Published online Jun 7, 2012. doi: 10.3748/wjg.v18.i21.2649
Revised: January 5, 2012
Accepted: April 10, 2012
Published online: June 7, 2012
AIM: To investigate the association between autoimmune pancreatitis (AIP) and systemic autoimmune diseases (SAIDs) by measurement of serum immunoglobulin G4 (IgG4).
METHODS: The serum level of IgG4 was measured in 61 patients with SAIDs of different types who had not yet participated in glucocorticosteroid treatment. Patients with an elevated IgG4 level were examined by abdominal ultrasonography (US) and, in some cases, by computer tomography (CT).
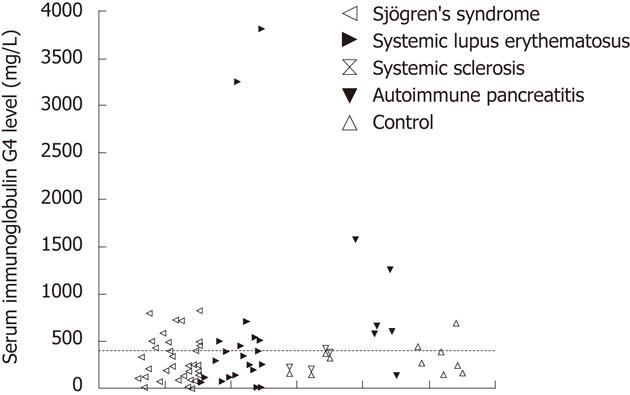
RESULTS: Elevated serum IgG4 levels (919 ± 996 mg/L) were detected in 17 (28%) of the 61 SAID patients. 10 patients had Sjögren’s syndrome (SS) (IgG4: 590 ± 232 mg/L), 2 of them in association with Hashimoto’s thyroiditis, and 7 patients (IgG4: 1388 ± 985.5 mg/L) had systemic lupus erythematosus (SLE). The IgG4 level in the SLE patients and that in patients with SS were not significantly different from that in AIP patients (783 ± 522 mg/L). Abdominal US and CT did not reveal any characteristic features of AIP among the SAID patients with an elevated IgG4 level.
CONCLUSION: The serum IgG4 level may be elevated in SAIDs without the presence of AIP. The determination of serum IgG4 does not seem to be suitable for the differentiation between IgG4-related diseases and SAIDs.
- Citation: Terzin V, Földesi I, Kovács L, Pokorny G, Wittmann T, Czakó L. Association between autoimmune pancreatitis and systemic autoimmune diseases. World J Gastroenterol 2012; 18(21): 2649-2653
- URL: https://www.wjgnet.com/1007-9327/full/v18/i21/2649.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i21.2649
Autoimmune pancreatitis (AIP) is an increasingly recognized type of chronic pancreatitis that is clearly distinct from other types of chronic pancreatitis. It is characterized by its morphology, immunologic features, pathology and glucocorticosteroid responsiveness[1-4].
Immunological examinations in AIP patients have demonstrated high incidences of hypergammaglobulinemia (43%), increased serum levels of immunoglobulin G (IgG) (62%-80%) and IgG4 (68%-92%), and the presence of antinuclear antibodies (40%-64%) and rheumatoid factor (25%). Among all the serological diagnostic features, an elevated serum level of IgG4 has the highest individual diagnostic value; however, it is not disease specific. Furthermore, an elevated serum IgG4 level correlates with the activity of AIP[5,6]. Kamisawa et al[7] reported an association between serum IgG4 level and extrapancreatic lesions in patients with AIP. AIP patients with a serum IgG4 level ≥ 2200 mg/L frequently exhibit extrapancreatic lesions.
The immunologic and histologic features of AIP and the glucocorticosteroid responsiveness suggest an autoimmune mechanism for the development of the disease[8]. AIP is accompanied by other autoimmune diseases (sclerosing cholangitis, sclerosing sialadenitis, retroperitoneal fibrosis, enlarged celiac and hilar lymph nodes, chronic thyroiditis and interstitial nephritis, etc.) in 50%-63% of cases, suggesting that AIP may be a systemic disorder[1-4]. The occurrence of autoimmune diseases in association with AIP is well documented[9,10], but the incidence of such associations has not been reported.
The aim of the present study was to assess the presence of AIP in different systemic autoimmune diseases (SAIDs) through measurement of the serum IgG4 level and examination of the morphology of the pancreas.
Serum samples were obtained from 61 patients with different SAIDs who had been admitted to our Department of Rheumatology and had not participated in glucocorticosteroid treatment during the past 2 years. One male and 60 females (mean age 54.5 years, range 29-82 years) were recruited.
Autoimmune diseases were diagnosed according to standard diagnostic criteria[11-14]. The diagnosis of AIP was based on the HISORt criteria[15]. The most frequent diagnosis was Sjögren’s syndrome (SS), but systemic lupus erythematosus (SLE), Hashimoto thyroiditis, Raynaud’s syndrome, polymyositis and systemic sclerosis also occurred (Table 1).
| No. of patients | Male/female | Age mean (range) | |
| Sjögren's syndrome | 35 | 1/34 | 56.7 (29-82) |
| Systemic lupus erythematosus | 22 | 0/22 | 50.2 (31-68) |
| Systemic sclerosis | 4 | 0/4 | 59.5 (45-80) |
| Normal subjects | 7 | 4/3 | 68 (56-80) |
| Autoimmune pancreatitis | 6 | 3/3 | 53.7 (27-75) |
Serum samples were additionally obtained from 7 age- and sex-matched healthy subjects, and 6 patients with AIP. In one AIP patient, the AIP was accompanied by rheumatoid arthritis and ankylosing spondylitis.
All participants provided their written informed consent. The study protocol was approved by the ethics committee at the University of Szeged and was carried out in full accordance with the most recent revisions of the Helsinki Declaration.
After collection, serum samples were stored at -70 °C until analyzed. The IgG4 subclass was determined by the radial immunodiffusion (RID) method (The Binding Site Limited, Birmingham, United Kingdom). The diameters of precipitation rings were measured after 72 h. The results were read using the RID reference table. The lowest detection limit was 22.4 mg/L. The intra- and inter-assay coefficients of variation were 3.26 and 0.89 CV%, respectively, as stated by the manufacturer. A cutoff value of 400 mg/L was employed.
Patients with a serum IgG4 level of > 400 mg/L were examined by a gastroenterologist. The clinical and laboratory data were reviewed and abdominal ultrasonography (US) and computed tomography (CT) were performed.
The presence of anti-SS-A/SS-B autoantibodies was determined by means of commercial enzyme-linked immunosorbent assays, conducted according to the protocols provided by the manufacturers.
Experimental data were evaluated statistically with the independent-samples t test. P-values < 0.05 were accepted as being statistically significant. Statistical data is expressed as mean ± SD.
An elevated serum IgG4 level (mean value 919 ± 996 mg/L) was detected in 17 (28%) of the 61 SAID patients (Figure 1). Ten of the 17 patients had SS (mean serum IgG4 590 ± 232 mg/L) (2 cases were associated with Hashimoto’s thyroiditis), 7 (mean serum IgG4 1388 ± 985.5 mg/L) were diagnosed with SLE. Two SLE patients showed markedly elevated IgG4 levels (> 3000 mg/L). In one case, SLE was associated with Raynaud’s syndrome, while the other patient suffered from xerophtalmia and bronchial asthma. The serum IgG4 level was elevated (mean serum IgG4 783 ± 522 mg/L) in 5 (83%) of the 6 AIP patients. The patient with a normal level of IgG4 had typical pancreatic histology and his condition improved with steroid therapy. The IgG4 levels in these SLE and SS patients were not significantly different from that in the AIP patients.
US examination revealed a normal pancreas in 11 of the 17 SAID patients with elevated serum IgG4 levels, but raised the suspicion of AIP by demonstrating a gracile pancreas in 2 cases (both suffered from SS), and widening of the body or the tail of pancreas, each in a further one patient (both suffered from SLE). However, in none of these 4 cases was AIP confirmed by an abdominal CT scan. The US examinations indicated pancreatic steatosis in 2 additional cases. None of the SAID patients had pancreatic duct dilatation.
The presence of anti-SS-A/SS-B autoantibodies and the potential relation of this to an elevated IgG4 level were examined in the patients with SS. Both anti-SS-A-positivity and anti-SS-B-positivity was detected in 22 patients; 7 of them exhibited an elevated IgG4 level. The anti-SS-A was positive and the anti-SS-B was negative in 9 cases; 2 of these patients had a high IgG4 level. In 4 patients with SS, neither anti-SS-A-positivity, nor anti-SS-B-positivity was found; an elevated IgG4 level was detected in only one of these cases.
The present study has demonstrated that the serum IgG4 level may be elevated in SAIDs, without the presence of AIP.
AIP can be complicated by a variety of extrapancreatic lesions, which appear synchronously or metachronously with the pancreatic lesion, share the same pathological conditions, and show a favorable response to glucocorticosteroid therapy, characteristics indicative of a common pathophysiological background. Among the variety of extrapancreatic diseases, lachrymal and salivary gland lesions are some of the most frequent, found in 23%-39% of patients with AIP[16,17]. Extrapancreatic lesions may mimic or be misdiagnosed as primary lesions of the corresponding organs, e.g., lachrymal and salivary gland lesions for SS. It is therefore necessary to differentiate between IgG4-related diseases and inherent diseases of the corresponding organ. When the pancreatic lesion is obscured, it may be difficult to detect these presumably IgG4-related extrapancreatic lesions[4].
IgG4 is the rarest of the 4 IgG subclasses in humans, with an incidence of about 4%. IgG antibodies are predominantly involved in the secondary immune response; complement activation is possibly their most important biological function. The main role of IgG4 is presumably to protect against the biological effects of the complement-fixing IgG subclasses and to act in parasitic infestation or various forms of atopy[18-20]. Serum IgG4 levels are frequently and significantly elevated in AIP patients[6] and an elevated level of serum IgG4 has been included among the laboratory criteria for the diagnosis of AIP[4,15]. AIP patients with 3 extrapancreatic lesions have been reported to have significantly higher IgG4 levels than those lacking such lesions[16]. The optimal cutoff value for discriminating AIP patients with extrapancreatic lesions from those without was demonstrated on the basis of receiver operator characteristic curves to be 2200 mg/L[7].
The serum IgG4 level was measured in 61 SAID patients in our study, 28% of whom proved to have an elevated serum level of IgG4. However, none of them could be diagnosed with AIP according to the HISORt criteria. What could be the reason for this?
One explanation is the composition of our patient cohort. In Japan AIP predominantly affects men, with a male:female ratio of 2.85:1[16]. Moreover, there was a male preponderance in the United Kingdom, European and US studies (100%, 66% and 65% male, respectively), similar to in reports from Japan[21-24]. In contrast, there was only one male in our patient population.
Lachrymal and salivary lesions associated with AIP were previously considered to be complications of SS. However, in contrast to those accompanying SS, the lachrymal and salivary gland lesions associated with AIP yield negative results for anti-SS-A/SS-B autoantibodies and show numerous IgG4-positive plasma cell infiltrations in the affected tissues. These lesions are currently thought to correspond to Mikulicz’s disease[25]. The explanation for our negative results may be that there was only one patient with negative SS-A/SS-B autoantibodies in our study group.
Another point is that autoantibodies against FcεRIα are detected in the sera of patients with different autoimmune diseases (such as SLE, dermatomyositis, pemphigus and pemphigoid); these antibodies are from subclasses IgG2 and IgG4, but they are functionally inactive[26]. In our study, elevated IgG4 levels were found in 7 patients treated for SLE.
Moreover, our 17 SAID patients with elevated IgG4 levels included 6 who suffered from different concomitant diseases which could cause the increase in the serum level of IgG4. In one patient, nodular sclerosis Hodgkin lymphoma (HL) was diagnosed histologically. HL cells frequently express interleukin 13 (IL-13) and its receptor. Besides exerting several effects on B cells (e.g., promoting their survival and proliferation), IL-13 switches the Ig class to IgG4 and IgE[27]. In another patient, bullous pemphigoid was identified, which is among the most common blistering autoimmune skin lesions. One of the features of the disease is the presence of autoantibodies against hemidesmosomal antigens (i.e., bullous pemphigoid antigen 1 and 2) in the serum and in affected areas of the skin. The major types of these autoantibodies are IgG4 and IgE[28]. In a third patient, cutaneous lymphocytic vasculitis was diagnosed, which could also explain the serum IgG4 elevation[29]. In 2 patients, the underlying disease was accompanied by Hashimoto’s thyroiditis, which can elevate the IgG4 level since thyroglobulin autoantibodies are from subclasses IgG2 and IgG4[30]. There was also one patient with bronchial asthma, in which disease elevated titers of IgG4 can be found[31].
Finally, SS was diagnosed in the remaining 4 patients, one of whom was seronegative, while the others were seropositive. The elevated serum IgG4 level in patients with seronegative SS may possibly be explained by the presence of Mikulicz’s disease[32]. Furthermore, an elevated serum IgG4 level has also been reported in SS[33].
However, not all AIP patients display elevated serum IgG and IgG4 levels. IgG4-negative AIP patients seem to occur more frequently in Europe[34]. Furthermore, some AIP cases improve spontaneously[4]. Hence, it cannot be ruled out that our SAID cohort included AIP patients who were not diagnosed by the measurement of serum IgG4 or in whom the morphology of the pancreas had already normalized by the time of our examination.
Overall, it can be concluded that the serum IgG4 level may be elevated in SAIDs, but as a consequence of the concomitant SAID rather than of AIP. The determination of serum IgG4 does not seem to be suitable for the differentiation between IgG4-related diseases and SAIDs.
Autoimmune pancreatitis (AIP) is frequently associated with some other autoimmune disease, suggesting that it may be a systemic disorder. The determination of serum immunoglobulin G4 (IgG4) is a sensitive marker to diagnose AIP and IgG4-related diseases.
IgG4 is a sensitive marker in the diagnosis of AIP. The association of AIP and systemic autoimmune diseases (SAIDs), and the usefulness of the determination of serum IgG4 in the diagnosis of AIP in patients with SAIDs are not defined.
The authors revealed that the serum IgG4 level may be elevated in SAIDs without the presence of AIP. The determination of serum IgG4 does not seem to be suitable for the differentiation between IgG4-related diseases and SAIDs.
This study provides important data about the serum level of IgG4 in SAIDs. The determination of serum IgG4 does not seem to be suitable for the differentiation between IgG4-related diseases and SAIDs. The diagnosis of AIP in SAIDs should be made on the results of morphological and histological examination.
AIP is an increasingly recognized distinct type of chronic pancreatitis with a presumed autoimmune etiology. Immunoglobulin G (IgG) has four subclasses (IgG1 through IgG4) and the IgG4 subclass accounts for 3%-6% of total serum IgG.
This paper discusses the relationship between autoimmune pancreatitis and an elevated serum IgG4 level in autoimmune diseases, and presents interesting and potentially important information. The patients used in this study are few and biased (1 male and 60 female cases), but it is highly significant as a clinical pilot study. This study is well-designed and written, but there are some points to be clarified.
Peer reviewer: Toshiyuki Ishiwata, Associate Professor, Department of Pathology, Integrative Oncological Pathology, Nippon Medical School, 1-1-5 Sendagi, Bunkyo-ku, Tokyo 113-8602, Japan
S- Editor Cheng JX L- Editor Cant MR E- Editor Xiong L
| 1. | Detlefsen S, Drewes AM. Autoimmune pancreatitis. Scand J Gastroenterol. 2009;44:1391-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Shimosegawa T, Kanno A. Autoimmune pancreatitis in Japan: overview and perspective. J Gastroenterol. 2009;44:503-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Park DH, Kim MH, Chari ST. Recent advances in autoimmune pancreatitis. Gut. 2009;58:1680-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 4. | Okazaki K, Kawa S, Kamisawa T, Ito T, Inui K, Irie H, Irisawa A, Kubo K, Notohara K, Hasebe O. Japanese clinical guidelines for autoimmune pancreatitis. Pancreas. 2009;38:849-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, Fukushima M, Nikaido T, Nakayama K, Usuda N. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344:732-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2026] [Cited by in RCA: 1876] [Article Influence: 78.2] [Reference Citation Analysis (0)] |
| 6. | Choi EK, Kim MH, Lee TY, Kwon S, Oh HC, Hwang CY, Seo DW, Lee SS, Lee SK. The sensitivity and specificity of serum immunoglobulin G and immunoglobulin G4 levels in the diagnosis of autoimmune chronic pancreatitis: Korean experience. Pancreas. 2007;35:156-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Kamisawa T, Imai M, Egawa N, Tsuruta K, Okamoto A. Serum IgG4 levels and extrapancreatic lesions in autoimmune pancreatitis. Eur J Gastroenterol Hepatol. 2008;20:1167-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Okazaki K, Uchida K, Koyabu M, Miyoshi H, Takaoka M. Recent advances in the concept and diagnosis of autoimmune pancreatitis and IgG4-related disease. J Gastroenterol. 2011;46:277-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 204] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 9. | Kamisawa T, Okamoto A. Autoimmune pancreatitis: proposal of IgG4-related sclerosing disease. J Gastroenterol. 2006;41:613-625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 362] [Cited by in RCA: 341] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 10. | Kamisawa T, Okazaki K, Kawa S, Shimosegawa T, Tanaka M. Japanese consensus guidelines for management of autoimmune pancreatitis: III. Treatment and prognosis of AIP. J Gastroenterol. 2010;45:471-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 11. | Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, Daniels TE, Fox PC, Fox RI, Kassan SS. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4161] [Cited by in RCA: 3921] [Article Influence: 170.5] [Reference Citation Analysis (0)] |
| 12. | Gill JM, Quisel AM, Rocca PV, Walters DT. Diagnosis of systemic lupus erythematosus. Am Fam Physician. 2003;68:2179-2186. [PubMed] |
| 13. | Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569-2581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6830] [Cited by in RCA: 6189] [Article Influence: 412.6] [Reference Citation Analysis (0)] |
| 14. | Walker JG, Pope J, Baron M, Leclercq S, Hudson M, Taillefer S, Edworthy SM, Nadashkevich O, Fritzler MJ. The development of systemic sclerosis classification criteria. Clin Rheumatol. 2007;26:1401-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Zhang L, Clain JE, Pearson RK, Petersen BT, Vege SS. Diagnosis of autoimmune pancreatitis: the Mayo Clinic experience. Clin Gastroenterol Hepatol. 2006;4:1010-1016; quiz 934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 656] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 16. | Hamano H, Arakura N, Muraki T, Ozaki Y, Kiyosawa K, Kawa S. Prevalence and distribution of extrapancreatic lesions complicating autoimmune pancreatitis. J Gastroenterol. 2006;41:1197-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 227] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 17. | Naitoh I, Nakazawa T, Ohara H, Ando T, Hayashi K, Tanaka H, Okumura F, Miyabe K, Yoshida M, Sano H. Clinical significance of extrapancreatic lesions in autoimmune pancreatitis. Pancreas. 2010;39:e1-e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Nirula A, Glaser SM, Kalled SL, Taylor FR. What is IgG4? A review of the biology of a unique immunoglobulin subtype. Curr Opin Rheumatol. 2011;23:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 209] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 19. | van der Zee JS, van Swieten P, Aalberse RC. Inhibition of complement activation by IgG4 antibodies. Clin Exp Immunol. 1986;64:415-422. [PubMed] |
| 20. | Aalberse RC, Schuurman J. IgG4 breaking the rules. Immunology. 2002;105:9-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 333] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 21. | Church NI, Pereira SP, Deheragoda MG, Sandanayake N, Amin Z, Lees WR, Gillams A, Rodriguez-Justo M, Novelli M, Seward EW. Autoimmune pancreatitis: clinical and radiological features and objective response to steroid therapy in a UK series. Am J Gastroenterol. 2007;102:2417-2425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Zamboni G, Lüttges J, Capelli P, Frulloni L, Cavallini G, Pederzoli P, Leins A, Longnecker D, Klöppel G. Histopathological features of diagnostic and clinical relevance in autoimmune pancreatitis: a study on 53 resection specimens and 9 biopsy specimens. Virchows Arch. 2004;445:552-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 440] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 23. | Raina A, Yadav D, Krasinskas AM, McGrath KM, Khalid A, Sanders M, Whitcomb DC, Slivka A. Evaluation and management of autoimmune pancreatitis: experience at a large US center. Am J Gastroenterol. 2009;104:2295-2306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 181] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 24. | Czakó L, Gyökeres T, Topa L, Sahin P, Takács T, Vincze A, Dubravcsik Z, Szepes A, Pap A, Földesi I. Autoimmune pancreatitis in Hungary: a multicenter nationwide study. Pancreatology. 2011;11:261-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Yamamoto M, Harada S, Ohara M, Suzuki C, Naishiro Y, Yamamoto H, Takahashi H, Imai K. Clinical and pathological differences between Mikulicz's disease and Sjögren's syndrome. Rheumatology (Oxford). 2005;44:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 188] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 26. | Fiebiger E, Hammerschmid F, Stingl G, Maurer D. Anti-FcepsilonRIalpha autoantibodies in autoimmune-mediated disorders. Identification of a structure-function relationship. J Clin Invest. 1998;101:243-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 178] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 27. | Skinnider BF, Elia AJ, Gascoyne RD, Trümper LH, von Bonin F, Kapp U, Patterson B, Snow BE, Mak TW. Interleukin 13 and interleukin 13 receptor are frequently expressed by Hodgkin and Reed-Sternberg cells of Hodgkin lymphoma. Blood. 2001;97:250-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 131] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 28. | Döpp R, Schmidt E, Chimanovitch I, Leverkus M, Bröcker EB, Zillikens D. IgG4 and IgE are the major immunoglobulins targeting the NC16A domain of BP180 in Bullous pemphigoid: serum levels of these immunoglobulins reflect disease activity. J Am Acad Dermatol. 2000;42:577-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Kawassaki AM, Haga H, Dantas TC, Musolino RS, Baldi BG, Carvalho CR, Kairalla RA, Mauad T. Adenopathy and pulmonary infiltrates in a Japanese emigrant in Brazil. Chest. 2011;139:947-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | Fukuma N, McLachlan SM, Petersen VB, Kau P, Bradbury J, Devey M, Bleasdale K, Grabowski P, Smith BR. Human thyroglobulin autoantibodies of subclasses IgG2 and IgG4 bind to different epitopes on thyroglobulin. Immunology. 1989;67:129-131. [PubMed] |
| 31. | Sprangers B, Claes K. IgG4-related disease should be considered in cases of hypocomplementemic immune-complex tubulointerstitial nephritis. Letters and Replies NDT Plus. 2010;3:326–334. [DOI] [Full Text] |
| 32. | Masaki Y, Sugai S, Umehara H. IgG4-related diseases including Mikulicz's disease and sclerosing pancreatitis: diagnostic insights. J Rheumatol. 2010;37:1380-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 33. | Suzuki S, Kida S, Ohira Y, Ohba T, Miyata M, Nishimaki T, Morito T, Kasukawa R, Hojyo H, Wakasa H. [A case of Sjögren's syndrome accompanied by lymphadenopathy and IgG4 hypergammaglobulinemia]. Ryumachi. 1993;33:249-254. [PubMed] |
| 34. | Kamisawa T, Takuma K, Tabata T, Inaba Y, Egawa N, Tsuruta K, Hishima T, Sasaki T, Itoi T. Serum IgG4-negative autoimmune pancreatitis. J Gastroenterol. 2011;46:108-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |