Published online May 7, 2012. doi: 10.3748/wjg.v18.i17.2099
Revised: August 19, 2011
Accepted: August 27, 2011
Published online: May 7, 2012
AIM: To investigate differences in clinical features between diffuse- and focal-type autoimmune pancreatitis (AIP).
METHODS: Based on radiological findings by computed tomography and/or magnetic resonance imaging, we divided 67 AIP patients into diffuse type (D type) and focal type (F type). We further divided F type into head type (H type) and body and/or tail type (B/T type) according to the location of enlargement. Finally, we classified the 67 AIP patients into three groups: D type, H type and B/T type. We compared the three types of AIP in terms of clinical, laboratory, radiological, functional and histological findings and clinical course.
RESULTS: There were 34 patients with D-type, 19 with H-type and 14 with B/T-type AIP. Although obstructive jaundice was frequently detected in D-type patients (88%) and H-type patients (68%), no B/T-type patients showed jaundice as an initial symptom (P < 0.001). There were no differences in frequency of abdominal pain, but acute pancreatitis was associated more frequently in B/T-type patients (36%) than in D-type patients (3%) (P = 0.017). Serum immunoglobulin G (IgG)4 levels were significantly higher in D-type patients (median 309 mg/dL) than in B/T-type patients (133.5 mg/dL) (P = 0.042). Serum amylase levels in B/T-type patients (median: 114 IU/L) were significantly greater than in H-type patients (72 IU/L) (P = 0.049). Lymphoplasmacytic sclerosing pancreatitis (LPSP) was histologically confirmed in 6 D-type, 7 H-type and 4 B/T-type patients; idiopathic duct-centric pancreatitis was observed in no patients. Marked fibrosis and abundant infiltration of CD20-positive B lymphocytes with few IgG4-positive plasma cells were detected in 2 B/T-type patients. Steroid therapy was effective in all 50 patients (31 D type, 13 H type and 6 B/T type). Although AIP relapsed during tapering or after stopping steroids in 3 D-type and 3 H-type patients, no patients relapsed in B/T type. During follow-up, radiological features of 6 B/T-type patients were not changed and 1 B/T-type patient improved naturally.
CONCLUSION: Clinical features of H-type AIP were similar to those of D-type, but B/T-type differed from D and H types. B/T-type may involve diseases other than LPSP.
- Citation: Tabata T, Kamisawa T, Takuma K, Hara S, Kuruma S, Inaba Y. Differences between diffuse and focal autoimmune pancreatitis. World J Gastroenterol 2012; 18(17): 2099-2104
- URL: https://www.wjgnet.com/1007-9327/full/v18/i17/2099.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i17.2099
Autoimmune pancreatitis (AIP) is a type of pancreatitis that is thought to have an autoimmune etiology. AIP responds dramatically to steroid therapy, therefore, differentiating AIP from pancreatic cancer is important to avoid unnecessary pancreatic resection[1-3]. According to the Asian Diagnostic Criteria for AIP[4], AIP is diagnosed based on the following items: diffuse or focal enlargement of the pancreas and narrowing of the main pancreatic duct; increased serum immunoglobulin G (IgG) or IgG4 levels or the presence of autoantibodies in the serum; histological findings of lymphoplasmacytic infiltration and fibrosis in the pancreas [lymphoplasmacytic sclerosing pancreatitis (LPSP)[5]]; and responsiveness to steroids. AIP occurs frequently in elderly men. The primary initial symptom is obstructive jaundice, and diabetes mellitus occurs in half of patients. AIP is rarely associated with acute pancreatitis or ulcerative colitis. AIP is associated with several sclerosing extrapancreatic lesions such as sclerosing cholangitis, sclerosing sialadenitis, and retroperitoneal fibrosis, and is currently recognized as a pancreatic lesion of IgG4-related systemic disease. AIP responds well to steroid therapy, but it sometimes relapses[1,2].
Recently, another histological pattern of AIP, characterized by ductal epithelial granulocytic infiltration, has been recognized[6,7]. This pattern is referred to as idiopathic duct-centric pancreatitis (IDCP)[6]. Clinical features of IDCP patients are different from those of LPSP patients. The terms, type 1 and type 2 AIP, are proposed to describe the clinical features associated with LPSP and IDCP, respectively[8-11].
Radiologically, AIP is classified into diffuse type (D type) and focal type (F type)[1-3,12-14]. Although diffuse enlargement of the pancreas is rather specific to AIP, F-type AIP should be strictly differentiated from pancreatic cancer. However, only a few studies[13,14] have investigated the differences between D- and F-type AIP, and it is unknown whether F-type AIP is an initial stage of D-type AIP, or a different type of AIP. This study aimed to clarify differences in clinical features between D- and F-type AIP.
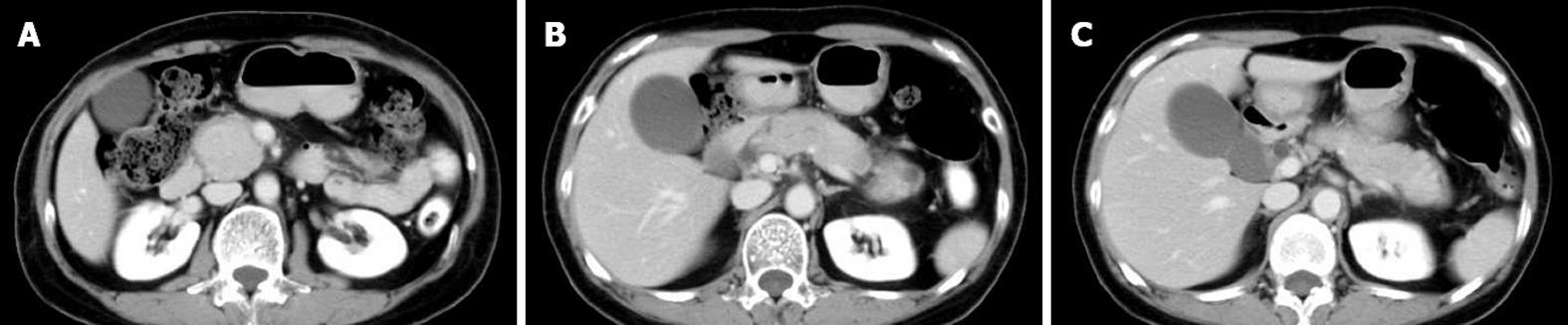
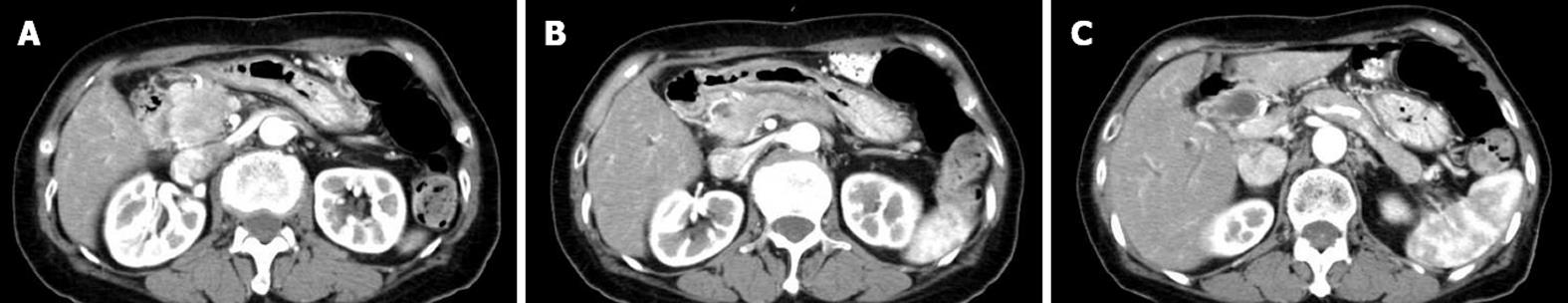
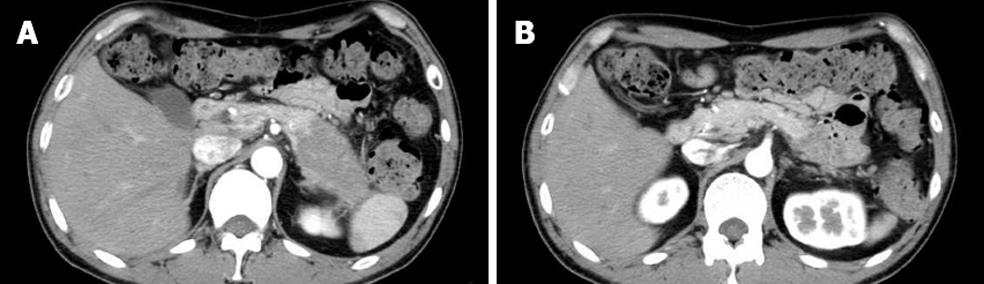
From 1988 to 2010, 67 AIP patients (47 men and 20 women; median age: 60.3 years; range: 27-83 years) were diagnosed according to the Asian Diagnostic Criteria for AIP[4] in Tokyo Metropolitan Komagome Hospital. Based on radiological findings by computed tomography and/or magnetic resonance imaging, we divided the AIP patients into D type and F type. We further divided F type into head type (H type) and body and/or tail type (B/T type) according to the location of enlargement. Finally, we classified the 67 AIP patients into three groups: D type, H type, and B/T type. D-type AIP patients showed diffuse enlargement of the pancreas (Figure 1A-C), H-type patients showed focal enlargement of only the pancreatic head (Figure 2A-C), and B/T-type patients showed enlargement of only the pancreatic body and/or tail (Figure 3A and B). We compared clinical, laboratory, radiological, functional, and histological findings and clinical course among the three types of AIP.
Clinical assessments were as follows: age at time of diagnosis; sex; drinking and smoking habits; presence and/or history of allergic diseases; initial symptoms such as obstructive jaundice and abdominal pain; and associated diseases such as diabetes mellitus, acute pancreatitis and ulcerative colitis. Drinking habit was defined as drinking > 80 g/d alcohol for > 7 years. Smoking habit was defined as smoking > 20 pack-years. Diabetes mellitus was diagnosed if fasting serum glucose levels and/or hemoglobin A1c levels were higher than normal levels (126 mg/dL and 6.1%, respectively)[15]. Acute pancreatitis was diagnosed when both severe abdominal pain and elevation of serum amylase level (> 3 times normal; normal: 115 IU/L) were seen.
Stenosis of the lower bile duct was evaluated on endoscopic retrograde cholangiopancreatography and/or magnetic resonance cholangiopancreatography. Three extrapancreatic lesions that are frequently associated with AIP (sclerosing cholangitis of the hilar or intrahepatic bile duct, swelling of salivary glands, and retroperitoneal fibrosis) were evaluated radiologically.
Laboratory findings were assessed including serum IgG (n = 67), IgG4 (n = 65) and immunoglobulin E (IgE) (n = 43) levels, peripheral eosinophil count (n = 56), serum amylase levels (n = 66), and autoantibodies including antinuclear antigen (n = 60) and rheumatoid factor (n = 58). Serum IgG4 levels were measured by nephelometry using IgG subclass (BS-NIA) kits. A cutoff value of 135 mg/dL, which is widely accepted, was used.
The pancreas was assessed by surgical resection (n = 7), surgical or ultrasound-guided biopsy (n = 6), and endoscopic ultrasonography-guided fine-needle aspiration (EUS-FNA, n = 10) and examined histologically and immunohistochemically using anti-CD3, anti-CD20, and anti-IgG4 antibodies.
Ten patients were initially treated with surgical procedures on suspicion of pancreatic cancer (pancreatoduodenectomy, n = 6; distal pancreatectomy, n = 1; choledochoduodenostomy with pancreatic biopsy, n = 3). Fifty patients, including one surgically treated, were treated with oral steroids. Prednisolone at an initial dose of 30-40 mg/d was given for 2-3 wk. It was then tapered by 5 mg every 1-3 wk to 5 mg/d. Maintenance therapy (2.5-5 mg/d, 1-3 years) was used in all patients. Recurrence of AIP was defined as the reappearance of symptoms with the development or reappearance of pancreatic and/or extrapancreatic abnormalities on imaging studies. Eight patients were followed up conservatively without steroid therapy.
Differences between groups were analyzed using Fisher’s exact probability test, and Mann-Whitney’s U test. P values were corrected by Bonferroni’s method, and P < 0.05 was considered statistically significant.
Sixty-seven AIP patients were classified into D-type (n = 34), H-type (n = 19) and B/T-type (n = 14) AIP. There were no significant differences in age, sex, drinking, smoking habits and history of allergic diseases among the groups. Although obstructive jaundice was frequently detected in D-type (88%) and H-type (68%) patients, no B/T-type patients showed jaundice as an initial symptom (P < 0.001). Although there were no differences in frequency of abdominal pain, acute pancreatitis was seen more frequently in B/T-type (36%) than in D-type (3%) patients (P = 0.017). Although stenosis of the lower bile duct was seen frequently in D-type (94%) and H-type (95%) patients, no B/T-type patients showed stenosis of the lower bile duct (P < 0.001). There were no differences between groups in associated extrapancreatic lesions (Table 1).
| D type, n = 34 | H type, n = 19 | B/T type, n = 14 | P value, D vs H | P value, D vs B/T | P value, H vs B/T | |
| Median age (yr) (quartile range) | 67.5 (59-71.5) | 64.0 (56-71) | 61.5 (50.3-72.5) | > 0.99 | > 0.99 | > 0.99 |
| Male/female | 24/10 | 15/4 | 8/6 | > 0.99 | > 0.99 | > 0.99 |
| Drinking habit +/- (%) | 4/27 (15) | 4/15 (21) | 2/12 (14) | > 0.99 | > 0.99 | > 0.99 |
| Smoking habit +/- (%) | 16/27 (59) | 12/16 (75) | 5/13 (39) | > 0.99 | > 0.99 | 0.198 |
| Allergies +/- (%) | 8/25 (32) | 6/18 (33) | 7/13 (54) | > 0.99 | 0.885 | 0.879 |
| Jaundice +/- (%) | 30/4 (88) | 13/6 (68) | 0/14 (0) | 0.420 | < 0.001 | < 0.001 |
| Abdominal pain +/- (%) | 6/28 (18) | 7/13 (37) | 5/9 (36) | 0.546 | 0.771 | > 0.999 |
| Diabetes +/- (%) | 10/24 (29) | 11/8 (58) | 4/10 (29) | 0.231 | > 0.999 | > 0.999 |
| Acute pancreatitis +/- (%) | 1/33 (3) | 1/18 (5) | 5/9 (36) | > 0.999 | 0.017 | 0.183 |
| Ulcerative colitis +/- (%) | 1/33 (3) | 0/19 (0) | 1/13 (7) | > 0.999 | > 0.999 | > 0.999 |
| Stenosis of the lower vile duct +/- (%) | 32/34 (94) | 18/19 (95) | 0/14 (0) | > 0.999 | < 0.001 | < 0.001 |
| Extrapancreatic lesions +/- (%) | 13/21 (38) | 6/13 (32) | 5/9 (36) | > 0.999 | > 0.999 | > 0.999 |
Serum IgG4 levels were significantly higher in D-type (median: 309 mg/dL) than in B/T-type (133.5 mg/dL) patients (P = 0.042). Serum IgG4 levels were more frequently elevated in D-type (88%) than in B/T-type (50%) patients (P = 0.030). There were no significant differences in serum IgE levels and peripheral eosinophil count among the groups. Serum amylase levels in B/T-type patients (median: 114 IU/L) were significantly greater than in H-type patients (72 IU/L) (P = 0.049). There were no differences among the groups in terms of the ratio of antinuclear antigen and rheumatoid factor (Table 2).
| D type, n = 34 | H type, n = 19 | B/T type, n = 14 | P value, D vs H | P value, D vs B/T | P value, H vs B/T | |
| Median IgG, mg/dL | 2163.5 (1637.5-2682.8) | 1920 (1398.5-2300) | 1619 (1369.5-2364.5) | 0.642 | > 0.999 | > 0.999 |
| (quartile range) | ||||||
| Median IgG4, mg/dL | 309 (181-1015) | 351 (228-780) | 133.5 (60.8-326.5) | > 0.999 | 0.042 | 0.057 |
| (quartile range) | ||||||
| IgG4 > 135, mg/dL (%) | 28/32 (88) | 16/19 (84) | 7/14 (50) | > 0.999 | 0.030 | 0.168 |
| Median IgE, IU/L | 406.9 (265.5-877) | 249.0 (74.75-589.75) | 264.0 (134.1-856) | 0.447 | > 0.999 | > 0.999 |
| (quartile range) | ||||||
| IgE > 580, (IU/L) (%) | 8/20 (40.0) | 3/12 (25.0) | 4/11 (36.4) | > 0.999 | > 0.999 | > 0.999 |
| Median eosinophil, /μL | 180 (72-440) | 238.5 (152.5-447.75) | 473 (114.5-621.5) | > 0.999 | 0.354 | > 0.999 |
| (quartile range) | ||||||
| Eosinophil > 600 /μL (%) | 3/27 (11) | 2/16 (13) | 3/13 (23) | > 0.999 | > 0.999 | > 0.999 |
| Median amylase (IU/L) | 86 (49.5-119.5) | 72 (43-100) | 114 (73.3-589.3) | > 0.999 | > 0.999 | 0.049 |
| (quartile range) | ||||||
| Amylase > 115 (IU/L) (%) | 8/33 (24) | 4/19 (21) | 7/14 (50) | > 0.999 | 0.300 | 0.408 |
| Positive antinuclear antigen (%) | 14/29 (48) | 4/18 (22) | 6/13 (46) | 0.366 | > 0.999 | 0.738 |
| Positive rheumatoid factor (%) | 10/28 (36) | 2/17 (12) | 1/13 (7) | 0.288 | 0.378 | > 0.999 |
LPSP was histologically confirmed with abundant infiltration of CD3-positive T lymphocytes and IgG4-positive plasma cells in 6 D-type, 7 H-type and 4 B/T-type patients. In the pancreas of 2 B/T-type patients (1 surgical biopsy specimen and 1 EUS-FNA specimen), marked fibrosis and abundant infiltration of CD20-positive B lymphocytes rather than T lymphocytes were detected, but few IgG4-positive plasma cells or neutrophils were detected. IDCP was not observed in any patients. EUS-FNA of 4 AIP patients could not confirm the histological diagnosis due to insufficient specimens (Table 3).
| D type | H type | B/T type | |
| Resection | LPSP (n = 3) | LPSP (n = 3) | LPSP (n = 1) |
| Biopsy | LPSP (n =2) | LPSP (n = 3) | Fibrosis with abundant infiltration of B lymphocytes (n = 1) |
| EUS-FNA | LPSP (n = 1) | LPSP (n = 1) | LPSP (n = 3) |
| Inadequate material (n = 1) | Inadequate material (n = 2) | Fibrosis with abundant infiltration of B lymphocytes (n = 1) | |
| Inadequate material (n = 1) |
Steroid therapy was effective in all 50 patients (31 D type, 13 H type and 6 B/T type). AIP relapsed during tapering or after stopping steroids in 3 D-type and 3 H-type patients. In 8 conservatively followed-up patients (1 H type and 7 B/T type), radiological features were not changed in seven patients, but enlargement of the pancreatic tail improved naturally in 1 B/T-type patient. During the course prior to our hospitalization, focal enlargement of the pancreatic head developed to diffuse enlargement in three cases, and focal enlargement of the pancreatic tail developed to diffuse enlargement in one case.
Radiologically, AIP is classified into diffuse and focal forms. Diffuse enlargement of the pancreas, called sausage-like enlargement, is a typical feature of AIP. However, F-type AIP, sometimes forming a mass, is frequently difficult to differentiate from pancreatic cancer[1-3,12-14]. Possible differences in clinical presentations between the D and F types of AIP are unclear.
In the present study, we classified 67 AIP patients into D type (n = 34), H type (n = 19) and B/T type (n = 14). D and H types showed similar clinical features. However, the B/T-type was different from D and H types in several aspects. Obstructive jaundice and stenosis of the lower bile duct were frequently detected in D- and H-type patients, but no B/T-type patients showed these features. According to Ghazale et al[16], stenosis of the lower bile duct was present in 70% of 53 AIP patients. Hirano et al[17] have stated that both pancreatic edema due to inflammation of the pancreatic head and biliary wall thickening influence stenosis of the lower bile duct in AIP, based on EUS findings and the fact that 93% of pancreatic head lesion-positive AIP patients had stenosis of the lower bile duct, compared with only 17% of lesion-negative patients.
In this study, the serum IgG4 level was significantly lower in B/T-type than in D-type patients, and elevation of serum IgG4 levels was less in B/T-type than in D-type patients. Acute pancreatitis was seen more frequently in B/T-type than in D-type patients, and serum amylase levels were significantly higher in B/T-type than in H-type patients. As to the diagnosis and frequency of acute pancreatitis, the high percentage in B/T type patients seems to depend mostly on the higher amylase levels. Acute pancreatitis or some acute inflammatory attack in chronic pancreatitis cases may not be associated with high amylase levels because of the atrophic acinar tissue. There was no difference in the frequency of abdominal pain, therefore, some cases in D-type and H-type might have been overlooked.
No B/T-type patients relapsed after steroid therapy, compared with 10% of D-type and 23% of H-type patients. It has become clear that there are two histological subtypes (LPSP and IDCP) in AIP[6-11]. Most AIP patients in Asia have LPSP, and half of AIP patients in Europe have IDCP[8,10]. The clinical features of these two subtypes differ substantially. It is generally reported that IDCP patients are younger at diagnosis, and IDCP patients are less likely to show elevated serum IgG4 levels. IDCP is more likely associated with acute pancreatitis and inflammatory bowel disease. According to a comparative study of LPSP and IDCP patients by Sah et al[9], IDCP patients tended to have more focal features than LPSP (84% vs 60%), and no relapse of IDCP was seen in any patient, whereas LPSP relapsed in 47% of patients. To diagnose IDCP, histological examination of an adequate pancreatic specimen is needed, and the need for histological examination to diagnose IDCP at present makes clinical diagnosis difficult.
In histological examination of the present cases, LPSP was confirmed in 17, but IDCP was not detected. Clinical files of D-type and H-type AIP were compatible with those of LPSP patients. However, considering low serum IgG4 levels and frequent association with acute pancreatitis, there is a possibility that some IDCP cases may be involved in B/T-type cases. For example, a 32-year-old man with B/T-type AIP showed normal serum IgG4 levels, association with ulcerative colitis, and good responsiveness to steroid therapy, but histological diagnosis by EUS-FNA could not be confirmed due to an insufficient biopsy specimen. Interestingly, histology of two B/T-type patients included abundant infiltration of B cells with little infiltration of IgG4-positive cells or neutrophils, which might be another type of AIP other than LPSP and IDCP. B/T-type AIP may involve a disease other than LPSP.
Three H-type and one B/T-type patient progressed to D type during the natural course. It has also been reported that H-type AIP progresses to D-type[18,19], and B/T-type AIP to D-type[20]. There have also been reports of relapse in the remnant pancreas after resection, such as relapse in the remnant pancreatic head 1 year after distal pancreatectomy[21], and relapse in the remnant pancreatic body and tail 4 mo after pancreatoduodenectomy[22]. Steroid therapy was effective for those lesions that had progressed or relapsed. These findings indicate that the focal inflammation may advance to subsequent diffuse changes throughout the pancreas or develop repeatedly at different sites and at different times in some AIP patients. Diffuse change in the pancreas may be the final appearance of AIP, and whether this inflammatory process affects the gland diffusely or focally may merely reflect the stage of the disease. In seven conservatively followed up B/T-type patients, six showed no change and one improved naturally. Kubota et al[23] have reported that all eight AIP patients who improved spontaneously showed focal pancreatic enlargement. B/T-type AIP may involve a disease other than D-type and H-type AIP.
In conclusion, although clinical features of H-type AIP are similar to those of D-type AIP, B/T-type AIP differed in several aspects from D and H types. B/T-type AIP may involve a disease other than LPSP.
Autoimmune pancreatitis (AIP) is a particular type of pancreatitis that is thought to have an autoimmune etiology. Recently, AIP has been radiologically classified into diffuse type (D type) and focal type (F type). However, the differences between D- and F-type AIP are not well known.
Differences in clinical, radiological, laboratory and histological findings between D- and F-type AIP were investigated.
AIP patients were divided into D type and F type. Furthermore, F type was divided into head type (H type) and body and/or tail type (B/T type). No B/T-type patients showed jaundice as an initial symptom, but acute pancreatitis was associated more frequently in B/T type than in D type. Serum amylase levels were significantly higher in B/T-type than in H-type patients. Serum immunoglobulin G (IgG)4 levels were significantly higher in D-type than in B/T-type patients. No B/T-type patients relapsed after steroid therapy, compared with 10% of D-type and 23% of H-type patients. Two B/T-type patients included abundant infiltration of B cells with little infiltration of IgG4-positive cells or neutrophils.
B/T-type AIP differed in several aspects from D and H types, and some B/T-type AIP may involve a disease other than lymphoplasmacytic sclerosing pancreatitis.
The study itself seems reasonable and the results are interesting and of etiological importance.
Peer reviewers: Shoichiro Sumi, MD, PhD, Associate Pro-fessor, Department of Organ Reconstruction, Institute for Frontier Medical Sciences, Kyoto University, Sakyo-ku, Kyoto 606-8507, Japan; Atsushi Masamune, MD, PhD, Division of Gastroenterology, Tohoku University Graduate School of Medicine, 1-1 Seiryo-machi, Aoba-ku, Sendai 980-8574, Japan
S- Editor Lv S L- Editor Kerr C E- Editor Li JY
| 1. | Okazaki K, Kawa S, Kamisawa T, Ito T, Inui K, Irie H, Irisawa A, Kubo K, Notohara K, Hasebe O. Japanese clinical guidelines for autoimmune pancreatitis. Pancreas. 2009;38:849-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 2. | Chari ST, Takahashi N, Levy MJ, Smyrk TC, Clain JE, Pearson RK, Petersen BT, Topazian MA, Vege SS. A diagnostic strategy to distinguish autoimmune pancreatitis from pancreatic cancer. Clin Gastroenterol Hepatol. 2009;7:1097-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 224] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 3. | Kamisawa T, Takuma K, Egawa N, Tsuruta K, Sasaki T. Autoimmune pancreatitis and IgG4-related sclerosing disease. Nat Rev Gastroenterol Hepatol. 2010;7:401-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 148] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 4. | Otsuki M, Chung JB, Okazaki K, Kim MH, Kamisawa T, Kawa S, Park SW, Shimosegawa T, Lee K, Ito T. Asian diagnostic criteria for autoimmune pancreatitis: consensus of the Japan-Korea Symposium on Autoimmune Pancreatitis. J Gastroenterol. 2008;43:403-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 310] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 5. | Kawaguchi K, Koike M, Tsuruta K, Okamoto A, Tabata I, Fujita N. Lymphoplasmacytic sclerosing pancreatitis with cholangitis: a variant of primary sclerosing cholangitis extensively involving pancreas. Hum Pathol. 1991;22:387-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 413] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 6. | Notohara K, Burgart LJ, Yadav D, Chari S, Smyrk TC. Idiopathic chronic pancreatitis with periductal lymphoplasmacytic infiltration: clinicopathologic features of 35 cases. Am J Surg Pathol. 2003;27:1119-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 382] [Article Influence: 17.4] [Reference Citation Analysis (1)] |
| 7. | Zamboni G, Lüttges J, Capelli P, Frulloni L, Cavallini G, Pederzoli P, Leins A, Longnecker D, Klöppel G. Histopathological features of diagnostic and clinical relevance in autoimmune pancreatitis: a study on 53 resection specimens and 9 biopsy specimens. Virchows Arch. 2004;445:552-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 440] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 8. | Park DH, Kim MH, Chari ST. Recent advances in autoimmune pancreatitis. Gut. 2009;58:1680-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 9. | Sah RP, Chari ST, Pannala R, Sugumar A, Clain JE, Levy MJ, Pearson RK, Smyrk TC, Petersen BT, Topazian MD. Differences in clinical profile and relapse rate of type 1 versus type 2 autoimmune pancreatitis. Gastroenterology. 2010;139:140-148; quiz 140-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 299] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 10. | Kamisawa T, Notohara K, Shimosegawa T. Two clinicopathologic subtypes of autoimmune pancreatitis: LPSP and IDCP. Gastroenterology. 2010;139:22-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Chari ST, Kloeppel G, Zhang L, Notohara K, Lerch MM, Shimosegawa T. Histopathologic and clinical subtypes of autoimmune pancreatitis: the honolulu consensus document. Pancreatology. 2010;10:664-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Irie H, Honda H, Baba S, Kuroiwa T, Yoshimitsu K, Tajima T, Jimi M, Sumii T, Masuda K. Autoimmune pancreatitis: CT and MR characteristics. AJR Am J Roentgenol. 1998;170:1323-1327. [PubMed] |
| 13. | Wakabayashi T, Kawaura Y, Satomura Y, Fujii T, Motoo Y, Okai T, Sawabu N. Clinical study of chronic pancreatitis with focal irregular narrowing of the main pancreatic duct and mass formation: comparison with chronic pancreatitis showing diffuse irregular narrowing of the main pancreatic duct. Pancreas. 2002;25:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Frulloni L, Scattolini C, Falconi M, Zamboni G, Capelli P, Manfredi R, Graziani R, D'Onofrio M, Katsotourchi AM, Amodio A. Autoimmune pancreatitis: differences between the focal and diffuse forms in 87 patients. Am J Gastroenterol. 2009;104:2288-2294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 15. | American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33 Suppl 1:S62-S69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3654] [Cited by in RCA: 4292] [Article Influence: 286.1] [Reference Citation Analysis (0)] |
| 16. | Ghazale A, Chari ST, Zhang L, Smyrk TC, Takahashi N, Levy MJ, Topazian MD, Clain JE, Pearson RK, Petersen BT. Immunoglobulin G4-associated cholangitis: clinical profile and response to therapy. Gastroenterology. 2008;134:706-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 667] [Cited by in RCA: 585] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 17. | Hirano K, Tada M, Isayama H, Yamamoto K, Mizuno S, Yagioka H, Yashima Y, Sasaki T, Kogure H, Togawa O. Endoscopic evaluation of factors contributing to intrapancreatic biliary stricture in autoimmune pancreatitis. Gastrointest Endosc. 2010;71:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Horiuchi A, Kawa S, Akamatsu T, Aoki Y, Mukawa K, Furuya N, Ochi Y, Kiyosawa K. Characteristic pancreatic duct appearance in autoimmune chronic pancreatitis: a case report and review of the Japanese literature. Am J Gastroenterol. 1998;93:260-263. [PubMed] [DOI] [Full Text] |
| 19. | Koga Y, Yamaguchi K, Sugitani A, Chijiiwa K, Tanaka M. Autoimmune pancreatitis starting as a localized form. J Gastroenterol. 2002;37:133-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Wakabayashi T, Kawaura Y, Satomura Y, Watanabe H, Motoo Y, Sawabu N. Long-term prognosis of duct-narrowing chronic pancreatitis: strategy for steroid treatment. Pancreas. 2005;30:31-39. [PubMed] |
| 21. | Motoo Y, Minamoto T, Watanabe H, Sakai J, Okai T, Sawabu N. Sclerosing pancreatitis showing rapidly progressive changes with recurrent mass formation. Int J Pancreatol. 1997;21:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Blank A, Maybody M, Isom-Batz G, Roslin M, Dillon EH. Necrotizing acute pancreatitis induced by Salmonella typhimurium. Dig Dis Sci. 2003;48:1472-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 62] [Reference Citation Analysis (1)] |