda E, Ruiz J, Cosme A, Lobo C. [Menetrier disease associated with ulcerative colitis. Response to the treatment with octreotide. Review of the diagnostic criteria and etiopathogenesis]. Gastroenterol Hepatol. 1997;20:175-179. [PubMed] |
| 11. | Green BT, Branch MS. Menetrier’s disease treated with octreotide long-acting release. Gastrointest Endosc. 2004;60:1028-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Gadour MO, Salman AH, El Samman el Tel W, Tadros NM. Menetrier’s disease: an excellent response to octreotide. A case report from the Middle East. Trop Gastroenterol. 2005;26:129-131. [PubMed] |
| 13. | Rothenberg M, Pai R, Stuart K. Successful use of octreotide to treat Ménétrier’s disease: a rare cause of abdominal pain, weight loss, edema, and hypoalbuminemia. Dig Dis Sci. 2009;54:1403-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Nalle SC, Turner JR. Menetrier’s disease therapy: rebooting mucosal signaling. Sci Transl Med. 2009;1:8ps10. [PubMed] |
| 15. | Dempsey PJ, Goldenring JR, Soroka CJ, Modlin IM, McClure RW, Lind CD, Ahlquist DA, Pittelkow MR, Lee DC, Sandgren EP. Possible role of transforming growth factor alpha in the pathogenesis of Ménétrier’s disease: supportive evidence form humans and transgenic mice. Gastroenterology. 1992;103:1950-1963. [PubMed] |
| 16. | Takagi H, Jhappan C, Sharp R, Merlino G. Hypertrophic gastropathy resembling Ménétrier’s disease in transgenic mice overexpressing transforming growth factor alpha in the stomach. J Clin Invest. 1992;90:1161-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 101] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Burdick JS, Chung E, Tanner G, Sun M, Paciga JE, Cheng JQ, Washington K, Goldenring JR, Coffey RJ. Treatment of Ménétrier’s disease with a monoclonal antibody against the epidermal growth factor receptor. N Engl J Med. 2000;343:1697-1701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 72] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Settle SH, Washington K, Lind C, Itzkowitz S, Fiske WH, Burdick JS, Jerome WG, Ray M, Weinstein W, Coffey RJ. Chronic treatment of Ménétrier’s disease with Erbitux: clinical efficacy and insight into pathophysiology. Clin Gastroenterol Hepatol. 2005;3:654-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Pinski J, Halmos G, Schally AV. Somatostatin analog RC-160 and bombesin/gastrin-releasing peptide antagonist RC-3095 inhibit the growth of androgen-independent DU-145 human prostate cancer line in nude mice. Cancer Lett. 1993;71:189-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Lahlou H, Guillermet J, Hortala M, Vernejoul F, Pyronnet S, Bousquet C, Susini C. Molecular signaling of somatostatin receptors. Ann N Y Acad Sci. 2004;1014:121-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 21. | Watt HL, Kharmate GD, Kumar U. Somatostatin receptors 1 and 5 heterodimerize with epidermal growth factor receptor: agonist-dependent modulation of the downstream MAPK signalling pathway in breast cancer cells. Cell Signal. 2009;21:428-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |