Published online Mar 14, 2012. doi: 10.3748/wjg.v18.i10.1048
Revised: June 20, 2011
Accepted: June 27, 2011
Published online: March 14, 2012
AIM: To compare the influence of different transplant sites in bone marrow mesenchymal stem cell (MSC)-based therapy for liver fibrosis.
METHODS: MSCs isolated from Sprague Dawley (SD) rats were induced into hepatocyte-like cells. Liver fibrosis in SD rats was induced with carbon tetrachloride. Following hepatocyte induction in vitro, 4’,6-diamidino-2-phenylindole (DAPI)-labeled MSCs were transplanted by intravenous, intrahepatic, and intraperitoneal injection. Histopathological staining, immunohistochemistry, and biochemical analysis were used to compare the morphological and functional liver regeneration among different MSC injection modalities. The expression differences of interleukins, growth factor, extracellular matrix, matrix metalloproteinases, and tissue inhibitor of metalloproteinase were examined by real-time reverse transcription-polymerase chain reaction (RT-PCR) and enzyme linked immunosorbent assay (ELISA).
RESULTS: Four days after exposure to hepatocyte differentiation medium, MSCs that did not express hepatocyte markers could express α-fetoprotein, albumin, and cytokeratin 18. The results of histopathological staining, immunohistochemistry, and biochemical analysis indicated that intravenous injection is more effective at rescuing liver failure than other injection modalities. DAPI-labeled cells were found around liver lobules in all three injection site groups, but the intravenous group had the highest number of cells. PCR and ELISA analysis indicated that interleukin-10 (IL-10) was highest in the intravenous group, whereas il1β, il6, tnfα and tgfβ, which can be regulated by IL10 and are promoters of liver fibrosis, were significantly lower than in the other groups.
CONCLUSION: MSC administration is able to protect against liver fibrosis. Intravenous injection is the most favorable treatment modality through promotion of IL10 expression.
- Citation: Zhao W, Li JJ, Cao DY, Li X, Zhang LY, He Y, Yue SQ, Wang DS, Dou KF. Intravenous injection of mesenchymal stem cells is effective in treating liver fibrosis. World J Gastroenterol 2012; 18(10): 1048-1058
- URL: https://www.wjgnet.com/1007-9327/full/v18/i10/1048.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i10.1048
Liver injury caused by chemical damage or viral infection often leads to liver fibrosis, which can lead to an impairment of liver function that requires medical intervention[1,2]. Although liver transplantation is by far the most effective treatment for liver cirrhosis, extensive clinical application of the technique is limited by the lack of donor organ availability[3]. Thus, it is very important to investigate different treatments and therapies for cirrhosis. Cell-based hepatocyte transplantation, a potential interventional procedure, provides an effective strategy and holds great promise for the treatment of impaired livers[4]. When compared to orthotopic liver transplantation, cell transplantation has the advantages of lower cost, lower risk, and a simpler procedure[5]. A single donor could serve multiple recipients, and excess cells could be cryopreserved for future use. However, the type of hepatocyte necessary for transplantation is less common in mature liver cells because of the paucity of cadaveric livers. Furthermore, with hepatocytes cultured in vitro, it is difficult to obtain mature and intact hepatocytes[6]. Thus, it is very important to investigate alternative, appropriate therapies for liver cirrhosis.
Mesenchymal stem cells (MSCs) are adherent, fibroblast-like, pluripotent and non-hematopoietic progenitor cells. They have been found to reside in most organs and tissues investigated to date, including bone marrow, adipose, dermis, muscular tissue, hair follicles, the periodontal ligament and the placenta. Previous studies[7,8] have demonstrated that MSCs can be differentiated into osteogenic, chondrogenic, adipogenic, myogenic, cardiomyogenic, and hematopoietic potential stromal cells. Moreover, apart from their differentiative abilities, MSCs play a supportive role in organ regeneration processes. In addition, several studies[9] have suggested that the use of MSCs in vivo should be safer than that of embryonic stem cells due to their higher chromosomal stability and lower tendency to form neoplasms in the recipient host. These reports indicate that MSCs are an attractive cell source for regenerative medicine.
A large number of in vitro studies indicate that bone marrow-derived MSCs can express the liver-specific marker α-fetoprotein (AFP), cytokeratin 18 (CK18), and albumin. In addition, they can be involved in urea production, show liver-specific functions of cytochrome P450 activity, and store glycogen when co-cultured with adult liver cells or cultured in the presence of cytokines, such as fibroblast growth factor and hepatocyte growth factor[10,11]. The functional hepatocyte-like cells derived from MSCs might serve as cell sources for liver-targeted cell therapy[12].
Hematopoietic stem cells (HSCs), another type of bone marrow-derived stem cell, also have multi-potent differentiation capabilities. The transplantation of HSCs can act as a substitute for hepatocyte transplantation in a murine model of tyrosinemia, and HSC transplantation can correct this metabolic liver disease[13,14]. However, the fusion process of hematopoietic stem cells with hepatocytes and the difficulty in maintaining hematopoietic stem cells hamper their wide application to human disease treatment[15,16]. Sato et al[17] examined the ability of fractionated human bone marrow-component MSCs to differentiate into hepatocytes in vivo by directly inoculating the cells into rat livers that had sustained chronic damage from alcohol treatment. Their results indicated that MSCs had a great ability to differentiate into hepatocytes without any evidence of fusion.
Besides treating acutely damaged tissue, MSCs also have the potential to reduce chronic fibrogenesis through the modulation of inflammation, collagen deposition, and remodeling. Although numerous studies have reported that bone marrow (BM)-derived MSCs can reduce carbon tetrachloride (CCl4)-induced liver fibrosis in mice, the mechanism by which MSCs repair the fibrosis is unclear, and the results are controversial[18-23]. For therapeutic applications, it will be important to understand the potency and possible repair mechanisms of MSCs. In the present study, we aimed to find and compare the best therapeutic effects among three different protocols of MSC engraftment (intraperitoneal, intravenous and intrahepatic transplantation) to treat CCl4-induced liver injury, as well as to elucidate the mechanisms that explain the differences between the effects of the cell transplant site.
MSCs were prepared from rat bone marrow as described previously. In brief, whole BM was flushed from the tibia and femur of Sprague Dawley (SD) rats (six-week-old males); cultured in Dulbecco’s Modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum, 1% penicillin/streptomycin, 2 mmol/L L-glutamine; and purified for up to five passages. MSCs were grown to confluency before being detached by trypsin/ethylenediaminetetraacetic acid treatment. After detachment, cells were incubated with four phycoerythrin-conjugated antibodies: CD34, a hematopoietic progenitor marker; CD45, a leukocyte marker; CD90, which is also known as Thy-1; and/or CD29. Fluorescence-activated cell sorting was performed on at least 10 000 cells/sample using Cell Quest software (Beckman Coulter).
Hepatic transplantation was performed as previously described[12]. Briefly, the cultured cells were harvested from the culture bottles with 0.25 g/L trypsin. Cultured cells at passage 3 were seeded in six-well cell culture plates. When the cells grew to 70% confluence, the control group was continuously cultured in DMEM supplemented with 10 mL/L fetal bovine serum (FBS), 100 U/mL penicillin, and 100 U/mL streptomycin. The hepatocyte differentiation group was cultured in α-MEM supplemented with 10 mL/L FBS, 20 ng/mL hepatocyte growth factor (HGF), 20 ng/mL fibroblast growth factor (FGF)-4, 20 ng/mL epidermal growth factor (EGF), 100 U/mL penicillin and 100 U/mL streptomycin. In each well, 2 mL of medium was added and changed every 4 d. The medium was stored at -20 °C until the albumin, AFP and urea assays were conducted. To determine the cell phenotype, the cultured cells were stained by anti-AFP and albumin (ALB) protein monoclonal antibodies according to the manufacturer’s protocol[17].
To establish the liver-damaged rat model, 0.5 mL/kg CCl4 was injected subcutaneously into adult male SD rats (320 ± 20 g) twice a week for 4 wk. Control (“normal”) animals were injected with the same volume of normal saline. The extent of hepatic damage was evaluated by biochemical analysis of blood samples and histopathological examination of liver tissue samples taken from sacrificed rats.
For in vivo tracking of transplanted cells, MSCs from the SD rats were labeled with 4,6-diamidino-2-phenylindole (DAPI). Forty-five rats that suffered liver injury induced by CCl4 were classified into three groups: intraperitoneal transplantation, intravenous transplantation, and intrahepatic transplantation. Each group’s hepatocyte differentiated-MSCs were resuspended in 0.1 mol/L phosphate buffer solution (PBS) at a concentration of 107 cells/mL, and every rat was injected with 300 μL. Rats were sacrificed at 28 d post-implantation. At that time, liver tissues and blood were obtained for analysis.
Total RNA was prepared using the RNeasy total RNA isolation kit (Invitrogen, United States). For cDNA synthesis, random hexamer primers (Invitrogen, United States) were used to prime reverse transcriptase reactions. The cDNA synthesis was carried out using Moloney-murine leukemia virus Superscript II reverse transcriptase (TaKaRa, Japan) following the manufacturer’s instructions. For the semiquantitative polymerase chain reaction (PCR) detection of AFP, ALB, and CK18, 5 μL of cDNA-template was mixed with 2.5 µL of 10× PCR buffer, 0.5 μL of 10 mmol/L dNTPs, 0.5 µL of each primer (50 ng/μL), and 0.5 μL of polymerase (TaKaRa, Japan) in a total volume of 25 μL for each probe. PCR was performed in a programmable Biometra Uno-Thermobloc (Biometra, Germany). All samples were analyzed on 1% agarose gels. The size of the PCR fragments was estimated using a 100-base-pair ladder.
At three days post-MSC transplantation, quantitative PCR was carried out using standard protocols with the Quantitec SYBR Green PCR Kit (TaKaRa, Japan). The PCR mix contained SYBR Green Mix, 0.5 μmol/L primers (Table 1), 1 ng of DNA template and nuclease-free water to a final volume of 25 μL. PCR was performed in an ABI Prism 7000 Detection System (Applied Biosystems, United States). The percentage of gene expression was normalized as a function of GAPDH gene expression. Oligonucleotide primers for real-time PCR were obtained from TaKaRa (Japan), including matrix metalloproteinases (mmp)2, mmp9, tissue inhibitor of metalloproteinase (timp)1, hgf, pdgf, il1β, il2, il6, il10, il13, infγ, tnfα and tgfβ1. Primer sequences are shown in Table 1.
| Gene | Sense | Anti-sense |
| afp | AACAGCAGAGTGCTGC-AAAC | AGGTTTCGTCCCTCAGAAAG |
| albumin | ATACACCCAGAAAGCA-CCTC | CACGAATTGTGCGAATG |
| ck18 | GGACCTCAGCAAGATC-ATGGC | CCACGATCTTACGGGTAGT-TG |
| mmp2 | CTATTCTGTCAGCACTT-TGG | CAGACTTTGGTTCTCCAACTT |
| mmp9 | AAATGTGGGTGTACAC-AGGC | TTCACCCGGTTGTGGAAACT |
| timp1 | ATATTCTGTCTGGATCG-GC | GCTTCGTCACTCCTGTTT |
| hgf | TGGTGTTTCACAAGCAA-TCCAGA | CCGTTGCAGGTCATGCATTC |
| pdgf | GGCCTTCTTAAAGATTGGTTCT | GCCTCATAGACCGCACCAAC |
| il1β | GCTGTGGCAGCTACCTA-TGTCTTG | AGGTCGTCATCATCCCACG-AG |
| il2 | GACGCTGGAAATTTCATCAGCA | GCTCATCATCGAATTGGCAC-TC |
| il6 | CCACTTCACAAGTCGGA-GGCTTA | GTGCATCATCGCTGTTCATACAATC |
| il0 | CAGACCCACATGCTCCG-AGA | CAAGGCTTGGCAACCCAAGTA |
| il13 | AGGAGCTTATTGAGGAG-CTGAAGCA | TGGAGATGTTGGTCAGGGA-ATCCA |
| infγ | AGGCCATCAGCAACAACATAAGTG | GACAGCTTTGTGCTGGATCTGTG |
| tnfα | AACTCGAGTGACAAGC-CCGTAG | GTACCACCAGTTGGTTGTCT-TTGA |
| tgfβ1 | TGCGCCTGCAGAGATTC-AAG | AGGTAACGCCAGGAATTGT-TGCTA |
Biochemical parameters including albumin, total bilirubin in serum (TBIL), and alanine aminotransferase (ALT) were analyzed with a biochemical analyzer (Roche Integra 800, Holliston, United States).
At the time of sacrifice, liver tissue samples were collected and fixed in 3.7% formaldehyde for two days. Tissues were then dehydrated, cleared, and infiltrated with a histoprocessor for 16 h. Serial 3-μm sections were hematoxylin and eosin (HE) and Van Gieson’s (VG) stained for histopathological analysis. For HE analysis, sectioned samples were stained with hematoxylin solution (Sigma-Aldrich, Germany) for 5 min followed by eosin for 5 min. For the VG stain, the sectioned samples were placed in hematoxylin solution for 5 min following a 10-min water wash, stained in VG (Biyuntian, China) solution for 3 min, and dehydrated in succession with 85%, 95% and 100% ethanol for 3 min.
Immunohistochemical staining was performed according to a previously reported method to evaluate certain markers including alpha-smooth muscle actin (α-SMA). After paraffin removal and microwave-based antigen retrieval, the sections were treated with 0.3% H2O2 in PBS to quench endogenous peroxidase activity and then incubated with 5% goat serum to block the non-specific sites. α-SMA (1:100) mAb was applied and incubated at 4 °C overnight (PBS was used as the negative control), followed by incubation with peroxidase-conjugated AffiniPure goat anti-mouse secondary antibody (Zhongshan Goldbridge, China) at 37 °C for 30 min. The specimens were then incubated with diaminobenzidine peroxidase substrate to obtain a brown stain and then subsequently counterstained with hematoxylin.
Blood of the MSC- and CCl4-treated rats from the three different injection groups was collected at days 3, 7 and 14 post-transplantation. Serum samples were assayed for IL10 production with an IL10 enzyme linked immunosorbent assay (ELISA) Quantitation kit (Invitrogen, United States) according to the manufacturer’s recommendation.
Data are shown as the means and standard deviations. The statistical differences were analyzed using Student’s t test for normally distributed values and by the t test for non-normally distributed values. Values of P < 0.05 were considered significant. The data represent mean ± SD from at least three independent experiments.
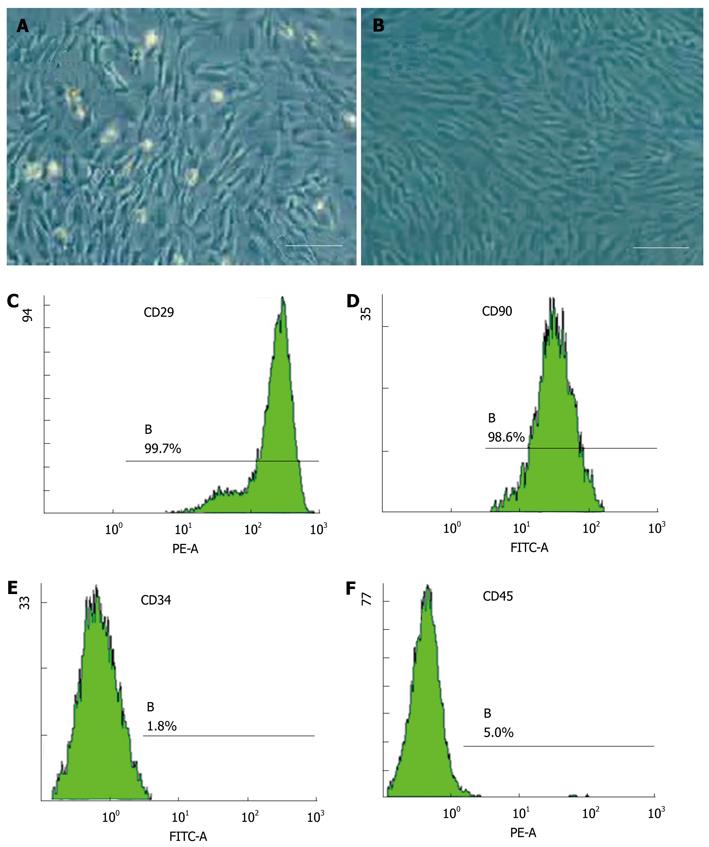
The cells were isolated by gradient density centrifugation. After approximately 3 d in culture, cells from the stromal fraction appeared as a monolayer of broad, flat cells (Figure 1A). As the cells approached 80% confluence, they differentiated into a more spindle-shaped, fibroblastic morphology (Figure 2B). More than 98% of cells expressed the MSC markers CD29 and CD90 (Figure 1C and D), and less than 5% of hematopoietic stem cells expressed leukocyte markers CD34 and CD45 (Figure 1E and F). They were easily expanded for up to 10 passages, maintaining the spindle-shaped, fibroblastic morphology and MSC markers of the undifferentiated state. Adipose-derived stem cells (ADSCs) did not spontaneously differentiate during in vitro culture.
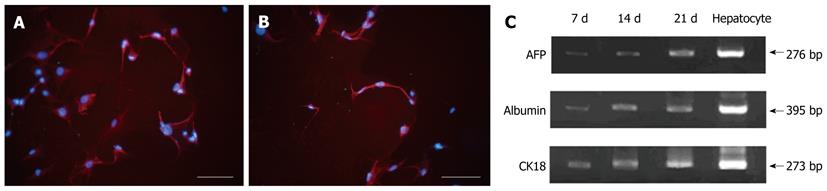
When the cultured cells reached 70% confluence, they were treated with hepatocyte differentiation medium containing 20 ng/mL HGF, 20 ng/mL FGF-4, and 20 ng/mL EGF. After 4 d of hepatocyte induction, ADSCs displayed changes in cellular morphology including shrinkage of the cytoplasm and diameter, as well as the formation of round cells. The fibroblasts, as control cells, did not exhibit any change in morphology after being treated with differentiation medium. Before induction, the MSCs did not express hepatocyte markers. Four days after exposure to differentiation medium, the MSCs stained positive for the AFP and ALB hepatocyte markers (Figure 2A and B). Hepatocyte marker expression after hepatocyte induction was further confirmed by RT-PCR. In addition, AFP, ALB and CK18 gene expression levels increased with prolonged exposure time in differentiation medium (Figure 2C). From these results, we can conclude that the MSCs differentiated into hepatocytes, and the proportion of differentiated cells account for approximately 70% of all induced cells.
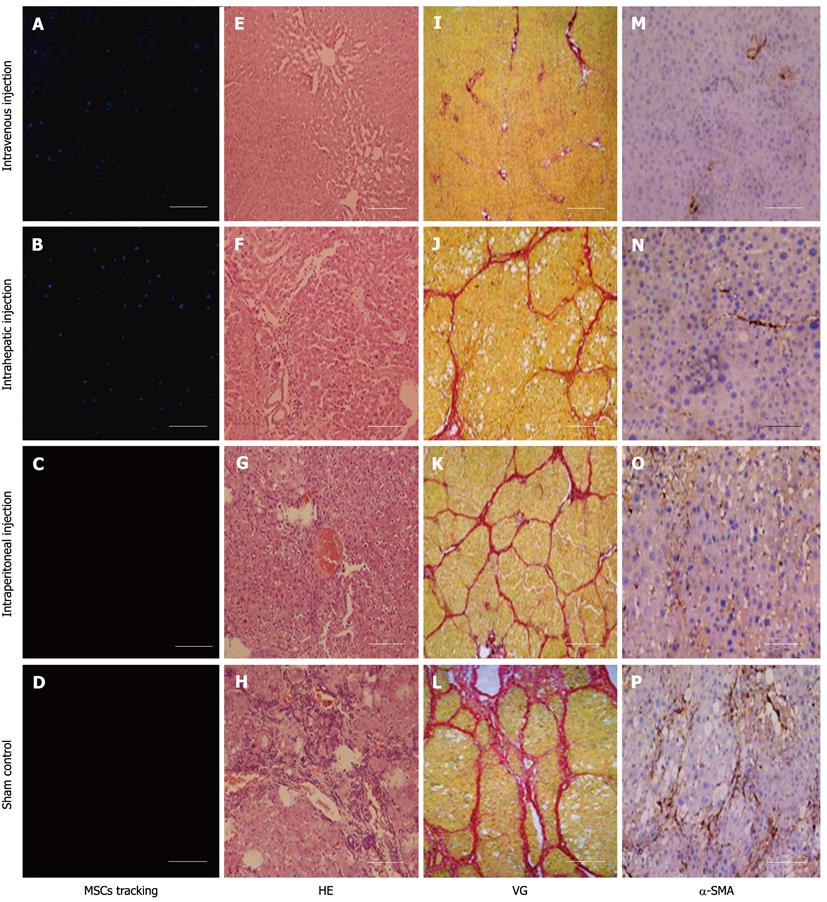
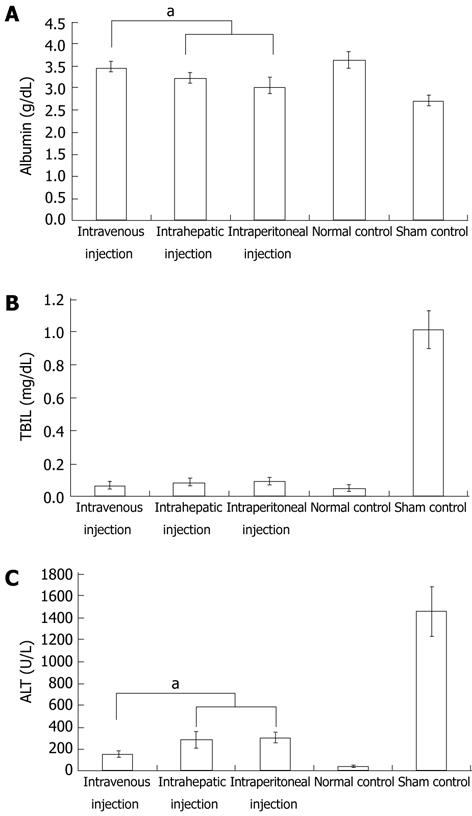
To explore cell homing, MSCs were labeled with DAPI before transplantation. Four days after transplantation, the number of surviving homing MSCs was highest in animals that received an intravenous injection. Surviving homing MSCs in animals that received an intrahepatic injection were more numerous than those observed in animals that received an intraperitoneal injection (Figure 3A-D). In addition, histological staining indicated that sham-injected, CCl4-treated rats suffered serious inflammation with non-normal liver lobules dispersed throughout the liver. The sham group also had large areas of collagen that were distributed along the edge of the liver lobules (Figure 3H and L). In the intraperitoneal and intrahepatic injection groups, the morphology of liver lobules was unclear, and collagen deposition was evident in the liver (Figure 3F, G, J and K). In the intravenous injection group, however, the liver lobules were normal (Figure 3E and I). Although there was mild inflammation in the portal or sinusoid areas, collagen accumulation was minimal in the liver. Immunohistochemistry of liver sections for α-SMA expression revealed intense staining patterns in sham- and intraperitoneal-injected mice (Figure 3O and P). However, α-SMA staining levels in intravenous- and intrahepatic-injected mice were significantly lower (Figure 3M and N). In addition, there were lower levels of α-SMA staining in intravenous-injected mice than in intrahepatic-injected mice. To further compare the functional restoration of MSCs following intraperitoneal, intravenous, and intrahepatic injection, we quantitatively analyzed the levels of AFP, albumin, and TBIL production. The serum from rats given CCl4 but not injected with MSCs, and from rats given neither MSCs nor CCl4, was used as control samples. Twenty-eight days post-injection, intravenously-injected MSCs were close to the level observed in normal rats. However, intraperitoneally-injected MSCs had not enough effective treatment when compared with MSCs intravenous injection group. The observed levels in the intrahepatic injection group fell between the other MSC-treated groups, but the levels were still closer to the levels observed in the intravenous injection group (Figure 4).
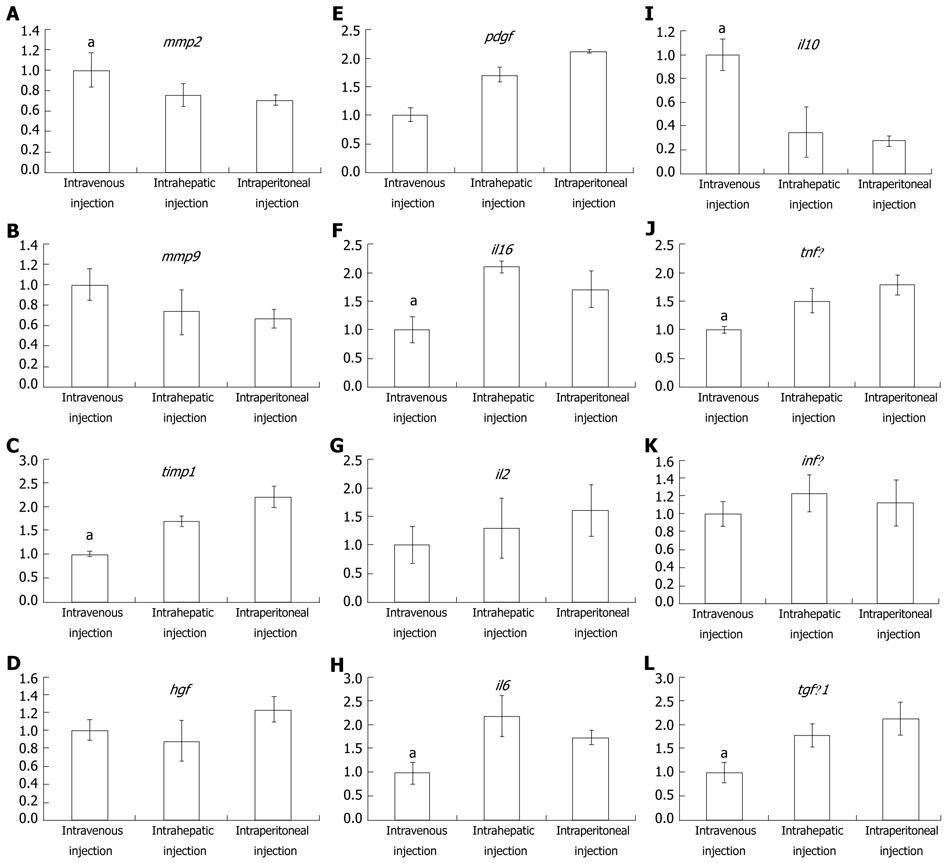
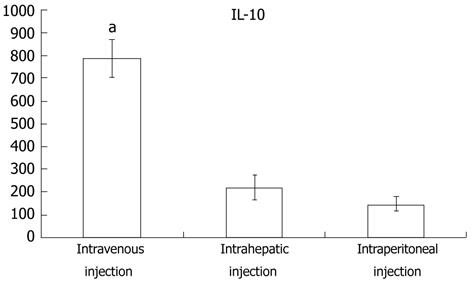
To further explore why intravenously-injected MSC treatment was more effective than either intraperitoneal or intrahepatic injection, real-time PCR was used to quantitatively analyze the expression difference among the 3 transplant methods. As seen in Figure 5A and B, mmp2 and mmp9 were expressed at their highest levels in the intravenous injection group, whereas timp1 expression was the lowest in this group. The intraperitoneal injection group, in contrast to the intravenous injection group, had the lowest mmp2 and mmp9 expression and the highest timp1 expression. These results were in accordance with the observation that the fibrosis observed in the intravenous injection group was low relative to the two other groups. Besides assaying mmp and timp expression, cytokine expression was also assayed to detect whether the immune response played a pivotal role in the differential treatment response among the three experimental groups. The expression of hgf was slightly higher in the intravenous injection group than in the other two groups (Figure 5D). platelet-derived growth factor (PDGF), an important fibrosis enhancer, was not significantly different among the three groups. Similarly, expression levels of pdgf, infγ and il2 were not significantly different among the three groups (Figure 5E, K and G). In the intravenous injection group, however, the expression levels of il1β, il6, tnfα and tgfβ were significantly lower than in other groups, and the intraperitoneal injection group had the highest expression of these genes (Figure 5F, H, J and L). Interestingly, in the intravenous injection group, il10, a key factor in the regulation of the Th1-mediated immune response and homeostasis between matrix metalloproteinase (MMP) and the tissue inhibitors of the MMP (TIMP), was significantly higher when compared to the intrahepatic and intraperitoneal injection groups. Furthermore, the blood ELISA assay also indicated that IL10 production in the intravenous injection group was higher than in the other two groups (Figure 6).
Liver fibrosis results from chronic injury to the liver in conjunction with excessive deposition of collagen and other components of the extracellular matrix (ECM), which is a characteristic of most types of chronic liver diseases[1]. If not effectively treated in time, liver fibrosis may transform into liver cirrhosis. Cirrhosis causes a number of complicating diseases, such as portal hypertension, hepatic encephalopathy, and gastrointestinal bleeding[2]. Thus, the discovery of a new approach to treating this disease would provide great clinical value. CCl4-induced hepatocyte necrosis has the ability to imitate human liver fibrosis disease in animals and has so far been a main method used in the development of animal treatment models[2,3]. In this study, CCl4 was used to induce liver fibrosis in SD rats according to previous protocols. Histological staining indicated collagen deposition in the liver, an indicator of fibrosis. Further liver function testing revealed that ALB, TBIL and ALT levels changed from their normal, baseline levels of 3.65 ± 0.18, 0.049 ± 0.019, and 48.70 ± 9.86 to 3.02 ± 0.22, 1.018 ± 0.114, and 1458.78 ± 230.07, respectively. These findings, in accordance with previous reports, all indicate that liver fibrosis was induced by CCl4.
Liver transplantation is currently considered the best treatment for liver cirrhosis caused by fibrosis. However, there are some disadvantages that limit the expansive use of the procedure, such as liver shortages, high risks associated with surgery, and the risk of further post-surgery complications[3]. Although hepatocyte therapy has been proven to improve liver function, immune rejection and hepatocyte disorder in in vitro cultures have provided obstacles to the expansive use of this type of therapy. MSCs, in recent years, have shown potential as a post-transplantation fibrosis cure[24,25]. MSCs derived from bone marrow also contain HSCs and multipotent adult progenitor cells. Thus, fluorescence-activated cell sorting in this study was performed to identify the type of cultured cells obtained. More than 98% of cells stained positive for CD29 and CD90, which is in accordance with the diagnostic characteristics of MSCs. The cells stained negative for CD34 and CD45, which are indicative of hematopoietic cell lines. These results demonstrate that the cultured cells derived from the rat bone marrow consisted of more than 98% MSCs. HSCs that produce all of the blood lineages and liver epithelium have been previously shown to be capable of acting as a substitute for hepatocytes in transplantation in a murine model of tyrosinemia[26,27]. The benefits to the regenerating liver in the transplant recipients are derived from donor HSCs that fuse with host hepatocytes, rather than from transdifferentiating hematopoietic stem cells or hepatic stem cells present in bone marrow[15,16]. In a study by Sato et al[17], unlike HSCs, MSCs grafted directly to livers that had sustained chronic damage from allyl alcohol treatment were more successful than MSC+ and non-MSC/CD34- cells in differentiating into hepatocytes without any evidence of fusion. In vitro, multiple studies, including those of our group, indicate that MSCs can also differentiate into hepatocytes when induced by cytokines, such as HGF, FGF-4 and EGF[28]. To assay the degree of hepatocyte differentiation of MSCs, real-time PCR and immunofluorescence staining were performed. High expression levels of AFP, ALB and CK18 were noted in induced MSCs when compared to non-induced MSCs. While some studies have examined only one marker, or a small number of them, most published data refer to multiple markers, of which expression is assessed at both the protein and mRNA levels. One of the most widely used markers is albumin secretion, together with the evaluation of AFP, metabolic enzymes, and cytoskeletal proteins[19]. AFP in the liver is a marker of immature liver cells or oval cells in adult livers. Albumin is a typical marker of mature hepatocytes. CK18 is expressed by several liver cell types, including biliary epithelial cells and hepatic oval cells. In particular, a “cytokeratin switch” can be observed as a later process in the maturation of hepatocytes from bipotential progenitors. In fact, bipotential hepatoblasts express both CK-18 and CK-19, while mature hepatocytes express CK-18 alone, and CK-19 specifically identifies cholangiocyte populations[17].
While MSCs are not the only reported adult stem cells that can treat liver fibrosis, they have advantages that make them more favorable for their use in clinical treatment, such as easy accessibility, minimal invasiveness, and fast proliferation[12]. In this study, we injected MSCs isolated from bone marrow into SD rats at three different body regions. We found that the liver function of the three groups was elevated compared to the control group. These observations were made by the biochemical analysis of ALB, TBIL and ALT and histological staining of collagen deposition. Although there was an elevation of liver function in all of the experimental groups, there were also differences among the groups. The expression of α-SMA, which plays a pivotal role in liver fibrosis, was also significantly reduced after MSC transplantation. These results are similar to those seen in many previous reports and suggest that, apart from expressing specific markers, differentiated cells are capable of carrying out the functional activities of mature hepatocytes, which are involved in the supportive functions needed for regenerative medicine applications. These enzymatic functions should also be considered as more reliable “markers” of the successful differentiation of MSCs.
So far, there are two primary methods, non-induced and hepatocyte-induced MSC injection, for the treatment of livers with MSCs. Although these two methods have a potent ability to reverse liver fibrosis, the mechanism of treatment for non-induced MSCs is still unknown. There may be two factors by which MSCs protect against liver fibrosis. One is that MSCs which differentiate into hepatocytes, because of the in vivo niche, secrete numerous growth factors that promote liver regeneration[29,30]. Another possibility is that MSCs suppress hepatic stellate cell activity and secrete MMP, thereby eliminating ECM deposition[31]. In contrast to the primary rationale for using non-induced MSCs to treat liver fibrosis, some studies have demonstrated that MSCs injected into rats with cirrhotic livers differentiate mainly into myofibroblasts and hepatic stellate cells, both of which are promoters of liver fibrosis[32,33]. In the present study, to avoid liver fibrosis aggravation, transplanted MSCs were induced to differentiate into hepatocytes. The results of this method appear to promote improved liver regeneration, rather than liver cirrhosis aggravation, in CCl4-induced fibrotic rats.
In previous reports, Kuo et al[12] demonstrated that intravenous MSC-derived hepatocyte transplantation was more effective in rescuing liver failure than intrasplenic transplantation. In this paper, we extended the effective comparison of MSC-engrafted sites, including intravenous, intraperitoneal, and intrahepatic transplantation. In agreement with the results of Kuo et al[12], intravenous transplantation in our experiments was better at rescuing liver fibrosis than transplantation via the other two methods. The effectiveness of intraperitoneal transplantation was better in the non-MSC-treated groups than in the other treated groups. The difference among the three groups was stratified by not only liver biochemical functional differences and collagen deposition but also by the distribution of MSC-derived hepatocytes in the recipient liver. Although the results of the present study and those of the Kuo group study are valuable for guiding MSC therapy for liver disease, they do not fully elucidate the causes of these transplant-site effectiveness differences.
Thus, we assayed for protein and mRNA expression differences of cytokines and interleukins that play an important role in fibrosis progression. HGF, a powerful mitotic promoter for hepatocytes, also has the ability to restrain collagen and transforming growth factor (TGF)β1 expression, thereby suppressing hepatic stellate cell activity[34,35]. PDGF can enhance liver fibrosis via stimulation of hepatic stellate cells expressing collagen and TIMP[36]. In this study, the mRNA expression of these two factors, however, was not significantly different among the three groups. Thus, they are not the factors leading to the observed difference caused by injection in different MSC transplant sites. Similarly, elevations of hgf, pdgf, infγ and il2 were also detected, but the differences did not reach statistical significance. il1β, il6, tnfα, and tgfβ were expressed at significantly lower levels after intravenous injection than after intraperitoneal or intrahepatic injection. The expression of il10, however, was highest in the intravenous injection group. These results demonstrate that IL10 expression may play a central role in mediating the superior effects of intravenously injected MSCs in ameliorating liver fibrosis. IL10 is an inhibitor of many cytokines that stimulate tissue fibrosis, such as IL6, tumor necrosis factor-alpha and TGFβ. In addition, IL10 can suppress TIMP-1 expression and thereby relieve MMP-1 to degrade liver collagen deposits[37,38]. The increased expression of timp1 following intravenous injection is in contrast to the expression following other injection modalities, which supports such a viewpoint (Figure 5C). According to previous reports, an intravenous injection of MSCs can beneficially modulate the host immune response by increasing the release of prostaglandin E2 from the BM-derived MSCs acting on the EP2 and EP4 receptors of the macrophages and by stimulating the production and release of IL10[39]. Blood ELISA assays indicated that IL10 expression in the intravenous injection group was higher than that in other groups. These results support the viewpoint that the main advantage provided by MSCs transplanted via injection into the venous blood is the stimulation of IL10 release, thereby supporting this method as an effective treatment of liver fibrosis.
In summary, MSC-derived hepatocytes are able to protect against liver fibrosis induced by CCl4. Intravenous injection, in contrast to the intraperitoneal and intrahepatic injection of MSCs, provides the most effective treatment to prevent fibrosis. These results are due to the fact that MSCs in the venous blood can stimulate IL10 release, which, in turn, can modulate the host immune response and homeostasis between TIMP and MMP.
Liver fibrosis causes many deadly diseases. Previous reports indicate that mesenchymal stem cells (MSCs) can facilitate recovery from chemically-induced liver damage and decrease liver fibrosis.
In recent years, MSCs have been reported in many studies to promote liver fibrosis regeneration at many different transplanted sites. However, there are no critical comparisons of how different transplant sites influence bone marrow MSC-based therapy for liver fibrosis, and the molecular mechanisms that influence the treatment differences are unknown.
This study compares the treatment effectiveness of MSCs for intravenous, intrahepatic and intraperitoneal injection. The results suggest that intravenous injection, in contrast to intraperitoneal and intrahepatic MSC injection, provides the most effective treatment to prevent fibrosis. These results are due to the fact that MSCs in the venous blood can stimulate interleukin-10 (IL-10) release, which, in turn, can modulate the host immune response and homeostasis between tissue inhibitor of metalloproteinase and matrix metalloproteinases.
This comparative study of treatment effectiveness influenced by transplanted sites supports the therapeutic principle of future MSC treatment.
Liver injury caused by chemical damage or viral infection often leads to liver fibrosis, which can lead to an impairment of liver function that requires medical intervention. MSCs that have been found to reside in most organs and tissues can be differentiated into hepatocyte-like cells in vitro, and rescue liver fibrosis. The authors found MSCs intravenously injected into the body impeded liver fibrosis caused by carbon tetrachloride more than intraperitoneal and intrahepatic injection. IL-10, which is a regulator of host immune response and metabolism homeostasis, may play an important role in mediating this phenomenon.
The authors compared the liver fibrosis treatment effectiveness among different MSCs injection modalities. The study revealed that MSCs intravenously transplanted evidently improved the functional protein expression and reduced the collagen deposition in injured liver more than other injection modalities. And further mechanism detection suggested intravenous injection of MSCs releases more IL10 that can ameliorate liver fibrosis by down-regulation of il1β, il6, tnfα, and tgfβ expression. The results may represent a molecular mechanism of MSC therapy in liver injury.
Peer reviewer: Andi Utama, PhD, Molecular Epidemiology Division, Mochtar Riady Institute For Nanotechnology, Jl. Boulevard Jend. Sudirman 1688, Lippo Karawaci-Tangerang, Banten 15810, Indonesia
S- Editor Sun H L- Editor Logan S E- Editor Li JY
| 1. | Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655-1669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1928] [Cited by in F6Publishing: 2053] [Article Influence: 128.3] [Reference Citation Analysis (0)] |
| 2. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3381] [Cited by in F6Publishing: 3806] [Article Influence: 200.3] [Reference Citation Analysis (3)] |
| 3. | Lee DS, Gil WH, Lee HH, Lee KW, Lee SK, Kim SJ, Choi SH, Heo JS, Hyon WS, Kim GS. Factors affecting graft survival after living donor liver transplantation. Transplant Proc. 2004;36:2255-2256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Lázaro CA, Croager EJ, Mitchell C, Campbell JS, Yu C, Foraker J, Rhim JA, Yeoh GC, Fausto N. Establishment, characterization, and long-term maintenance of cultures of human fetal hepatocytes. Hepatology. 2003;38:1095-1106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 143] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Chou SH, Kuo TK, Liu M, Lee OK. In utero transplantation of human bone marrow-derived multipotent mesenchymal stem cells in mice. J Orthop Res. 2006;24:301-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Lee RH, Hsu SC, Munoz J, Jung JS, Lee NR, Pochampally R, Prockop DJ. A subset of human rapidly self-renewing marrow stromal cells preferentially engraft in mice. Blood. 2006;107:2153-2161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Kassem M, Kristiansen M, Abdallah BM. Mesenchymal stem cells: cell biology and potential use in therapy. Basic Clin Pharmacol Toxicol. 2004;95:209-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 158] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Le Blanc K, Pittenger M. Mesenchymal stem cells: progress toward promise. Cytotherapy. 2005;7:36-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 167] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 9. | Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568-584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1203] [Cited by in F6Publishing: 1150] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 10. | Lee KD, Kuo TK, Whang-Peng J, Chung YF, Lin CT, Chou SH, Chen JR, Chen YP, Lee OK. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004;40:1275-1284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 668] [Cited by in F6Publishing: 646] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 11. | Fang B, Shi M, Liao L, Yang S, Liu Y, Zhao RC. Systemic infusion of FLK1(+) mesenchymal stem cells ameliorate carbon tetrachloride-induced liver fibrosis in mice. Transplantation. 2004;78:83-88. [PubMed] [Cited in This Article: ] |
| 12. | Kuo TK, Hung SP, Chuang CH, Chen CT, Shih YR, Fang SC, Yang VW, Lee OK. Stem cell therapy for liver disease: parameters governing the success of using bone marrow mesenchymal stem cells. Gastroenterology. 2008;134:2111-2121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 333] [Cited by in F6Publishing: 341] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 13. | Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229-1234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1764] [Cited by in F6Publishing: 1845] [Article Influence: 76.9] [Reference Citation Analysis (0)] |
| 14. | Theise ND, Nimmakayalu M, Gardner R, Illei PB, Morgan G, Teperman L, Henegariu O, Krause DS. Liver from bone marrow in humans. Hepatology. 2000;32:11-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 900] [Cited by in F6Publishing: 962] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 15. | Vassilopoulos G, Wang PR, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature. 2003;422:901-904. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1005] [Cited by in F6Publishing: 1044] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 16. | Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897-901. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1230] [Cited by in F6Publishing: 1276] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 17. | Sato Y, Araki H, Kato J, Nakamura K, Kawano Y, Kobune M, Sato T, Miyanishi K, Takayama T, Takahashi M. Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood. 2005;106:756-763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 449] [Cited by in F6Publishing: 487] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 18. | Zhao DC, Lei JX, Chen R, Yu WH, Zhang XM, Li SN, Xiang P. Bone marrow-derived mesenchymal stem cells protect against experimental liver fibrosis in rats. World J Gastroenterol. 2005;11:3431-3440. [PubMed] [Cited in This Article: ] |
| 19. | Abdel Aziz MT, Atta HM, Mahfouz S, Fouad HH, Roshdy NK, Ahmed HH, Rashed LA, Sabry D, Hassouna AA, Hasan NM. Therapeutic potential of bone marrow-derived mesenchymal stem cells on experimental liver fibrosis. Clin Biochem. 2007;40:893-899. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 222] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 20. | Li C, Kong Y, Wang H, Wang S, Yu H, Liu X, Yang L, Jiang X, Li L, Li L. Homing of bone marrow mesenchymal stem cells mediated by sphingosine 1-phosphate contributes to liver fibrosis. J Hepatol. 2009;50:1174-1183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 21. | di Bonzo LV, Ferrero I, Cravanzola C, Mareschi K, Rustichell D, Novo E, Sanavio F, Cannito S, Zamara E, Bertero M. Human mesenchymal stem cells as a two-edged sword in hepatic regenerative medicine: engraftment and hepatocyte differentiation versus profibrogenic potential. Gut. 2008;57:223-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 202] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 22. | Chang YJ, Liu JW, Lin PC, Sun LY, Peng CW, Luo GH, Chen TM, Lee RP, Lin SZ, Harn HJ. Mesenchymal stem cells facilitate recovery from chemically induced liver damage and decrease liver fibrosis. Life Sci. 2009;85:517-525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Sakaida I, Terai S, Yamamoto N, Aoyama K, Ishikawa T, Nishina H, Okita K. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology. 2004;40:1304-1311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 423] [Cited by in F6Publishing: 407] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 24. | Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, Hovell C, Arthur MJ. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538-549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 785] [Cited by in F6Publishing: 789] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 25. | Piryaei A, Valojerdi MR, Shahsavani M, Baharvand H. Differentiation of bone marrow-derived mesenchymal stem cells into hepatocyte-like cells on nanofibers and their transplantation into a carbon tetrachloride-induced liver fibrosis model. Stem Cell Rev. 2011;7:103-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 26. | Khurana S, Mukhopadhyay A. Characterization of the potential subpopulation of bone marrow cells involved in the repair of injured liver tissue. Stem Cell. 2007;25:1439-1447. [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, Novelli M, Prentice G, Williamson J, Wright NA. Hepatocytes from non-hepatic adult stem cells. Nature. 2000;406:257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 779] [Cited by in F6Publishing: 737] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 28. | Kang XQ, Zang WJ, Bao LJ, Li DL, Song TS, Xu XL, Yu XJ. Fibroblast growth factor-4 and hepatocyte growth factor induce differentiation of human umbilical cord blood-derived mesenchymal stem cells into hepatocytes. World J Gastroenterol. 2005;11:7461-7465. [PubMed] [Cited in This Article: ] |
| 29. | Takeda M, Yamamoto M, Isoda K, Higashiyama S, Hirose M, Ohgushi H, Kawase M, Yagi K. Availability of bone marrow stromal cells in three-dimensional coculture with hepatocytes and transplantation into liver-damaged mice. J Biosci Bioeng. 2005;100:77-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, Lu M, Zhu Z, Chopp M. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003;92:692-699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 524] [Cited by in F6Publishing: 495] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 31. | Yannaki E, Athanasiou E, Xagorari A, Constantinou V, Batsis I, Kaloyannidis P, Proya E, Anagnostopoulos A, Fassas A. G-CSF-primed hematopoietic stem cells or G-CSF per se accelerate recovery and improve survival after liver injury, predominantly by promoting endogenous repair programs. Exp Hematol. 2005;33:108-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 161] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 32. | Russo FP, Alison MR, Bigger BW, Amofah E, Florou A, Amin F, Bou-Gharios G, Jeffery R, Iredale JP, Forbes SJ. The bone marrow functionally contributes to liver fibrosis. Gastroenterology. 2006;130:1807-1821. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 370] [Cited by in F6Publishing: 338] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 33. | Carvalho AB, Quintanilha LF, Dias JV, Paredes BD, Mannheimer EG, Carvalho FG, Asensi KD, Gutfilen B, Fonseca LM, Resende CM. Bone marrow multipotent mesenchymal stromal cells do not reduce fibrosis or improve function in a rat model of severe chronic liver injury. Stem Cells. 2008;26:1307-1314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 34. | Yasuda H, Imai E, Shiota A, Fujise N, Morinaga T, Higashio K. Antifibrogenic effect of a deletion variant of hepatocyte growth factor on liver fibrosis in rats. Hepatology. 1996;24:636-642. [PubMed] [DOI] [Cited in This Article: ] |
| 35. | Sanz S, Pucilowska JB, Liu S, Rodríguez-Ortigosa CM, Lund PK, Brenner DA, Fuller CR, Simmons JG, Pardo A, Martínez-Chantar ML. Expression of insulin-like growth factor I by activated hepatic stellate cells reduces fibrogenesis and enhances regeneration after liver injury. Gut. 2005;54:134-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 36. | Adachi T, Togashi H, Suzuki A, Kasai S, Ito J, Sugahara K, Kawata S. NAD(P)H oxidase plays a crucial role in PDGF-induced proliferation of hepatic stellate cells. Hepatology. 2005;41:1272-1281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 133] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 37. | Zheng WD, Zhang LJ, Shi MN, Chen ZX, Chen YX, Huang YH, Wang XZ. Expression of matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-1 in hepatic stellate cells during rat hepatic fibrosis and its intervention by IL-10. World J Gastroenterol. 2005;11:1753-1758. [PubMed] [Cited in This Article: ] |
| 38. | Zhang LJ, Yu JP, Li D, Huang YH, Chen ZX, Wang XZ. Effects of cytokines on carbon tetrachloride-induced hepatic fibrogenesis in rats. World J Gastroenterol. 2004;10:77-81. [PubMed] [Cited in This Article: ] |
| 39. | Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1678] [Cited by in F6Publishing: 1742] [Article Influence: 108.9] [Reference Citation Analysis (1)] |