Published online May 7, 2012. doi: 10.3748/wjg.v18.i17.2084
Revised: June 25, 2011
Accepted: August 15, 2011
Published online: May 7, 2012
AIM: To compare rifaximin and insulin-like growth factor (IGF)-1 treatment of hyperammonemia and brain edema in cirrhotic rats with portal occlusion.
METHODS: Rats with CCl4-induced cirrhosis with ascites plus portal vein occlusion and controls were randomized into six groups: Cirrhosis; Cirrhosis + IGF-1; Cirrhosis + rifaximin; Controls; Controls + IGF-1; and Controls + rifaximin. An oral glutamine-challenge test was performed, and plasma and cerebral ammonia, glucose, bilirubin, transaminases, endotoxemia, brain water content and ileocecal cultures were measured and liver histology was assessed.
RESULTS: Rifaximin treatment significantly reduced bacterial overgrowth and endotoxemia compared with cirrhosis groups, and improved some liver function parameters (bilirubin, alanine aminotransferase and aspartate aminotransferase). These effects were associated with a significant reduction in cerebral water content. Blood and cerebral ammonia levels, and area-under-the-curve values for oral glutamine-challenge tests were similar in rifaximin-treated cirrhotic rats and control group animals. By contrast, IGF-1 administration failed to improve most alterations observed in cirrhosis.
CONCLUSION: By reducing gut bacterial overgrowth, only rifaximin was capable of normalizing plasma and brain ammonia and thereby abolishing low-grade brain edema, alterations associated with hepatic encephalopathy.
- Citation: Òdena G, Miquel M, Serafín A, Galan A, Morillas R, Planas R, Bartolí R. Rifaximin, but not growth factor 1, reduces brain edema in cirrhotic rats. World J Gastroenterol 2012; 18(17): 2084-2091
- URL: https://www.wjgnet.com/1007-9327/full/v18/i17/2084.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i17.2084
Hepatic encephalopathy (HE) is a complication of advanced hepatic insufficiency characterized by a wide range of neurological and neuropsychiatric symptoms, ranging from subclinical manifestations to hepatic coma[1]. When cirrhotic patients develop HE, their survival prognosis considerably worsens[2], and liver transplantation has to be considered[3].
It is well known that high plasma ammonia levels play a central role in the multifactorial network of mechanisms leading to HE[4-6]. In fact, ammonia reaches the liver via the portal vein from the intestine as a result of bacterial degradation of nitrogenous compounds and as a consequence of the metabolism of glutamine by the enzyme glutaminase[7]. In addition, urea cycle activity in cirrhotic patients is decreased due to the reduction of liver cell mass[8]. Both the presence of portosystemic shunts and the loss of parenchymal cells in the liver of cirrhotic patients lead to an increase in plasma ammonia levels and are key factors in the development of HE in these patients[9]. A traditional therapeutic approach to HE is to decrease plasma ammonia levels by decreasing ammoniagenic substrates, as well as inhibiting ammonia generation, reducing its intestinal absorption, and facilitating its elimination[10].
Non-absorbable antibiotics and/or non-absorbable disaccharides have been used as a standard treatment of HE in human cirrhosis[10-14]. Recent studies have demonstrated that rifaximin reduces the risk of hospitalization involving HE without producing side effects[11,15]. Rifaximin is a non-absorbable rifamycin derivative with activity against aerobic and anaerobic microorganisms, which are an important source of ammonia[7,11,16]. Furthermore, rifaximin is not absorbed by the gut, thereby allowing the antibiotic to reach high concentrations in the intestinal tract and to remain in the feces in its active form[10,17].
Insulin-like growth factor (IGF)-1 is a powerful anabolic hormone that exerts anabolic and trophic effects in many tissues, acting in an endocrine, paracrine and autocrine manner[18]. Levels of IGF-1 are markedly decreased in liver cirrhosis. Several studies have shown that the administration of low doses of IGF-1 (i.e., 4 μg/100 g body weight per day) reduces liver fibrosis, improves liver function, increases intestinal absorption of nutrients and corrects osteopenia and hypogonadism in experimental liver cirrhosis[19-22]. Previous work from our laboratory has demonstrated that IGF-1 therapy enhances intestinal barrier function, and reduces endotoxemia and bacterial translocation in cirrhotic rats[23]. Most of these alterations are considered to be precipitating factors leading to HE, therefore, the administration of IGF-1 could be a novel therapeutic approach for this condition.
The aim of this study was to compare the efficacy of rifaximin and IGF-1 in the treatment of HE using a combined model of intrahepatic hypertension (CCl4-induced cirrhosis plus ascites) and extrahepatic hypertension generated through portal vein occlusion - a proven new animal model of hyperammonemia and brain edema related to decompensated advanced liver cirrhosis, recently described in our laboratory[24], which exhibits most of the alterations present in type C HE.
Male Sprague-Dawley OFA rats weighing about 100 g were included in the study. All animals were caged individually at a constant room temperature of 21 °C, exposed to a 12/12-h light/dark cycle and provided free access to a standard rodent chow (A04; Harlan Ibérica S.A, Barcelona, Spain). Rats received 1.5 mmol/L phenobarbital, an inducer of cytochrome P450 enzymatic activity, in their drinking water. The study was conducted according to guidelines established by the Guide for the Care and Use of Laboratory Animals and was approved by the Ethical and Research Committee of our research institute.
Six groups of rats were studied. (1) Cirrhosis (group 1; n = 9): rats with CCl4-induced liver cirrhosis with ascites plus portal vein occlusion treated with placebo (saline); (2) Control (group 2; n = 10): sham-operated control rats treated with placebo; (3) Cirrhosis + IGF-1 (group 3; n = 9): rats with CCl4-induced liver cirrhosis with ascites plus portal vein occlusion treated with IGF-1 (2 μg/100 g s.c. twice daily for 14 d ); (4) Control + IGF-1 (group 4; n = 9): sham-operated control rats treated with IGF-1 (2 μg/100 g s.c. twice daily for 14 d); (5) Cirrhosis + R (group 5; n = 9): rats with CCl4-induced liver cirrhosis with ascites plus portal vein occlusion treated with rifaximin (50 mg/kg daily by gavage for 14 d); and (6) Control + R (group 6; n = 9): sham-operated control rats treated with rifaximin (50 mg/kg daily for 14 d).
Ascitic cirrhotic rats with portal occlusion were assessed as previously described[24]. Briefly, when animals reached a body weight of 200 g, cirrhosis was induced by intragastric administration of CCl4 through an orogastric stainless steel tube (Poper and Sons, New Hyde Park, NY, United States). The initial dose was 20 μL, and subsequent doses were adjusted based on changes in body weight[25]. Six weeks after starting cirrhosis induction, animals underwent partial portal vein occlusion (> 0.9 mm portal diameter) achieved by ligating around a 20 G needle, followed by complete portal vein occlusion 48 h later[26]. Surgical procedures were performed under strict aseptic conditions; animals were anesthetized for surgery using ketamine, diazepam and atropine, and were subsequently administered 30 μg (s.c.) buprenorphine (Buprex; Schering-Plough, Madrid, Spain) for 3 d. Cirrhosis induction was continued (CCl4 administration) until ascites developed. When ascites was diagnosed (by abdominal paracentesis), animals were randomized to receive the corresponding treatment (placebo, rifaximin or IGF-1) for 14 d. Control rats were subjected to sham operation and were also randomized in parallel. Twelve hours after finishing treatment, rats underwent an oral glutamine-challenge test, and immediately afterward were sacrificed by bilateral thoracotomy. Peripheral and portal blood, cecum fecal content, and solid tissue (brain and liver) were obtained.
A load of 100 mg/kg of L-glutamine (SHS S.A., Barcelona, Spain) was administered through an orogastric stainless steel tube. Venous blood samples (150 μL) from the femoral vein were drawn preload (baseline) and every 30 min for 4 h for ammonia determination. Body temperature was monitored and maintained between 36 °C and 38 °C using an infrared lamp. Samples were centrifuged in situ for 10 min at 2000 ×g, and plasma was stored at -80 °C until analysis. The area under the curve (AUC) of the ammonemia response was also calculated using Graph Pad Prism for Windows version 5.01 (La Jolla CA, United States).
Blood samples were obtained during sacrifice. Biochemical determinations [aspartate aminotransferase (AST), alanine aminotransferase (ALT), bilirubin, and glucose] were made using an autoanalyzer (Dimension Clinical Chemistry System, Dade Behring-Siemens, Madrid, Spain).
Endotoxemia was quantified in all groups of rats using a Limulus amebocyte lysate kinetic test (Endosafe Charles River, L’Abresle Cedex, France). Briefly, plasma samples were diluted 1:5 with endotoxin-free water and then heated to 70 °C for 5 min. Afterwards, samples were further diluted (final dilution, 1:50) and assessed.
During the course of laparotomy and after harvesting all other samples, the cecal region was identified and 1 mL of content was obtained by cecal puncture. Cecal bacterial content was measured by culturing serially diluted samples (1/8000 and 1/160 000) on non-selective blood agar plates. All samples were cultured in triplicate. After an incubation period of 48 h, the number of colony-forming units (CFU) was counted. The composition of isolated flora was determined using standard bacteriological identification techniques. The results were expressed as CFU/mL of cecal content. Cecal bacterial overgrowth was defined as a stool bacterial count greater than the mean of healthy control rats plus two standard deviations[27].
Ammonia was measured in plasma and cerebral cortex. Briefly, blood (150 μL) was drawn from the femoral vein and centrifuged in heparinized tubes. The resulting plasma samples were stored at -80 °C until analysis. Brain samples were weighed, homogenized, and deproteinized by adding five volumes of cold perchloric acid (6%) and then centrifugation at 12 000 ×g for 20 min. After neutralization with KHCO3 (25% w/v), samples were stored at -80 °C until analysis, which was performed using a commercial enzymatic Ammonia Assay Kit (Sigma-Aldrich, Madrid, Spain).
Low-grade brain edema was measured as brain water content. Briefly, a frontal left hemisphere brain sample from each rat was excised, weighed, and heated to 90 °C for 48 h in a drying oven to evaporate all water content. Then, dried samples were weighed again. The difference between initial and final weight was considered as the water content[28].
Liver samples for histological examination were collected in 4% formaldehyde, subsequently embedded in paraffin wax, sliced into 5-μm sections, and stained with hematoxylin and eosin. Liver samples were evaluated using the Scheuer scoring system[29].
Unless otherwise indicated, results are expressed as mean ± SE or proportions, as appropriate. Comparisons of means among groups were performed using one-way analysis of variance or corresponding non-parametric (Kruskal-Wallis) tests; post hoc comparisons to identify pairs of groups significantly different at the 0.05 level were made using the Duncan test or the Mann-Whitney U test, respectively. Differences in proportions among groups were compared using the χ2 test. Statistical analysis were performed with SPSS for Windows version 13.0 (Chicago, IL, United States).
No differences in any of the parameters studied, except for fecal bacterial count (as expected), were observed among the three sham-operated control groups. In contrast, all parameters were significantly altered in cirrhosis plus portal vein occlusion groups compared to control groups.
Body weight at sacrifice was similar in cirrhotic groups and was significantly lower than in controls (overall P = 0.017). No differences in the time elapsed between the first CCl4 dose and ascites development were observed among the groups (range: 8-15 wk). None of the ascitic rats showed any signs of infection or sepsis.
Liver function and liver damage parameters are summarized in Table 1. Liver cirrhosis plus portal vein occlusion resulted in a significant increase in serum AST, ALT and bilirubin, and a decrease in serum glucose concentrations. However, in rifaximin-treated cirrhotic rats, these alterations tended to be less marked. In fact, no differences in bilirubin or transaminases were observed in this group compared to controls. By contrast, all of these biochemical parameters remained significantly altered in the IGF-1-treated group compared to control groups, indicating that IGF-1 treatment was unable to improve liver function.
| Endotoxin (EU) | Glucose (mmol/L) | Bilirubin (μmol/L) | ALT (UI/L) | AST (UI/L) | |
| Cirrhosis | 0.582 ± 0.069a | 9.41 ± 2.17a | 22.0 ± 6.71ac | 181.2 ± 16.1a | 333.2 ± 84.2a |
| Controls | 0.374 ± 0.037 | 27.63 ± 2.39 | 2.9 ± 0.15 | 68.9 ± 9.9 | 228.3 ± 24.6 |
| Cirrhosis + IGF-1 | 0.432 ± 0.033 | 9.43 ± 0.70a | 10.2 ± 2.96a | 210.3 ± 58.6a | 482.3 ± 121.8a |
| Controls + IGF-1 | 0.363 ± 0.032 | 25.17 ± 2.42 | 3.2 ± 0.10 | 94.4 ± 22.1 | 179.2 ± 30.1 |
| Cirrhosis + R | 0.410 ± 0.041 | 17.75 ± 3.06e | 5.0 ± 1.23 | 121.4 ± 18.4 | 262.1 ± 49.1 |
| Controls + R | 0.368 ± 0.027 | 29.39 ± 2.08 | 3.1 ± 0.20 | 97.7 ± 23.4 | 236.2 ± 43.2 |
Portal blood endotoxin levels were significantly increased only in placebo-treated cirrhotic rats (0.582 ± 0.069 vs 0.374 ± 0.037; P = 0.044). By contrast, both IGF-1 and rifaximin treatments normalized endotoxemia levels, producing similar values relative to their respective controls (Table 1).
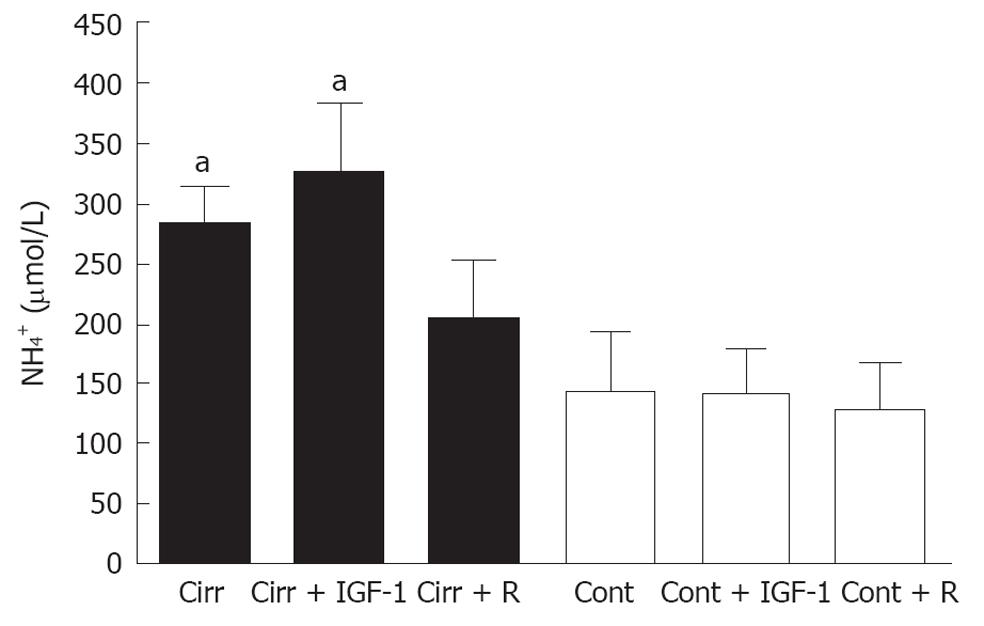
Liver cirrhosis plus portal vein occlusion resulted in hyperammonemia. Blood ammonia levels were increased in placebo-treated cirrhotic rats compared to placebo-treated controls (284 ± 29 μmol/L vs 144 ± 47 μmol/L; P = 0.007). Rifaximin treatment improved ammonemia in cirrhotic rats, reducing ammonia to levels similar to those observed in rifaximin-treated controls (205 ± 47 μmol/L vs 128 ± 37 μmol/L; P = 0.122). By contrast, IFG-1 treatment failed to reduce plasma ammonia levels, which remained significantly increased compared to those observed in controls (323 ± 58 μmol/L vs 142 ± 35 μmol/L; P = 0.004; Figure 1).
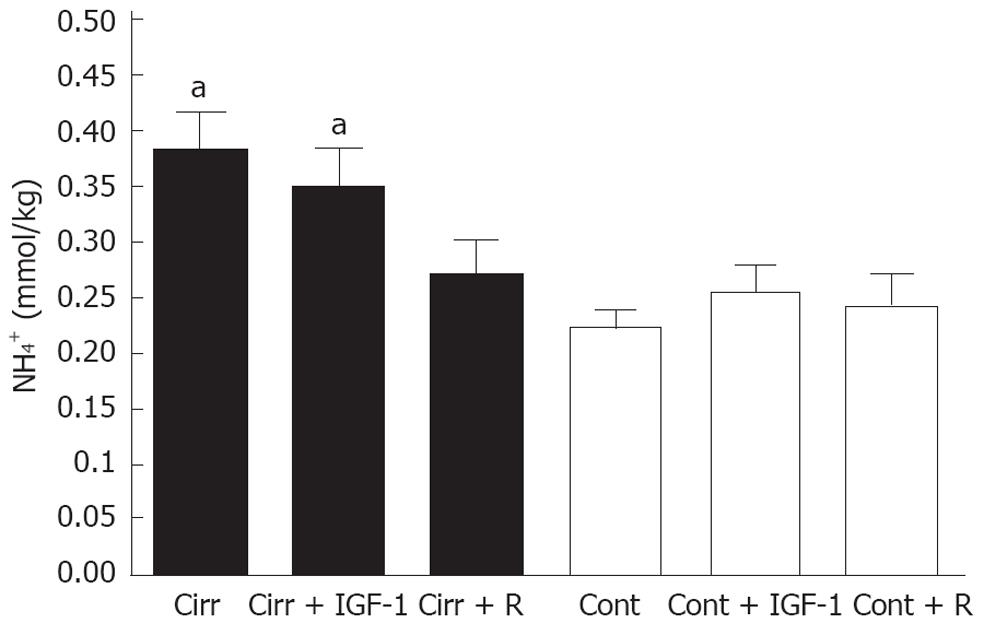
Similarly to ammonemia, brain ammonia levels were significantly higher in placebo-treated cirrhotic rats than in placebo-treated controls (0.38 ± 0.03 mmol/kg vs 0.22 ± 0.01 mmol/kg; P = 0.006). Rifaximin treatment normalized brain ammonia levels in cirrhosis, yielding values similar to those observed in rifaximin-treated control rats (0.27 ± 0.03 mmol/kg vs 0.24 ± 0.03 mmol/kg; P = 0.429). Again, IGF-1 treatment was ineffective; brain ammonia levels in IGF-1-treated cirrhotic rats were significantly higher than those in controls (0.35 ± 0.04 mmol/kg vs 0.25 ± 0.02 mmol/kg; P = 0.039; Figure 2).
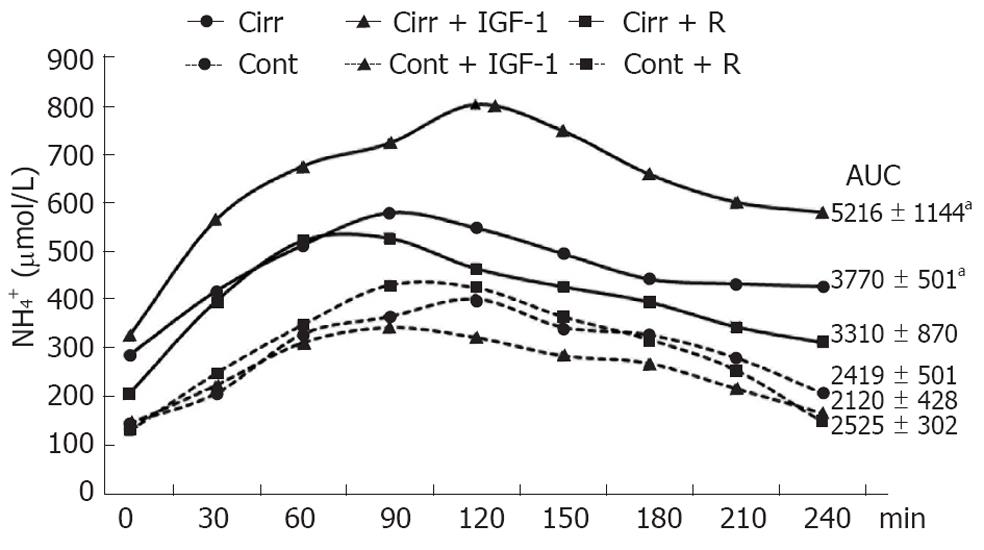
Further analysis of these results (Figure 3) showed that AUCs after oral glutamine-challenge tests were significantly increased in placebo and IGF-1 treatments in cirrhotic rats compared with each control group (3770 ± 501 vs 2419 ± 501; P = 0.043 and 5216 ± 1144 vs 2120 ± 428; P = 0.021). By contrast, the AUC in rifaximin-treated cirrhotic rats was similar to that observed in controls (3310 ± 870 vs 2525 ± 302; P = 0.240).
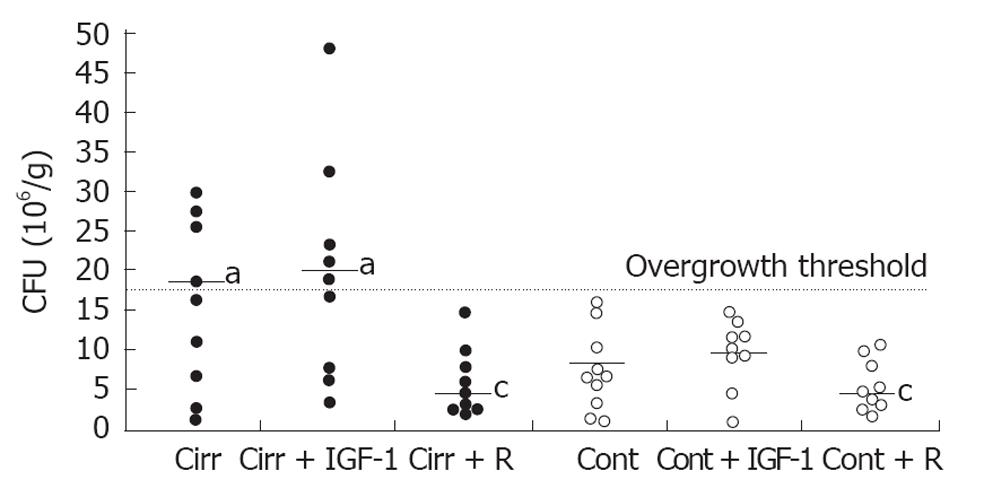
Cecal bacterial content was significantly increased in both the placebo-treated cirrhosis group and cirrhotic rats treated with IGF-1 compared with their respective controls (18.6 ± 3.6 × 106 CFU/mL vs 8.4 ± 1.3 × 106 CFU/mL; P = 0.042 and 20.4 ± 4.6 × 106 CFU/mL vs 8.9 ± 1.4 × 106 CFU/mL; P = 0.047). Only rifaximin treatment reduced bacterial content in cirrhotic rats; the values in rifaximin-treated cirrhotic rats were similar to those observed in the rifaximin-treated control group (4.4 ± 1.4 × 106 CFU/mL vs 4.4 ± 1.3 × 106 CFU/mL; P = 0.931), and were significantly lower than those in the placebo-treated cirrhosis group (P = 0.003) and cirrhosis + IGF-1 group (P = 0.002). Moreover, as shown in Figure 4, rifaximin eliminated bacterial overgrowth (threshold value, 17.64 × 106 CFU/mL) in cirrhotic rats, whereas almost 50% of rats in the placebo-treated cirrhosis group (4/9 rats) and cirrhosis + IGF-1 group (5/9 rats) showed bacterial overgrowth (overall P < 0.05 vs each control and cirrhosis + rifaximin groups).
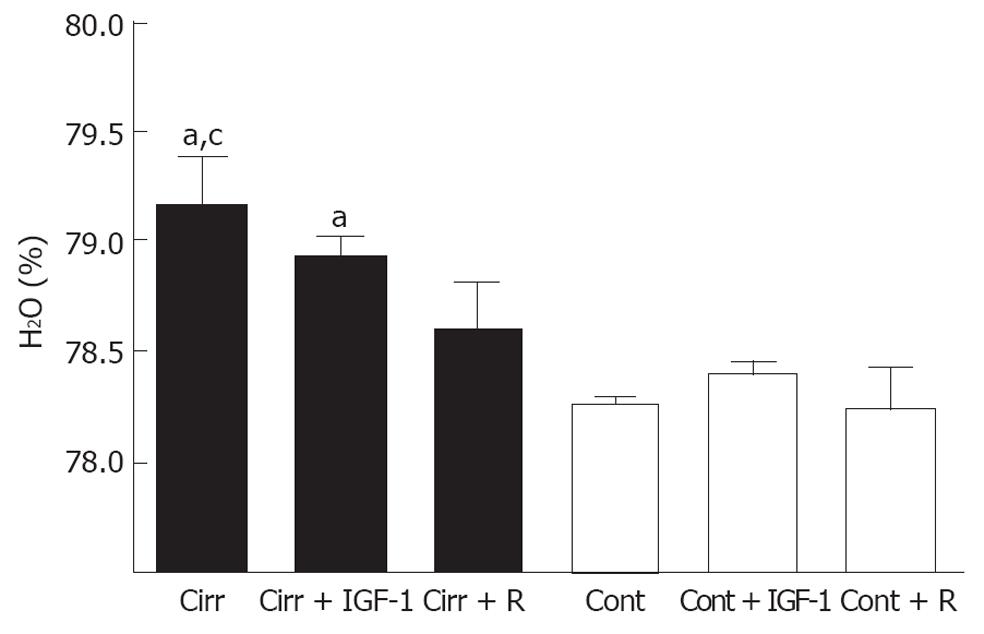
Liver cirrhosis plus portal vein occlusion resulted in a significant increase in brain water content compared to placebo-treated controls (79.17% ± 0.22% vs 78.26% ± 0.04%; P = 0.002). Rifaximin treatment was accompanied by a significant reduction in low-grade brain edema, as demonstrated by the fact that brain water content in this group of cirrhotic rats was similar to that measured in rifaximin-treated control rats (78.61% ± 0.31% vs 78.24% ± 0.19%; P = 0.233) and significantly lower than that observed in placebo-treated cirrhotic rats (P = 0.046). IGF-1 treatment did not diminish brain edema; brain water content in the cirrhosis + IGF-1 group was similar to that observed in the placebo-treated cirrhosis group (P = 0.566) and was significantly higher than that observed in the control + IGF-1 group (78.94% ± 0.08% vs 78.34% ± 0.19%; P = 0.009; Figure 5).
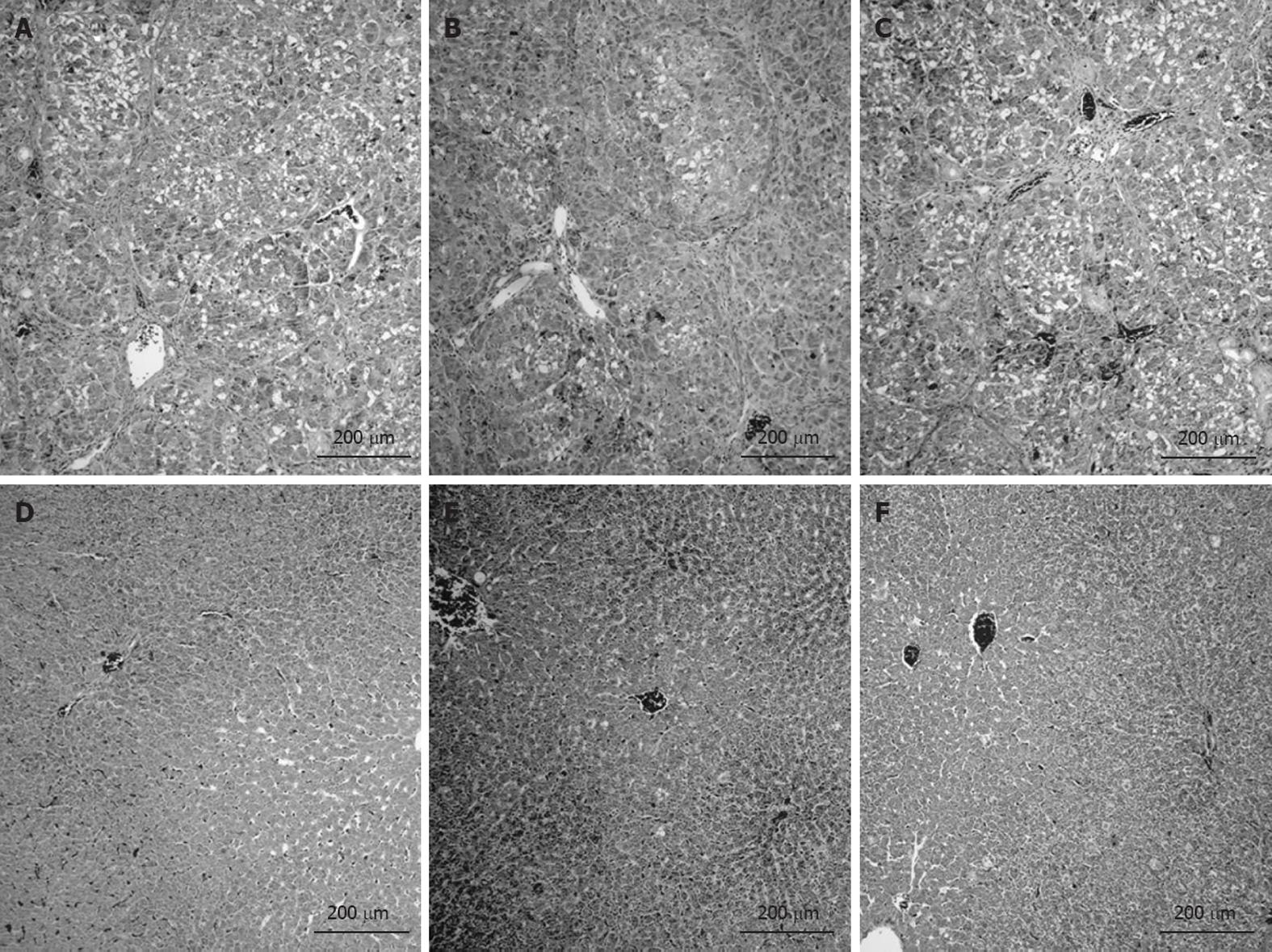
All ascitic cirrhotic rats with portal vein occlusion developed liver cirrhosis with regeneration nodules, necrosis, and steatosis regardless of treatment received. All CCl4-treated animals scored F4 with the Scheuer system. As expected, all control groups showed normal hepatic histology (Figure 6).
In this study, we demonstrated the effectiveness of rifaximin in normalizing both ammonemia and brain ammonia levels, and consequently averting the appearance of low-grade brain edema in ascitic cirrhotic rats with portal vein occlusion; an experimental model of hyperammonemia related to decompensated advanced cirrhosis. These alterations play a central role in the multifactorial mechanisms leading to HE as a result of chronic liver disease.
Although administration of low doses of IGF-1 has been proposed as a promising therapy for cirrhotic patients on the basis of preclinical data showing that this hormone displays hepatoprotective and antifibrogenic activities[7,9,21], we observed virtually no positive effect of IGF-1 on most of the alterations that lead to HE in this experimental model.
Given that the major precipitating factor leading to HE in cirrhosis is the presence of large amounts of ammonia, not only in the bloodstream but especially in the brain, and further considering that the main source of this ammonia is production by enteric bacteria, the two key factors that warrant particular attention are: (1) Deranged function of the liver, which introduces ammonia into the urea cycle and is the main ammonia-detoxifying organ; and (2) The presence of bacterial overgrowth related to disturbed intestinal transit.
In this context, rifaximin treatment was accompanied by a slight, but significant, improvement of some parameters of liver function, such as glucose, bilirubin and ALT. This improvement cannot be mainly attributed to a decrease in endotoxin levels, which is known to promote activation and release of proinflammatory cytokines such as tumor necrosis factor (TNF)-α[30,31], because these parameters were also diminished in the IGF-1 treated group (group 3) without producing any positive effect on liver function.
Notwithstanding these observations, a recent study has observed a direct effect of norfloxacin, another “non-absorbable” antibiotic widely used for selective intestinal bacterial decontamination, in cirrhotic patients. Norfloxacin actively accumulates in polymorphonuclear cells, leading to a decrease in plasma TNF-α and interferon-γ levels, and a reduction in oxidative stress[32]. Although these mechanisms were not explored in the present study, we cannot rule out the possibility that a similar action of rifaximin could explain our results. Further studies are needed to examine this possibility.
However, we did not observe the hepatoprotective effects of IGF-1 administration in experimental cirrhosis that have been reported by others[20]. In our study, hepatic function (glucose, bilirubin, AST, ALT) in IGF-1-treated cirrhotic rats was similar to that observed in untreated cirrhotic rats. We attribute these differences to the fact that, in our study, all animals presented with well-established cirrhosis plus ascitic decompensation.
As mentioned previously, cirrhotic patients present several alterations in gut motility that could lead to an increase in gut bacterial content[33]. A close cause and effect relationship between bacterial overgrowth and plasma ammonia levels has been reported, reflecting the fact that enteric bacterial fermentation is the main source of ammonia. In our study, cirrhotic groups treated with placebo or IGF-1 (groups 1 and 2) showed a significant increase in cecal bacterial content compared with control groups. By contrast, rifaximin treatment dramatically reduced cecal bacterial content, not only in cirrhotic rats but also in control rats (groups 3 and 6), as reported in other studies[34,35]. As a consequence of this reduction in bacterial content, plasma ammonia levels in rifaximin-treated cirrhotic rats (group 3) remained similar to those observed in controls (groups 4-6). Similarly, brain ammonia levels were normalized in rifaximin-treated rats. In keeping with this, low-grade brain edema was absent in this group of rats. Again, consistent with its inability to modify cecal bacterial content, IGF-1 failed to improve any of these parameters.
In conclusion, our data indicate that, by reducing gut bacterial overgrowth and improving liver function, rifaximin may be useful in the treatment of most alterations associated with HE in experimental cirrhosis, whereas the administration of low doses of IGF-1 is not indicated in this condition.
Hepatic encephalopathy (HE) in cirrhosis appears as a consequence of hepatic failure and/or the presence of portosystemic shunting that leads to the passage of nitrogenate compounds such as ammonia from the gut to the systemic circulation, which in turn, lead to an increase of brain water content. Non-absorbable antibiotics and/or non-absorbable disaccharides have been used as a standard treatment of HE in human cirrhosis.
Rifaximin has been reported to be useful for its activity against aerobic and anaerobic microorganisms, which are an important source of ammonia. However, no data regarding the effect of rifaximin on low-grade brain edema in experimental HE in cirrhotic rats has been reported. Also, insulin-like growth factor (IGF)-1 could be useful for the treatment of that pathology for its antifibrogenic and anabolic effects.
For the first time, authors have demonstrated in an experimental model of hyperammonemia related to decompensated cirrhosis that the effectiveness of rifaximin for the treatment of HE is mainly due to its efficacy in reducing low-grade brain edema. On the contrary, low doses of IGF-1 have no effects in preventing liver damage or hyperammonemia.
By understanding unequivocally that rifaximin is a useful therapy in the treatment of hyperammonemia and most of the alterations associated with HE in cirrhosis. The data suggest that the use of non-absorbable antibiotics could be a good therapeutic strategy.
Rifaximin is a non-absorbable rifamycin derivative, with activity against enteric aerobic and anaerobic microorganisms. IGF-1 is a powerful anabolic hormone with diverse endocrine, paracrine and autocrine effects. In cirrhosis, the reduction of functional hepatocellular mass causes a marked fall in IGF-1 serum levels.
This is an experimental study comparing the efficacy of rifaximin and IGF-1 in the treatment of some of the alterations observed in HE such as hyperammonemia and low-grade brain edema. The results showed that only rifaximin, by abolishing bacterial overgrowth, is capable of reducing hyperammonemia and low-grade brain edema in cirrhotic rats. By contrast, low doses of IGF-1 are not indicated for this pathology.
Peer reviewers: Sanaa Ahmed Ali, Assistant Professor, Department of Theraputic Chemistry, National Research Centre, El Behooth St., Cairo 12622, Egypt; Dr. Lisheng Zhang, Beckman Research Institute, City of Hope/National Medical Center, 1500 E, Duarte, CA 91010, United States
S- Editor Lv S L- Editor Kerr C E- Editor Li JY
| 1. | Butterworth RF. Pathogenesis of hepatic encephalopathy: new insights from neuroimaging and molecular studies. J Hepatol. 2003;39:278-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Córdoba J, Mínguez B. Hepatic encephalopathy. Semin Liver Dis. 2008;28:70-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 3. | O'Leary JG, Lepe R, Davis GL. Indications for liver transplantation. Gastroenterology. 2008;134:1764-1776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 134] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 4. | Felipo V, Butterworth RF. Neurobiology of ammonia. Prog Neurobiol. 2002;67:259-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 454] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 5. | Olde Damink SW, Jalan R, Redhead DN, Hayes PC, Deutz NE, Soeters PB. Interorgan ammonia and amino acid metabolism in metabolically stable patients with cirrhosis and a TIPSS. Hepatology. 2002;36:1163-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 161] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Butterworth RF, Giguère JF, Michaud J, Lavoie J, Layrargues GP. Ammonia: key factor in the pathogenesis of hepatic encephalopathy. Neurochem Pathol. 1987;6:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 289] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 7. | Hoover WW, Gerlach EH, Hoban DJ, Eliopoulos GM, Pfaller MA, Jones RN. Antimicrobial activity and spectrum of rifaximin, a new topical rifamycin derivative. Diagn Microbiol Infect Dis. 1993;16:111-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Bachmann C. Mechanisms of hyperammonemia. Clin Chem Lab Med. 2002;40:653-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 101] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Romero-Gómez M, Jover M, Galán JJ, Ruiz A. Gut ammonia production and its modulation. Metab Brain Dis. 2009;24:147-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Mas A, Rodés J, Sunyer L, Rodrigo L, Planas R, Vargas V, Castells L, Rodríguez-Martínez D, Fernández-Rodríguez C, Coll I. Comparison of rifaximin and lactitol in the treatment of acute hepatic encephalopathy: results of a randomized, double-blind, double-dummy, controlled clinical trial. J Hepatol. 2003;38:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 152] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 11. | Neff GW, Kemmer N, Zacharias VC, Kaiser T, Duncan C, McHenry R, Jonas M, Novick D, Williamson C, Hess K. Analysis of hospitalizations comparing rifaximin versus lactulose in the management of hepatic encephalopathy. Transplant Proc. 2006;38:3552-3555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Williams R, James OF, Warnes TW, Morgan MY. Evaluation of the efficacy and safety of rifaximin in the treatment of hepatic encephalopathy: a double-blind, randomized, dose-finding multi-centre study. Eur J Gastroenterol Hepatol. 2000;12:203-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Bucci L, Palmieri GC. Double-blind, double-dummy comparison between treatment with rifaximin and lactulose in patients with medium to severe degree hepatic encephalopathy. Curr Med Res Opin. 1993;13:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 90] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Alcorn J. Review: rifaximin is equally or more effective than other antibiotics and lactulose for hepatic encephalopathy. ACP J Club. 2008;149:11. [PubMed] |
| 15. | Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB, Sigal S, Sheikh MY, Beavers K, Frederick T. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 868] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 16. | Venturini AP, Marchi E. In vitro and in vivo evaluation of L/105, a new topical intestinal rifamycin. Chemioterapia. 1986;5:257-262. [PubMed] |
| 17. | Descombe JJ, Dubourg D, Picard M, Palazzini E. Pharmacokinetic study of rifaximin after oral administration in healthy volunteers. Int J Clin Pharmacol Res. 1994;14:51-56. [PubMed] |
| 18. | Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3-34. [PubMed] |
| 19. | Fernández-Rodriguez CM, Prada I, Andrade A, Moreiras M, Guitián R, Aller R, Lledó JL, Cacho G, Quiroga J, Prieto J. Disturbed synthesis of insulinlike growth factor I and its binding proteins may influence renal function changes in liver cirrhosis. Dig Dis Sci. 2001;46:1313-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 62] [Reference Citation Analysis (1)] |
| 20. | Castilla-Cortazar I, Garcia M, Muguerza B, Quiroga J, Perez R, Santidrian S, Prieto J. Hepatoprotective effects of insulin-like growth factor I in rats with carbon tetrachloride-induced cirrhosis. Gastroenterology. 1997;113:1682-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 105] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Muguerza B, Castilla-Cortázar I, García M, Quiroga J, Santidrián S, Prieto J. Antifibrogenic effect in vivo of low doses of insulin-like growth factor-I in cirrhotic rats. Biochim Biophys Acta. 2001;1536:185-195. [PubMed] |
| 22. | Castilla-Cortazar I, Prieto J, Urdaneta E, Pascual M, Nuñez M, Zudaire E, Garcia M, Quiroga J, Santidrian S. Impaired intestinal sugar transport in cirrhotic rats: correction by low doses of insulin-like growth factor I. Gastroenterology. 1997;113:1180-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Lorenzo-Zúñiga V, Rodríguez-Ortigosa CM, Bartolí R, Martínez-Chantar ML, Martínez-Peralta L, Pardo A, Ojanguren I, Quiroga J, Planas R, Prieto J. Insulin-like growth factor I improves intestinal barrier function in cirrhotic rats. Gut. 2006;55:1306-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Miquel M, Bartolí R, Odena G, Serafín A, Cabré E, Galan A, Barba I, Córdoba J, Planas R. Rat CCl(4)-induced cirrhosis plus total portal vein ligation: a new model for the study of hyperammonaemia and brain oedema. Liver Int. 2010;30:979-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Runyon BA, Sugano S, Kanel G, Mellencamp MA. A rodent model of cirrhosis, ascites, and bacterial peritonitis. Gastroenterology. 1991;100:489-493. [PubMed] |
| 26. | Lebrec D. Animal models of portal hypertension. Portal hypertension. Clinical and Physiological aspects. Tokyo: Springer-Verlag 1991; 101-113. |
| 27. | Guarner C, Runyon BA, Young S, Heck M, Sheikh MY. Intestinal bacterial overgrowth and bacterial translocation in cirrhotic rats with ascites. J Hepatol. 1997;26:1372-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 165] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 28. | Vogels BA, van Steynen B, Maas MA, Jörning GG, Chamuleau RA. The effects of ammonia and portal-systemic shunting on brain metabolism, neurotransmission and intracranial hypertension in hyperammonaemia-induced encephalopathy. J Hepatol. 1997;26:387-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991;13:372-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1130] [Cited by in RCA: 1198] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 30. | Jirillo E, Caccavo D, Magrone T, Piccigallo E, Amati L, Lembo A, Kalis C, Gumenscheimer M. The role of the liver in the response to LPS: experimental and clinical findings. J Endotoxin Res. 2002;8:319-327. [PubMed] |
| 31. | Paik YH, Lee KS, Lee HJ, Yang KM, Lee SJ, Lee DK, Han KH, Chon CY, Lee SI, Moon YM. Hepatic stellate cells primed with cytokines upregulate inflammation in response to peptidoglycan or lipoteichoic acid. Lab Invest. 2006;86:676-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Zapater P, Caño R, Llanos L, Ruiz-Alcaraz AJ, Pascual S, Barquero C, Moreu R, Bellot P, Horga JF, Muñoz C. Norfloxacin modulates the inflammatory response and directly affects neutrophils in patients with decompensated cirrhosis. Gastroenterology. 2009;137:1669-1679.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Pardo A, Bartolí R, Lorenzo-Zúñiga V, Planas R, Viñado B, Riba J, Cabré E, Santos J, Luque T, Ausina V. Effect of cisapride on intestinal bacterial overgrowth and bacterial translocation in cirrhosis. Hepatology. 2000;31:858-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 123] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 34. | Miglioli PA, Allerberger F, Calabrò GB, Gaion RM. Effects of daily oral administration of rifaximin and neomycin on faecal aerobic flora in rats. Pharmacol Res. 2001;44:373-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Yang J, Lee HR, Low K, Chatterjee S, Pimentel M. Rifaximin versus other antibiotics in the primary treatment and retreatment of bacterial overgrowth in IBS. Dig Dis Sci. 2008;53:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |